Abstract
The bottlenose dolphin (Tursiops truncatus Montagu, 1821) is a regularly observed species in the Mediterranean Sea, but its network organization has never been investigated on a large scale. We described the network macrostructure of the bottlenose dolphin (meta)population inhabiting the Pelagos Sanctuary (a wide protected area located in the north-western portion of the Mediterranean basin) and we analysed its connectivity in relation to the landscape traits. We pooled effort and sighting data collected by 13 different research institutions operating within the Pelagos Sanctuary from 1994 to 2011 to examine the distribution of bottlenose dolphins in the Pelagos study area and then we applied a social network analysis, investigating the association patterns of the photo-identified dolphins (806 individuals in 605 sightings). The bottlenose dolphin (meta)population inhabiting the Pelagos Sanctuary is clustered in discrete units whose borders coincide with habitat breakages. This complex structure seems to be shaped by the geo-morphological and ecological features of the landscape, through a mechanism of local specialization of the resident dolphins. Five distinct clusters were identified in the (meta)population and two of them were solid enough to be further investigated and compared. Significant differences were found in the network parameters, suggesting a different social organization of the clusters, possibly as a consequence of the different local specialization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The social structure of wild populations can be studied by means of network analysis, investigating and measuring the association level of the units identified; the units can be considered as nodes or vertices in a network space, connected by flow links (Sade and Dow 1994; Lusseau et al. 2003; Lusseau and Newman 2004; Borgatti et al. 2009). This can help us to investigate the connectivity level within the network and the genetic/cultural flow through the same units (individuals, clusters, sub-populations). The network structure can be characterized through its social parameters (density, clustering coefficient, average shortest path, etc.), with the aid of specific software such as Netdraw (Borgatti 2002), Ucinet (Borgatti et al. 2002), SOCPROG (Whitehead 2009).
When associated with geographical and landscape parameters, network analysis can also be used to test connectivity between units across the landscape (Urban and Keitt 2001; Storfer et al. 2010; Fletcher et al. 2011). In fact, landscape can play an important role in shaping the network macrostructure of a wild population (or meta-population) and, as a consequence, its genetic structure on a fine level (Manel et al. 2003; Kopps et al. 2015). This is particularly true in philopatric populations, with local units being able to specialize on the residency habitat (or micro-habitat) through culturally and genetically inherited behaviours (Hoelzel and Dover 1991; Knudsen et al. 2010; Kopps et al. 2015).
In this respect two main types of geographical population structure can be identified: continuous clines and sharp boundaries (Manel et al. 2003). In a continuous cline pattern, geographical distance tends to correlate with the genetic/cultural distance of the units and differences are maximum at the extremes of the continuum. However, geo-morphological features of the landscape, such as mountains or rivers, may represent a sharp boundary, a breakage in the habitat continuum which, in turn, can produce a discontinuity in the genetic/cultural structure of the population (Storfer et al. 2010; Kopps et al. 2015).
The bottlenose dolphin (Tursiops truncatus Montagu, 1821) is a cosmopolitan Delphinidae, living in tropical and temperate waters of both hemispheres. Its wide distribution, associated with the resident behaviour (Wells et al. 1987; Wells 1991, 2003), tends to produce a remarkable morphometric differentiation among populations, as a consequence of local selection pressure and genetic drift (Natoli et al. 2004, 2005). The bottlenose dolphin is also known for its opportunistic attitude, being able to exploit the food resources with behavioural local strategies which are culturally transmitted through a matrilineal route (Barros and Odell 1990; Kopps et al. 2015). This opportunistic behaviour often involves human fishing activities as well, such as the regular exploitation of trawlers (Corkeron et al. 1990; Fertl and Leatherwood 1997; Chilvers and Corkeron 2001; Pace et al. 2003) or gillnets (Lauriano et al. 2004; Díaz López 2006; Brotons et al. 2008; Wells and Scott 2009).
The plasticity in foraging behaviour is accompanied with a plasticity in the pattern of association, a flexible social model which was defined as “fission–fusion society” (Connor et al. 2000). This consists of groups of variable size and composition, aggregating, breaking-up and re-aggregating at frequent intervals (Conradt and Roper 2005). This kind of social organization may limit the effect of internal groups rivalry by allowing for the herd to split during periods of high competition (Dunbar 1992; Kummer 1995). It may also improve cooperative behaviour through social cohesion when ecological costs of aggregating are low and/or benefits of sociality are high (Takahata et al. 1994; van Schaik 1999; Wittemyer et al. 2005).
The bottlenose dolphin is considered a commonly occurring species in the Mediterranean Sea (Pilleri and Gihr 1969; Cagnolaro et al. 1983; Notarbartolo di Sciara and Demma 1994) and can be found in most coastal waters of the basin (Bearzi and Fortuna 2006). The Pelagos Sanctuary is a wide protected area located in the North-Western portion of the Mediterranean Sea, which is characterized by a remarkable geomorphological (and ecological) diversity. The bottlenose dolphin is regularly present in this area, with a continuous distribution over the continental shelf (Gnone et al. 2011); the specimens here show a clear philopatric behaviour, performing maximum displacements of about 50 km (on average) and tend to form local units (Gnone et al. 2011).
Based on the above assumptions [(a) the bottlenose dolphin is regularly present in the Pelagos area, with a continuous distribution over the continental shelf, fragmented in discrete neighbouring units showing some sort of local specializations; (b) the landscape is characterized by high geomorphological diversity with sharp boundaries between habitats) our hypothesis is that the network macrostructure of this (meta)population could be shaped by the landscape traits (and its breakages). According to this hypothesis we expect that: (1) network breakages should partially overlap with landscape habitat borders; (2) since the specialization in the foraging activity may influence the aggregation patterns (also considering the plasticity of bottlenose dolphin sociality in the fission–fusion theory), the different units possibly identified on the two sides of the habitat breakage could have a different social organization.
