Abstract
Although rare, extra-pulmonary inflammatory myofibroblastic tumors (IMTs) are becoming increasingly recognized. While surgical resection is currently an effective and accepted treatment for IMTs, the optimal management of unresectable or residual IMTs remains a clinical dilemma. We present the case of an incompletely resected IMT treated successfully with anti-inflammatory therapy alone, and describe the rationale for this approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extra-pulmonary inflammatory myofibroblastic tumors (IMTs) are rare entities that are increasingly recognized in the medical literature [1]. IMTs are composed of a characteristic spindle cell proliferation with associated inflammatory components and are named from the myofibroblasts which constitute the spindle cell population [2]. As the majority of these unusual tumors have been reported in children [3], the guidelines for the treatment of IMTs in adults are speculative and sparse. The inability to adequately predict the clinical behavior of IMTs, which ranges from relatively benign to highly aggressive and metastatic, further complicates treatment recommendations [1, 4]. An additional impediment to treatment guidance is that the term IMT has been used synonymously with inflammatory pseudotumor, benign myofibroblastic tumor, inflammatory fibrosarcoma, inflammatory myofibrohistiocytic proliferation, omental–mesenteric myxoid hamartoma, plasma cell pseudotumor, and plasma cell granuloma in the historical literature [5]. The exact etiology of IMTs has yet to be defined, but a known chromosomal rearrangement involving 2p23 and subsequent alteration of the ALK gene have been associated with approximately 50% of IMT cases [1]. Based upon reports of clinically aggressive disease and known genetic abnormalities, IMTs are recommended to be treated as true neoplastic processes.
Although examples of abdominal IMTs are infrequent, reported cases have demonstrated surgical resection as a potentially curative treatment with recurrences dependent upon the completeness of excision [2, 6]. However, the therapy for patients with unresectable or incompletely resected tumors is a clinical dilemma. Interestingly, IMTs have been shown to respond to anti-inflammatory therapy and to occasionally exhibit complete regressions [7, 8]. Given this background, we present the case of a 28-year-old female diagnosed and treated for an unresectable mesenteric IMT.
Case report
A 28-year-old female presented with complaints of intermittent abdominal pain that increased over the course of 2 years. In 2008, she underwent a laparoscopic cholecystectomy with only mild relief of her symptoms. In February 2010, her abdominal pain began to escalate significantly and was associated with episodes of nausea and vomiting. Due to the progression of her symptoms, she underwent a computed tomography (CT) scan that demonstrated a 5.5-cm, well-marginated, yet heterogeneous-appearing mass located near the root of the small bowel mesentery (Fig. 1a). The mass was relatively lower in CT attenuation centrally. Abdominal magnetic resonance imaging (MRI) subsequently performed for further characterization revealed that the central component of this mass was decreased in T1 and T2 signal, which could represent internal blood products or fibrous tissue (Fig. 1b). There were slightly more mesenteric lymph nodes than usual located along the inferior margin of the mass, and these appeared subcentimeter and rounded. There was no additional abdominal lymphadenopathy, ascites, or abnormal small or large bowel. The radiological differential diagnosis included lymphoma, malignant fibrous histiocytoma, desmoid tumor, or a sarcoma. Her gastrointestinal symptoms were believed to be secondary to a partial mechanical small bowel obstruction.