In order to verify our hypothesis, we pooled the sighting data collected by 13 different research institutions operating within the Pelagos Sanctuary from 1994 to 2011. We analysed the network macrostructure of the bottlenose dolphin (meta)population (and its connectivity), investigating the association patterns of the photo-identified dolphins; we overlapped the results to the ecological and geo-morphological traits of the landscape, with special attention to the amplitude and slope gradient of the continental shelf. Subsequently we identified the clusters inhabiting different shelf habitats and we analysed their network parameters, with the objective of identifying possible differences in the social organization.
Materials and methods
Study area
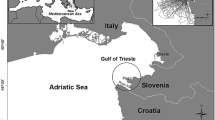
Data were collected in the Pelagos Sanctuary (and immediate adjacent areas), located in the northern part of the western Mediterranean basin (Fig. 1).
The Sanctuary covers an area of 87,500 km2, extending over the waters of Italy, France and Principality of Monaco, including the coasts of Corsica and northern Sardinia (Notarbartolo di Sciara et al. 2008). The Sanctuary bathymetric profile is extremely variable, with a range of oceanographic and physiographic features, from shallow waters with an extended continental shelf (as in the Gulf of La Spezia, the coasts of Tuscany and the Tuscany Archipelago) to deep water zones and steep continental slopes located near the shore (as in the western Ligurian coast, the Côte d’Azur and western Corsica).
Local abundance estimates of bottlenose dolphins in this area have been conducted based on mark-recapture and photo identification techniques (Defran et al. 1990; Wűrsig and Jefferson 1990; Wilson et al. 1999). The most recent and complete study estimated a total population of 954 ± 70 individuals (Gnone et al. 2011) using Chao (Chao et al. 1992) mark-recapture model for closed population.
Data collection
We pooled data gathered from 1994 to 2011 (excluding 1998 for lack of data) by 13 research groups working in different parts of the Sanctuary, during boat-based surveys carried out all year around (Table 1). All surveys were conducted in good light conditions and in calm waters (Douglas scale ≤3); effort tracks and sightings positions were recorded by GPS. Animals were photographed and later identified through natural marks on their dorsal fins (Würsig and Würsig 1977; Wilson et al. 1999).
All the data were used to analyse the distribution of bottlenose dolphins in relation to the sighting effort. When available, photographic data were integrated to produce a unique photo-ID database (Table 1). Photographs were selected based on criteria indicated in Gnone et al. (2011): only high-quality photographs were used and included in the database (Laska et al. 2011) and only adult animals with permanent marks such as notches, deformities, particular pigmentations and unusual fin shapes (Wilson et al. 1999; Chilvers and Corkeron 2003) were considered for photo-identification.
Effort/sighting distribution and capture success
GIS (ESRI 2007) was used to visualize all the sightings of the target species over space in relation to the research effort (Figs. 1, 2). In order to measure the capture success over time and space and to investigate the displacement behaviour of the photo-identified dolphins, the Pelagos area was subdivided in 6 sub-areas according to the traditional study zones of the research groups involved (Fig. 1); the number of individuals photo-identified in each sub-area was calculated over time.
Analysis of the connectivity through the network and landscape
The connectivity through the social network was measured using the association patterns of the photo-identified dolphins. For association analysis, two animals were assumed to be associated if present in the same group (Lusseau et al. 2006; Wiszniewski et al. 2009) and a group was defined as all the individuals within a 100 m radius behaving in a co-ordinated fashion (Irvine et al. 1981; Wells et al. 1987; Shane 1990; Möller et al. 2006). Replicated groups were excluded if sighted more than once in the same day (Smolker et al. 1992; Rossbach and Herzing 1999; Wiszniewski et al. 2009).
We calculated strength of relationships among dyads in the population using the half-weight index (HWI) (Cairns and Schwager 1987; Ginsberg and Young 1992; Bejder et al. 1998) in SOCPROG 2.4 (Whitehead 2009), estimating the proportion of times that two individuals associate in a pair (Whitehead 2009).
where, x is the number of encounters for which both dolphin ‘a’ and ‘b’ were in the same group; y a is the number of encounters including dolphin ‘a’ but not dolphin ‘b’ in the same group; y b is the number of encounters including dolphin ‘b’ but not dolphin ‘a’ in the same group; y ab is the number of encounters including dolphin ‘a’ and ‘b’ in different clusters of groups at the same time.
This index is commonly used in bottlenose dolphin studies (Wells et al. 1987; Smolker et al. 1992; Lusseau et al. 2006; Wiszniewski et al. 2009; Foley et al. 2010) because it accounts for missing members of groups not identified due to implicit bias of sampling techniques (Cairns and Schwager 1987; Whitehead 2008).
In order to obtain an overall representation of the network macrostructure of the population and its connectivity we used the stochastic spring embedding algorithm (Eades 1984; Fruchterman and Reingold 1991) as implemented in Cytoscape 2.8 (Smoot et al. 2011).
In order to investigate the connectivity of the network through the landscape, the spring embedding visualization was integrated with the geographical data and each single spot (corresponding to a different photo-identified individual) was symbolized according to the sighting sub-areas (Fig. 1). Individuals sighted in more than one area were identified with a combination of symbols.
We performed an assortativity analysis per symbol phenotype to measure the propensity of the photo-ID dolphins to pair with individuals coming from the same sub-area (same symbol) (assortnet R package—Farine 2014). Associations between individuals were weighted according to the HWI values (Farine 2014). Dolphins captured in more than one sub-area were assigned to the sub-area with more captures; individuals with equal captures in two or more sub-areas were removed from the analysis.
The same geographical data were used to calculate the HWI between sub-areas, as an expression of the number of links connecting different sighting regions.
Aiming at the verification of the influence of the landscape traits on the connectivity through the network, the bathymetric traits of the continental shelf were considered. To this scope a map of sea bottom slope, calculated as ratio among distance from coast and depth, was employed (Fig. 3).
Identification and social characterization of the clusters in the network
Following this first connectivity analysis, we tried to identify possible (social) clusters within the network. To mitigate the randomness associated to the capture event, this part of the analysis was carried out on a selection of individuals, based on the capture frequency of the photo-identified dolphins (see the “Results”).
We performed a Manly Bejder permutation test on this data selection (Manly 1995; Bejder et al. 1998; Miklós and Podani 2004; Whitehead 2008) in SOCPROG 2.4 (Whitehead 2009) in MatLab 7.0.1 (Matlab 2014) to examine if individuals associate randomly. We increased the number of permutations until the P value stabilized (Whitehead 2009). In case of preferred associations, the SD of the randomized network is lower of the real one in a significant (>95 %) number of permutations (Whitehead 2009).
We used the same spring embedding algorithm to visualize the network of the selected individuals, using the same symbols of the sighting sub-areas.
The Girvan–Newman algorithm, based on edge betweenness measurements (Freeman 1977; Girvan and Newman 2002; Lusseau and Newman 2004; Newman and Girvan 2004), was used to detect the community structure within the network and to identify possible clusters. The best division for the network was identified using a modularity index Q (Newman and Girvan 2004), where the highest Q value indicates the best division (Efron 1979; Newman and Girvan 2004). This index, based on a previous measure of assortative mixing proposed by Newman (2003), varies between 0 (community structure no better than in a random network) and 1 (strong community structure) and it can be considered meaningful if it falls in the range 0.3–0.7 (Chen et al. 2009).
Betweenness centrality was also used to identify possible brokers within the network, measuring the number of geodesic path lengths passing through each vertex. Animals with high betweenness usually connect discrete clusters and may play a central role in spreading genetic and cultural information within the population, together with potential diseases (Lusseau and Newman 2004).
Kernel density estimation (KDE) of the clusters’ home ranges
In relation to the possible clusters identified by the Girvan–Newman analysis, we used ArcGIS 9.3 (ESRI 2007) with the Hawth Tools extension (Beyer 2004) to estimate kernel densities and to identify the cluster’s home ranges and core areas (Formica et al. 2010). Home ranges were considered as the surface contour including 90 % of the cluster sighting events, while core areas were considered as the surface contour including 50 % of the cluster’s sighting events (Silverman 1986; Worton 1995; Kernoham et al. 1998). Least square cross validation was used to smooth the kernel density estimation (Worton 1989; Seaman and Powell 1996).
Social organization of the clusters
Following the hypothesis that isolation between neighbouring (sub)populations (or clusters) may be the consequence of a different habitat specialisation, which may also produce a different social structure, we first calculated the mean size of the herds in the sub-areas where the home ranges of the main clusters identified are located (we averaged all the sightings included within the virtual borders of the same sub-areas).
Subsequently we analysed the same clusters in relation to 4 social network parameters (see below). Differences among the 4 parameters were tested by means of F and Z statistic test and Chi square. All network metrics were calculated and all networks were drawn using Cytoscape 2.8 (Smoot et al. 2011).
Half Weight Index (HWI)
We calculated the cluster average HWI (the mean of the HWI characterizing each link between individuals within the same cluster) and the average HWI for each individual of the clusters (the mean of the HWI characterizing each link connecting a single dolphin to any other individual in the cluster).
Density (Δ)
Density is one of the main descriptive statistics, often used as the primary indicator of the degree of cohesion of the network. The density is defined as the proportion between the ties actually linking the N nodes of the network and the maximum number of links as possible (it varies from 0 to 1).
Clustering coefficient (C)
The clustering coefficient is a measure of the level of individuals sociality (Croft et al. 2004; Whitehead 2008); it gives the probabilities that, if an individual a is connected to two other individuals b and c, b and c are linked as well. We used the average clustering coefficient calculated per individual (it varies from 0 to 1).
Average shortest path from geodesic path length (l)
Considering any pair of individuals of the network, their geodesic path length is the minimum number of edges (or individuals) to step through when moving from one individual to the other (in case two individuals are directly linked, their geodesic path length is 1). The average shortest path l is the average geodesic path length for all possible pairs of individuals in the network.
Results
Effort/sighting analysis and capture success
Effort analysis
A total of 213,651 km of effort tracks were covered during the entire research period (1994–2011). The effort distribution tends to be heterogeneous over space; effort coverage is higher along the coasts and decreases from north to south and from east to west, according to the traditional study areas of the research groups involved. The continental shelf however (<200 m) is well covered by the effort tracks, with the exception of the east side of Corsica and northwest coast of Sardinia (Fig. 2).
The total annual effort increased significantly since 1999 and was maximum in 2005–2006, mainly as a consequence of the addition of new sampling activities in the study area (Table 2). This effort activity produced a total of 986 sightings between 1994 and 2011 (Fig. 1).
The sightings’ position confirms the continuous, shelf limited distribution of the bottlenose dolphin in the study area, with a clear preference for shallow waters below 100 m in depth: over 986 sightings, 951 occurred within the 200 m isobath and 871 within the 100 m isobath. This distribution is not an artefact of a coast limited survey effort, since the effort coverage greatly overcomes the continental shelf, with virtually no results in terms of sighting of the target species.
Capture success
Over a total of 986 sightings, 605 were characterised by data useful for photo-identification purposes. This allowed to reliably identify 806 adult bottlenose dolphins.
The capture distribution over time (years) and space (sub-areas) is summarized in Table 2. The capture success reflects the heterogeneous effort distribution over time/space and the table allows to identify those years with the best capture coverage among sub-areas. Table 2 also shows the high recapture rate within each single sub-area and the low recapture rate between different sub-areas, confirming the philopatric behaviour of the photo-identified dolphins.
The frequency per capture class of the totality of the individuals photo-identified, shows that about 40 % of the dolphins were captured only once and about 66 % were captured 3 times or less. On average, the dolphins were captured 4 times.
Analysis of the connectivity through the network and landscape
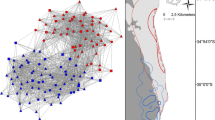
The majority of the individuals (748 of the 806 photo-identified dolphins in 605 sightings) are linked together in one single network, while 58 individuals are apparently excluded (Fig. 4). However the connectivity level within the network follows a complex pattern, with bottlenecks and breakages between units.