a Enhanced abdominal CT at initial time of presentation reveals a 5.5-cm rounded well-marginated centrally lower attenuation mass near the root of the small bowel mesentery (white arrow); a SMA, v SMV. b Axial T2 fat-saturated MRI image demonstrates decreased signal intensity centrally within the mesenteric mass, suggesting fibrous tissue (white arrow)
At an outside facility, she underwent an exploratory laparotomy with the intent of resecting the mass for tissue diagnosis and potential relief of her symptoms. Complete resection was unsuccessful secondary to the fibrotic nature of the tumor and adherence to the root of the mesentery near the superior mesenteric artery. Frozen sections were consistent with a desmoid tumor or possibly gastrointestinal stromal tumor. Final pathological review showed a moderately dense bland spindled to stellate population of neoplastic cells with minimal to no atypia set in a hyaline stroma with frequent scattered inflammatory cells (Fig. 2a). Mitotic figures were rare to absent and necrosis was not identified. An immunohistochemical stain for smooth muscle actin (Clone 1A4, DakoCytomation, Carpenteria, CA, USA) was diffusely positive in the neoplastic cells (Fig. 2b), while a stain for ALK-1 (Anti-CD246, Clone ALK1, DakoCytomation) was negative (Fig. 2c), as is typical for most cases of IMT. Additional immunohistochemistry for cyclooxygenase-2 (COX-2) (Clone SP-1, Neomarkers, Fremont, CA, USA) demonstrated weak cytoplasmic reactivity in lesional spindled cells with strong reactivity in infiltrating mononuclear inflammatory cells (Fig. 2d), suggesting COX-2 as a potential therapeutic target.
a Moderately dense spindle proliferation with minimal atypia and frequent associated inflammatory cells typical of IMT (H&E, ×4, inset ×20), b positive immunohistochemical staining for marker of smooth muscle differentiation (smooth muscle actin, ×40), c negative reaction for ALK-1 (×40), and d COX-2 immunohistochemistry reveals weak cytoplasmic reactivity in lesional spindled cells (×20) with strong reactivity in infiltrating mononuclear inflammatory cells (see inset, ×10)
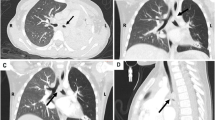
After referral to our institution, 2 weeks after her aborted surgery the patient underwent a repeat CT scan that indicated an increase in the size of her tumor, to approximately 5 × 6 cm (Fig. 3a, b). Stranding had developed within the periphery of the mass and the mesentery, indicative of inflammation. The tumor also appeared to be in close proximity to the superior mesenteric artery, making complete resection difficult or unlikely. Secondary to the persistence of her symptoms of nausea, vomiting, and severe and often debilitating abdominal pain, she was evaluated for a partial small bowel obstruction; however, an upper gastrointestinal study performed did not reveal evidence of obstruction.
a Coronal and b axial maximum intensity projection (MIP) reconstructed images from arterial phase enhanced CT through mesentery depict superior mesenteric artery (black arrow) and jejunal branches (white arrowheads) in relation to the mass (white arrows). Note small bowel staple line inferior to mass from prior incomplete surgical resection
Given these radiographic and pathological findings, the patient was placed on a course of steroids (prednisone 20 mg/day for 3 weeks), a nonsteroidal anti-inflammatory drug (celecoxib 200 mg/day), and a proton pump inhibitor (pantoprazole 40 mg/day) for gastritis prophylaxis. Initially, adequate pain control required narcotic medications adjusted by the pain management team. Gradually over the course of 8 weeks, she was successfully weaned off all narcotic pain medications, had significant improvement in her symptoms, and began to resume her normal activities. A short-interval CT scan performed 3 months after the initiation of anti-inflammatory therapy revealed near-complete resolution of the prior mesenteric inflammation and decrease in size of the mass to 3.5 × 4.2 cm (Fig. 4). Symptomatic and radiographic improvement suggested the effectiveness of the anti-inflammatory therapy in this COX-2-positive tumor within a relatively short time period.The patient was therefore continued on a daily regimen of celecoxib, and subsequent surveillance imaging has revealed stable disease. The celecoxib, which was well-tolerated, will be continued until there is either complete radiographic resolution or a prolonged period of radiographic and symptom stability.
Discussion
Conclusive literature regarding the non-surgical management of extra-pulmonary IMTs is nonexistent. While complete surgical excision is recommended as the definitive therapy, the optimal treatment for an unresectable IMT remains a dilemma. There are no specific radiographic findings associated with IMTs [5], thus histological evaluation of tissue is required for confirmation and to exclude malignancy. Our patient presented to an outside facility and obtained a tissue biopsy during a failed attempt at resection. While the radiographic features can be deceptive, the pathological features of IMTs are fairly consistent, with stromal or spindle cells interspersed with some type of inflammatory cells being most frequently described [2]. Tissue for definitive diagnosis could have been obtained by a core needle biopsy at initial presentation, and this is the approach we would have employed at our institute. As the imaging indicated a low probability of complete resection given its location, knowing the pathological diagnosis would have likely spared this patient a laparotomy and an exacerbation of her inflammatory process and associated symptoms.