The Pelagos bottlenose dolphin network visualized with the spring embedding layout (806 individuals in 605 sightings). The different symbols correspond to the different sub-areas in Fig. 1; in parentheses the number of individuals captured in the same sub-area. Individuals captured in more than one sub-area are represented with a combination of symbols. White points (OUT) represent individuals captured outside the sub-areas
The assortment analysis by capture sub-area was implemented on a total of 775 individuals over 806 photo-identified (25 dolphins were excluded since it was impossible to assign them to a specific sub-area; 6 more were excluded since they were never captured within the sub-areas’ borders). The analysis provides very high values (0.95 weighted assortment) confirming that bottlenose dolphins tend to assort with individuals originating from the same sub-area (same symbol in Fig. 4).
According to the HWI analysis per sub-area (Table 3), the connection level between different capture sub-areas is very diverse and sometimes unexpected, if we only consider the geographical distance between units: C and D are linked by many (60) connections and have the highest HWI (0.222), while only 1 connection links D and E sub-areas (HWI = 0.005), despite their geographical proximity. The HWI of the other sub-areas is usually low (or equal to 0) and only in three occasions overcomes 0.05 (AE, BD and EF). The overall pattern seems to confirm the resident behaviour of the dolphins with diverse connection levels between neighbouring units. When considering the geographical position of the residency sub-areas, in relation to the bathymetric traits of the continental shelf (Fig. 3) we may notice that connectivity bottlenecks and breakages tend to coincide with the geomorphological (and ecological) borders; i.e., sub-area D is positioned over the large continental shelf typical of the eastern portion of the Sanctuary, characterized by a very gentle slope gradient, while sub-area E includes the north-west coast of Corsica, characterized by a narrow continental shelf with a very steep slope. If we consider together all the sub-areas located on the western portion of the study area (A, B, E), which is characterised by a steep rocky platform, and those located on the eastern portion of the Pelagos Sanctuary (C, D) which is characterized by a wide platform with muddy sea floors, we only obtain 22 links over a total population of 692 photo-identified dolphins (HWI = 0.06). Among these, only 1 connection pass through the southern portion of the area, where the habitat breakage is sharper, while 18 links connect the two areas on the north, passing through the C sub-area, which presents intermediate ecological traits (in 3 cases it was impossible to understand the route of the link, since the dolphins were sighted in sub-areas A, B and D but neither in sub-area C nor in sub-area E). This is even more remarkable if we consider that the dolphins sighted and photo-identified on the west coast of Corsica (154 individuals) account for about the 73 % of the total western (sub)population.
Identification and characterization of the clusters in the network
To identify the social clusters within the network we selected those individuals with at least 4 captures (the average capture class of the total photo-identified population, 272 dolphins in 540 sightings, about 34 % of the original network). The data selection produced a different reduction in the number of individuals per sub-area, possibly as a consequence of the unequal distribution of the sampling effort (see Fig. 5).
Network visualization, using the spring embedding layout algorithm, of the bottlenose dolphins captured at least 4 times and Girvan–Newman based clusters characterization. The network is split in 2 main clusters (α and β) plus 3 minor units (γ, δ, ε). The nodes (individuals) are designed as in Fig. 4. Black circles highlights the nodes with highest betweenness (i.e., brokers)
We performed a permutation test on this selection to check the network association pattern; the standard deviation (SD) of the randomized network is lower of the real one in a significant number of permutations (>95 %), confirming that dolphins do have preferred associations.
The Girvan–Newman analysis, implemented on the same data selection, splits the population in five clusters (best division for the network was identified with the value of Q max = 0.374) (Fig. 5); we can easily recognize two major clusters (α, β) plus 3 smaller units (γ, δ, ε) (Fig. 5).
Girvan–Newman algorithm was performed also inside α and β but results show no significant splitting values and are not reported in this paper.
Kernel density estimation (KDE) of the clusters home ranges
We used kernel density estimation analysis to investigate the possible relationships between clusters and their geographical distribution in the Pelagos area (Fig. 6).
Fixed kernel density distribution for the five-bottlenose dolphins clusters visualized in Fig. 5. Volume contour lines represent the 50 % (core areas), and 90 % (home range) for each cluster
All clusters seem to have a well-distinct core area in a particular region of the Pelagos Sanctuary, with the exception of cluster ε: cluster α ranges from Genoa to northern Tuscany, while β core area is located in western Corsica. Core areas of clusters γ and δ are located respectively in the south of Corsica (Strait of Bonifacio) and in the Tuscan Archipelago. Cluster ε has a core area in the Hyères islands, but its individuals can be found in a wide area, extending from Côte d’Azur to Corsica.
All well-defined clusters’ core areas (50 % kernel density estimation) are located inside the continental shelf, with the single exception of cluster ε (Fig. 6).
Brokers
Three dolphins are considered as potential brokers (Lusseau and Newman 2004) based on their high betweenness and relative position in the network (see Fig. 5); these are all adult, well-marked individuals. We could not sex these dolphins through the photos collected, however none of them was never observed in association with newborns or calves, suggesting they are either males or non-reproductive females. Their parameters are summarized in Table 4 and compared with the mean parameters of the network.
The betweenness level of these individuals is very high if compared with the mean of the network, while the degree is slightly lower. The HWI is similar to the mean of the network or lower (individual P276).
Social organization of the clusters
Due to data shortage, we are not able to make further considerations on clusters γ, δ and ε and so only clusters α and β are considered for the following analysis.
We calculated the mean size of the herds sighted in the two sub-areas (C and E) where the home ranges of the two main clusters (α and β) are located. The mean size in sub-area C (n = 501) is 12.9 ± 12.47 (SD), while the mean size in sub-area E (n = 46) is 8.2 ± 6.65 (SD). To compare the two mean size values, we randomly extracted 46 sightings from sample C and we tested the difference with sample E using a non-parametric test (one-way ANOVA Kruskal–Wallis; P < 0.001).
In relation to the network parameters characterizing the two main clusters, results are summarized in Table 5.
The average value of HWI (links) shows that bonds among individuals in cluster β (HWI = 0.23, SD = 0.12) are on average significantly stronger than those among individuals in cluster α (HWI = 0.16, SD = 0.11) (z test = 18.80, P ≪ 0.01).
If we observe the distribution of the HWI (individuals) in the two clusters, again we notice a relevant difference, with β values shifted toward higher value of the HWI (Fig. 7).
Density values indicate that in α approximately 34 % of possible ties are present, while 32 % of possible ties are recorded in β (χ2 = 4.04, P = 0.044).
In both α and β clusters, C values appear quite high (respectively 0.63 and 0.57), suggesting a good sociability level. However C results significantly higher in cluster α (see Table 5).
l values are quite low in both clusters (respectively 1.67 in α and 1.74 in β), despite the different number of nods.
We used Chi square test to weight the difference of the shortest path class frequency (1, 2, 3) in the two clusters (Table 6). Despite the bigger size, cluster α seems to have a better connection between individuals (χ2 = 439, P ≪ 0.01).
Discussion
The analysis of the network connectivity, based on the association pattern of the dolphins photo-identified, confirms our hypotheses.
The nodes (corresponding to the photo-identified individuals) tend to aggregate consistently with the geographical capture areas, possibly as a consequence of the resident behaviour of the animals; the assortative analysis confirms that bottlenose dolphins associate with individuals coming from the same sub-area. However the HWI between different symbols (which reflects the connection level between different sighting sub-areas) is not always consistent with the geographical distance. Sub-areas C and D, for example, have a high number of connections (and the highest HWI), while sub-areas D and E (which are as close) have only 1 link (and an HWI close to 0). This phenomenon is not the artefact of a heterogeneous sampling effort or a bias in the individual recognition over time; the different sub-areas were sampled simultaneously for many years and data gaps between contiguous sub-areas (when present) were relatively short in time, especially considering that bottlenose dolphins are long living animals and that photo-ID regards mostly adult, well-marked individuals, with a low mortality rate.
To explain this network connectivity we should then consider the landscape traits and the ecological distance between different sub-areas together with the geographical one. Sub-areas D and E are very close (just a few km), but their borders do coincide with a remarkable habitat breakage, delimited by the Corsica’s “finger” (Cape Corso): the western coast of the French island (sub-area E) is characterized by a narrow rocky platform and a steep slope, while the Tuscany Archipelago (sub-area D) presents typical shallow water and sandy/muddy ecosystems. On the contrary sub-area C and sub-area D are located along a continuous habitat extension.
Similar results were found in a large-scale study by Natoli et al. (2005) who identified the genetic diversity of bottlenose dolphin populations along a contiguous distributional range from the Black Sea to the eastern North Atlantic and found that the boundaries of the population structure coincided with transitions between habitat regions, suggesting that local populations of bottlenose dolphins are habitat dependent. On a much finer scale, Kopps et al. (2015), studying the bottlenose dolphin population inhabiting Shark Bay (Western Australia) got the same results.
This habitat preference could be related to the ecological specialization in the foraging activity (Hastie et al. 2004; Kopps et al. 2015) which may also include opportunistic feeding on different fishing tools, as reported by Chilvers and Corkeron (2001) in Shark Bay (Australia) and by Gnone et al. (2011) in the same Pelagos area.
The Girvan–Newman analysis, applied on a selection of 272 individuals which were captured at least four times, produces a network clearly split in two main clusters (α and β), which contain together 90 % of the selected individuals, plus three minor units (γ, δ, ε).
Although only preliminary appraisals can be made about clusters γ, δ and ε, due to the data shortage, it is interesting to note how cluster ε seems to play a central role in the network, reinforcing connection between clusters α and β. Animals belonging to this cluster are long travellers and can be found in a wide area, from the Côte d’Azur to south-western of Corsica; they are the only ones showing a home range partially overcoming the continental shelf border (see Fig. 6).
In relation to the network social parameters, the two main clusters (α and β) seem to be structured in a similar way, which reflects the fission–fusion model proposed by Connor et al. (2000), characterised by high density and low HWI; the high clustering coefficient (C) and low average shortest path (l) also suggest a good connection level among individuals. However we found significant differences between the two clusters: α dolphins tend to aggregate in larger groups with a lower binding between individuals, while β ones form smaller herds with a tighter bond between components. Cluster α also has a significant higher clustering coefficient (C) and a lower low average shortest path (l), which reflects more complex (and promiscuous) connections between individuals.
These differences may be the consequence of the local habitat specialization (including different foraging strategies); the wider continental shelf, characterizing the eastern portion of Pelagos Sanctuary, could support bigger groups and/or the foraging activity in α residency area could be favoured by a higher cooperation among individuals. The different foraging strategy may also account for a different cohesion between individuals; according to Díaz López and Bernal Shirai (2008) the cohesion decreased in opportunistic strategy and increased in non-opportunistic foraging. Opportunistic feeding on trawlers is regularly observed in α individuals (Nuti et al. 2006; J. Alessi et al., unpublished data; Bellingeri et al. 2008), while β individuals do not have this opportunity (since trawling is poorly practiced in sub-area E) and direct their opportunistic foraging mainly on gillnets (Rocklin et al. 2009). However many other local variables could contribute in shaping the size of the groups and the association pattern among individuals and a superficial analysis may drive to erroneous considerations. A complete understanding of these items needs a more specific effort and goes beyond the objectives of the present research.
In the population network we identified some individuals that seem to behave as brokers (Lusseau and Newman 2004) between communities (see Fig. 5). The most important edge of the whole network, taking into consideration the betweenness, is represented by the only link connecting directly cluster α to β (P 276); the majority of the shortest paths of the network passes from this link. No brokers were found between cluster β and cluster δ, despite of their geographical closeness.
We could not directly verify the sex of these individuals, but the big size, the high presence of marks on the dorsal fin and, most of all, the complete absence of association with new-borns or calves over time, suggest these could be adult males, possibly looking for reproductive opportunities. This behaviour could favour a genetic flow between neighbouring (sub)populations or clusters (together with potential pathogenic agents).
In the end, we were able to confirm the forecasts associated to our initial hypothesis (see the introduction): (a) the connectivity through the network seems to retrace the landscape traits and its habitat breakages; (b) the units identified on the two sides of the habitat border show significant differences in the social organization.
Following our analysis, we claim that the social network macrostructure of the Pelagos population could be shaped by the geo-morphological and ecological characteristics of the landscape. Local specializations, especially (but not only) in the feeding techniques, seem to produce a segregation between neighbouring dolphins and a clusterization of the (meta)population; the different clusters show differences in the social organization. A few long travelling individuals, possibly adult erratic males looking for new reproductive territories, seem to play a role in connecting neighbouring clusters.