It has been hypothesized that several inciting agents may generate an exaggerated response to tissue injury resulting in IMT formation [1]. Previous case reports demonstrate the association of local inflammation in the progression of IMTs which supports a potential role for anti-inflammatory treatments. There are infrequent reports of adjuvant therapy with steroids, radiotherapy, chemotherapy and nonsteroidal anti-inflammatory drugs [2]. Currently, there is no consensus regarding the most effective nonsurgical therapeutic course. A recent small study reported that the majority of sampled IMTs expressed vascular endothelial growth factor (VEGF) and COX-2, arguing for the use of anti-inflammatory medications as an appropriate first-line choice in medical management [9]. An additional report of two patients with abdominal IMTs demonstrated the complete regression of an extensive retroperitoneal IMT and stabilization of disease with the use of anti-inflammatory agents alone [8]. In patients with prohibitive risks for surgical resection, medical management with the use of nonsteroidal anti-inflammatory medications is a viable option, and perhaps even a first-line option, that should be further explored in this patient group. Our presented case supports the rationale for targeting COX-2 with medical therapy, as it was effective in obviating surgical intervention.
In summary, en bloc surgical resection of extra-pulmonary IMTs remains the mainstay of treatment, as complete resection has been historically associated with a low rate of recurrence. However, in patients with either recurrent or non-resectable IMTs, treatment with anti-inflammatory agents may be definitive and substantially less morbid than surgery, chemotherapy, or radiotherapy. Furthermore, it can be inferred that anti-inflammatory therapy may serve as a useful adjunct even in resectable IMTs, as it may promote tumor down-sizing prior to surgery or eliminate the need for invasive procedures.
References
Gleason BC, Hornick JL (2008) Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol 61:428–437
Kovach SJ, Fischer AC, Katzman PJ et al (2006) Inflammatory myofibroblastic tumors. J Surg Oncol 94:385–391
Meis JM, Enzinger FM (1991) Inflammatory fibrosarcoma of the mesentery and retroperitoneum. A tumor closely simulating inflammatory pseudotumor. Am J Surg Pathol 15:1146–1156
Dehner LP (2000) The enigmatic inflammatory pseudotumours: the current state of our understanding, or misunderstanding. J Pathol 192:277–279
Narla LD, Newman B, Spottswood SS et al (2003) Inflammatory pseudotumor. Radiographics 23:719–729
Imabun S, Namba M, Kina M et al (1998) Inflammatory pseudotumor of the abdomen: report of a case. Surg Today 28:1172–1174
Mattei P, Barnaby K (2008) Rapid regression of duodenal inflammatory myofibroblastic tumor after intravenous ketorolac: case report and review of the literature. J Pediatr Surg 43:1196–1199
Przkora R, Bolder U, Schwarz S et al (2004) Regression of nonresectable inflammatory myofibroblastic tumours after treatment with nonsteroidal anti-inflammatory drugs. Eur J Clin Invest 34:320–321
Applebaum H, Kieran MW, Cripe TP et al (2005) The rationale for nonsteroidal anti-inflammatory drug therapy for inflammatory myofibroblastic tumors: a Children’s Oncology Group study. J Pediatr Surg 40:999–1003 (discussion 1003)
Acknowledgments
The authors would like to recognize the Pathology Coordinating Office of the Cancer and Leukemia Group B (CALGB) for providing the COX-2 immunohistochemistry.
Conflict of interest
The authors have no financial relationship with any commercial entity that has an interest in the subject matter or materials discussed in the manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Shatzel, J., Wooten, K., Ankola, A. et al. Inflammatory myofibroblastic tumor of the mesentery: a clinical dilemma. Int J Clin Oncol 17, 380–384 (2012). https://doi.org/10.1007/s10147-011-0297-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-011-0297-0