These findings are new and original for the Mediterranean bottlenose dolphin population and provide an insight on how this species distributes and specializes itself according to the geo-morphological and ecological characteristics of the landscape. Furthermore this work contributes to better understand which may be the preferential routes of genetic, cultural and disease flows inside the Pelagos bottlenose dolphin population.
These same findings may serve as a model to investigate the spatial distribution and the network organization of this species in other geographical contexts.
Genetic studies, overlapping the association pattern analysis, would provide further insight on the observed pattern of distribution, producing quantitative data on the isolation/integration level of the clusters identified; this would allow to identify the genetic borders between (sub)populations.
References
Barros NB, Odell DK (1990) Food habits of bottlenose dolphins in the southeastern United States. In: Leatherwood S, Reeves RR (eds) The Bottlenose Dolphin. Academic Press, San Diego, pp 309–328
Bearzi G, Fortuna C (2006) Common bottlenose dolphin (Mediterranean subpopulation). In: Reeves RR, Notarbartolo di Sciara G (compilers and eds) The status and distribution of cetaceans in the Black Sea and Mediterranean Sea. IUCN Centre for Mediterranean Cooperation, Malaga, pp 64–73
Bejder L, Fletcher D, Brager S (1998) A method for testing association patterns of social mammals. Anim Behav 56:719–725
Bellingeri M, Fossa F, Gnone G, Saporiti F, Frigerio L (2008) Different behaviour of bottlenose dolphin (Tursiops truncatus) related with trawlers in the Eastern Ligurian Sea. Eur Res Cetaceans 22
Beyer HL (2004) Hawth’s analysis tools for ArcGIS. http://www.spatialecology.com/htools Accessed 10 Jan 2006
Borgatti SP (2002) NetDraw software for network visualization. Analytic Technologies, Lexington
Borgatti SP, Everett MG, Freeman LC (2002) Ucinet for Windows: software for social network analysis. Analytic Technologies, Harvard
Borgatti SP, Mehra A, Brass DJ, Labianca G (2009) Network analysis in the social sciences. Science 323:892–895
Brotons JM, Grau AM, Rendell L (2008) Estimating the impact of interactions between bottlenose dolphins and artisanal fisheries around the Balearic Islands. Mar Mamm Sci 24:112–127
Cagnolaro L, Di Natale A, Notarbartolo di Sciara G (1983) Cetacei. Guide per il riconoscimento delle specie animali delle acque lagunari e costiere italiane. AQ/1/224, 9. Consiglio Nazionale delle Ricerche, Genova (in Italian)
Cairns JS, Schwager SJ (1987) A comparison of association indices. Anim Behav 35:1454–1469
Chao A, Lee SM, Jeng SL (1992) Estimating population size for capture-recapture data when capture probabilities vary by time and individual animal. Biometrics 48:201–216
Chen J, Zaïane OR, Goebel R (2009) Detecting communities in social networks using max–min modularity. In: Apte C, Park H, Wang K, Zaki Mj (eds) Proceedings of the 2009 SIAM international conference on data mining. SIAM, pp 978–989 doi:10.1137/1.9781611972795.84
Chilvers BL, Corkeron PJ (2001) Trawling and bottlenose dolphins’ social structure. Proc R Soc B Biol Sci 268:1901–1905
Chilvers BL, Corkeron PJ (2003) Abundance of indo-pacific bottlenose dolphins, Tursiops aduncus, off point lookout, Queensland, Australia. Mar Mamm Sci 19:85–95
Connor RC, Wells RS, Mann J, Read AJ (2000) The bottlenose dolphin: social relationships in a fission-fusion society. In: Mann J, Conner RC, Tyack PL, Whitehead H (eds) Cetacean societies: field studies of dolphins and whales. University of Chicago Press, Chicago, pp 91–126
Conradt L, Roper TJ (2005) Consensus decision making in animals. Trends Ecol Evol 20:449–456
Corkeron PJ, Bryden MM, Hedstrom KE (1990) Feeding by bottlenose dolphins in association with trawling operations in Moreton Bay, Australia. In: Leatherwood S, Reeves RR (eds) The bottlenose dolphin. Academic Press, San Diego, pp 329–336
Croft DP, Krause J, James R (2004) Social network in the guppy (Poecilia reticulata). Proc R Soc B Biol Sci 271:516–519
Defran RH, Shultz GM, Weller DW (1990) A technique for the photographic identification and cataloging of dorsal fins of the bottlenose dolphin (Tursiops truncatus). Report of International Whaling Commission 12:53–55
Díaz López B (2006) Interactions between Mediterranean bottlenose dolphins (Tursiops truncatus) and gillnets off Sardinia, Italy. ICES J Mar Sci 63:944–951
Díaz López B, Bernal Shirai JA (2008) Marine aquaculture and bottlenose dolphins’ (Tursiops truncatus) social structure. Behav Ecol Sociobiol 62:887–894
Dunbar RM (1992) Time: a hidden constraint on the behavioral ecology of baboons. Behav Ecol Sociobiol 31:35–49
Eades P (1984) A heuristic for graph drawing. Congr Numer 42:149–160
Efron B (1979) Computers and the theory of statistics: thinking the unthinkable. SIAM Rev 21:460–480
ESRI (2007) ArcGIS Desktop: release 9.3. Environmental System Research Institute, Redlands
Farine DR (2014) Measuring phenotypic assortment in animal social networks: weighted associations are more robust than binary edges. Anim Behav 89:141–153
Fertl D, Leatherwood S (1997) Cetacean interactions with trawls: a preliminary review. J Northwest Atl Fish Sci 22:219–248
Fletcher RJ, Acevedo MA, Reichert BE, Pias KE, Kitchens WM (2011) Social network models predict movement and connectivity in ecological landscapes. Proc Natl Acad Sci USA 108:19282–19287
Foley A, McGrath D, Berrow S, Gerritsen H (2010) Social structure within the bottlenose dolphin (Tursiops truncatus) population in the Shannon Estuary, Ireland. Aquat Mamm 36:372–381
Formica VA, Augat ME, Barnard ME, Butterfield RE, Wood CW, Brodie ED III (2010) Using home ranges estimates to construct social networks for species with indirect behavioral interactions. Behav Ecol Sociobiol 64:1199–1208
Freeman LC (1977) A set of measures of centrality based upon betweenness. Sociometry 40:35–41
Fruchterman TMJ, Reingold EM (1991) Graph drawing by force-directed placement. Softw Pract Exp 21:1129–1164
Ginsberg JR, Young TP (1992) Measuring association between individuals or groups in behavioral studies. Anim Behav 44:377–379
Girvan M, Newman MEJ (2002) Community structure in social and biological networks. Proc Natl Acad Sci USA 99:7821–7826
Gnone G, Bellingeri M, Dhermain F, Dupraz F, Nuti S, Bedocchi D, Moulins A, Rosso M, Alessi J, McCrea RS, Azzellino A, Airoldi S, Portunato N, Laran S, David L, Di Meglio N, Bonelli P, Montesi G, Trucchi R, Fossa F, Wurtz M (2011) Distribution, abundance and movements of the bottlenose dolphin (Tursiops truncatus) in the Pelagos Sanctuary MPA (north-west Mediterranean Sea). Aquat Conserv 21:372–388
Hastie GD, Wilson B, Wilson LJ, Parsons KM, Thompson PM (2004) Functional mechanisms underlying cetacean distribution patterns: hotspots for bottlenose dolphins are linked to foraging. Mar Biol 144:397–403
Hoelzel AR, Dover GA (1991) Genetic differentiation between sympatric Killer whale populations. Heredity 66:191–195
Irvine AB, Scott MD, Wells RS, Kaufmann JH (1981) Movements and activities of the Atlantic bottlenose dolphin, Tursiops truncatus, near Sarasota, Florida. Fish Bull 79:671–678
Kernoham BJ, Millspaugh JJ, Jenks JA, Naugle DE (1998) Use of home range estimators in a GIS environment to identify habitat use patterns. J Environ Manag 53:83–89
Knudsen R, Primicerio R, Amundsen PA, Klemetsen A (2010) Temporal stability of individual feeding specialization may promote speciation. J Anim Ecol 79:161–168
Kopps AM, Ackermann CY, Sherwin WB, Allen SJ, Bejder L, Krützen M (2015) Cultural transmission of tool use combined with habitat specializations leads to fine-scale genetic structure in bottlenose dolphins. Proc R Soc B Biol Sci 281:20133245
Kummer H (1995) In quest of the sacred baboon: a scientist’s journey. Princeton University Press, Princeton
Laska D, Speakman T, Fair PA (2011) Community overlap of bottlenose dolphins (Tursiops truncatus) found in coastal waters near Charleston, South Carolina. J Mar Anim Ecol 4:10–18
Lauriano G, Fortuna CM, Motedo G, Notarbartolo di Sciara G (2004) Interactions between common bottlenose dolphins (Tursiops truncatus) and the artisanal fishery in Asinara Island National Park (Sardinia): assessment of catch damage and economic loss. J Cetacean Res Manag 6:165–173
Lusseau D, Newman MEJ (2004) Identifying the role that animals play in their social networks. Proc R Soc B Biol Sci 271:477–481
Lusseau D, Schneider K, Boisseau OJ, Haase P, Slooten E, Dawson SM (2003) The bottlenose dolphin community of Doubtful Sound features a large proportion of long-lasting associations. Can geographic isolation explain this unique trait? Behav Ecol Sociobiol 54:396–405
Lusseau D, Wilson B, Hammond PS, Grellier K, Durban JW, Parsons KM, Barton TR, Thompson PM (2006) Quantifying the influence of sociality on a population structure in bottlenose dolphins. J Anim Ecol 75:14–24
Manel S, Schwartz MK, Luikart G, Taberlet P (2003) Landscape genetics: combining landscape ecology and population genetics. Trends Ecol Evol 18:189–197
Manly BFJ (1995) A note on the analysis of species co-occurrences. Ecology 76:1109–1115
Matlab (2014) MATLAB and statistic toolbox release 2014b. The MathWorks Inc, Natick
Miklós I, Podani J (2004) Randomization of presence-absence matrices: comments and new algorithms. Ecology 85:86–92
Möller LM, Beheregaray LB, Allen SJ, Harcourt RG (2006) Association patterns and kinship in female Indo-Pacific bottlenose dolphins (Tursiops aduncus) of southeastern Australia. Behav Ecol Sociobiol 6:109–177
Natoli A, Peddemors VM, Hoelzel AR (2004) Population structure and speciation in the genus Tursiops based on microsatellite and mitochondrial DNA analyses. J Evol Biol 17:363–375
Natoli A, Birkun A, Aguilar A, Lopez A, Hoelzel AR (2005) Habitat structure and the dispersal of male and female bottlenose dolphins (Tursiops truncatus). Proc R Soc B Biol Sci 272:1217–1226
Newman MEJ (2003) Mixing patterns in networks. Phys Rev E 67:026126
Newman MEJ, Girvan M (2004) Finding and evaluating community structure in networks. Phys Rev E 69:026113
Notarbartolo di Sciara G, Demma M (1994) Guida dei mammiferi marini del Mediterraneo, 3rd edn. Franco Muzzio Editore, Padova (in Italaian)
Notarbartolo di Sciara G, Agardy T, Hyrenbach D, Scovazzi T, Van Klaveren P (2008) The PELAGOS Sanctuary for Mediterranean marine mammals. Aquat Cons 18:367–391
Nuti S, Bedocchi D, Chiericoni V, Giorli G, Tozzi S (2006) Alimentazione “opportunistica” di Tursiops truncatus (Montagu, 1821) in presenza di reti a strascico. 5° Convegno Nazionale per le Scienze del Mare, CoNISMa. http://www.conisma.it/V%20convegno/convegno.html (in Italian)
Pace DS, Pulcini M, Triossi F (2003) Interactions with fisheries: modalities of opportunistic feeding for bottlenose dolphins at Lampedusa Island. Eur Res Cetaceans 17:132–135
Pilleri G, Gihr M (1969) Uber adriatische Tursiops truncatus (Montagu, 1821) und vergleichende Untersuchungen über mediterrane und atlantische Tümmler. Invest Cetaceans 1:66–73 (in German)
Rocklin D, Santoni MC, Culioli JM, Tomasini JA, Pelletier D, Mouillot D (2009) Changes in the catch composition of artisanal fisheries attributable to dolphin depredation in a Mediterranean marine reserve. ICES J Mar Sci 66:699–707
Rossbach KA, Herzing DL (1999) Inshore and offshore bottlenose dolphin (Tursiops truncatus) communities distinguished by association patterns near Grand Bahama Island, Bahamas. Can J Zool 77:581–592
Sade DS, Dow M (1994) Primate social networks. In: Wasserman S, Galaskiewicz J (eds) Advances in social network analysis: research in the social and behavioral sciences. Sage, California, pp 152–166
Seaman DE, Powell RA (1996) An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology 77:2075–2085
Shane SH (1990) Behavioral ecology of the bottlenose dolphin at Sanibel Island. In: Leatherwood S, Reeves RR (eds) The bottlenose dolphin. Academic Press, San Diego, pp 245–265
Silverman BW (1986) Density estimation for statistics and data analysis. Chapman and Hall, London
Smolker RA, Richards AF, Connor RC, Pepper JW (1992) Sex differences in patterns of association among Indian Ocean bottlenose dolphins. Behaviour 123:38–69
Smoot M, Ono K, Ruscheinski J, Wang PL, Ideker T (2011) Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics 27:431–432
Storfer A, Murphy MA, Spear SF, Holderegger R, Waits LP (2010) Landscape genetics: where are we now? Mol Ecol 19:3496–3514
Takahata Y, Suzuki S, Okayasu N, Hill D (1994) Troop extinction and fusion in wild Japanese macaques of Yakushima Island, Japan. Am J Primatol 33:317–322
Urban D, Keitt T (2001) Landscape connectivity: a graph-theoretic perspective. Ecology 82:1205–1218
van Schaik CP (1999) The socioecology of fission-fusion sociality in orangutans. Primates 40:69–86
Wells RS (1991) The role of long-term study in understanding the social structure of a bottlenose dolphin community. In: Pryor K, Norris KS (eds) Dolphin societies: discoveries and puzzles. University of California Press, Berkeley, pp 199–225
Wells RS (2003) Dolphin social complexity: lessons from long-term study and life history. In: de Waal FBM, Tyack PL (eds) Animal social complexity: intelligence, culture and individualize societies. Harvard University Press, Cambridge, pp 32–56
Wells RS, Scott MD (2009) Common bottlenose dolphin (Tursiops truncatus). In: Perrin WF, Würsig B, Thewissen JGM (eds) Encyclopedia of marine mammals, 2nd edn. Elsevier Inc., San Diego, pp 249–255
Wells RS, Scott MD, Irvine AB (1987) The social structure of free ranging bottlenose dolphins. In: Genoways HH (ed) Current mammalogy. Plenum Press, New York, pp 247–305
Whitehead H (2008) Analyzing animal societies: quantitative methods for vertebrate social analysis. University of Chicago Press, Chicago
Whitehead H (2009) SOCPROG programs: analyzing social structures. Behav Ecol Sociobiol 63:765–778
Wilson B, Hammond PS, Thompson PM (1999) Estimating size and assessing trends in a coastal bottlenose dolphin population. Ecol Appl 9:288–300
Wiszniewski J, Allen SJ, Möller LM (2009) Social cohesion in a hierarchically structured embayment population of Indo-Pacific bottlenose dolphins. Anim Behav 77:1449–1457
Wittemyer G, Douglas-Hamilton I, Getz WM (2005) The socioecology of elephants: analysis of the processes creating multi-tiered social structures. Anim Behav 69:1357–1371
Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70:164–168
Worton BJ (1995) Using Monte-Carlo simulation to evaluate kernel-based home-range estimators. J Wildl Manag 59:794–800
Wűrsig B, Jefferson TA (1990) Methods of photo-identification for small cetaceans. Report of International Whaling Commission 12:43–52
Würsig B, Würsig M (1977) The photographic determination of group size, composition and stability of coastal porpoises, Tursiops truncatus. Science 198:755–756
Acknowledgments
We would like to thank all the Pelagos partners who contributed to the data collection. In particular: Frank Dhermain and Franck Dupraz from GECEM, Léa David and Nathalie di Meglio from écoOcéan Institut, Silvio Nuti from CE.TU.S., Sabina Airoldi and Arianna Azzellino from Tethys Research Institute, Patrizia Bonelli and Gionata Montesi from Ambiente Mare, Nicola Portunato from NURC, Roberta Trucchi from WWF Liguria, Sophie Laran, Giovanni Caltavuturo, Davide Bedocchi. We also would like to thank all the partners of GIONHA (Governance and Integrated Observation of Marine Natural Habitat, http://www.gionha.eu) sharing their data on the Intercet Platform: Ilaria Fasce from Regione Liguria, Fabrizio Serena from ARPAT, Sergio Ventrella from Regione Toscana, Elisabetta Secci from Regione Sardegna, Massimo Tognotti from Provincia di Livorno, Madeleine Cancemi from OEC (Office de l’Environnement de la Corse). Unità di Ricerca dei Cetacei (University of Genova), Cooperativa Pelagos, Area Marina Protetta Tavolara—Punta Coda Cavallo. We would also like to acknowledge the support given by the “Dottorato di Scienze Ambientali: Ambiente Marino e Risorse” of the University of Messina and Prof. Emilio de Domenico. Thanks to Giulia Mo, for her invaluable support in the review of the English language.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carnabuci, M., Schiavon, G., Bellingeri, M. et al. Connectivity in the network macrostructure of Tursiops truncatus in the Pelagos Sanctuary (NW Mediterranean Sea): does landscape matter?. Popul Ecol 58, 249–264 (2016). https://doi.org/10.1007/s10144-016-0540-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-016-0540-7