Abstract
Understanding the mechanisms that maintain genetic diversity within a population remains a primary challenge for evolutionary biology. Of the processes capable of maintaining variation, negative frequency-dependent selection (NFDS), under which rare phenotypes (or alleles) enjoy a high fitness advantage, is suggested to be the most powerful. However, few experimental studies have confirmed that this process operates in nature. Although a lot of suggestive evidence has separately been provided in various polymorphic systems, these are not enough to prove the existence of NFDS in each system. Here we present a general review of NFDS and point out some problems with previous works to develop reasonable alternative research strategies for testing NFDS. In the second half of this paper, we focused on NFDS in the common bluetail damselfly, Ischnura senegalensis, that shows female-limited genetic polymorphism. We show (1) the proximate causal mechanisms of the frequency-dependent process, (2) frequency-dependent inter-morph interaction, (3) rare morph advantage and (4) morph frequency oscillations in a natural population. These results provide unequivocal empirical support for NFDS in a natural system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frequency-dependent selection (FDS) is the term given to a process in which the survival/fitness advantage of a type is dependent on its relative frequency. It is usually the result of interactions between species (predation, parasitism, or competition) or between morphs (or alleles) within species. Because most behavioral, ecological and genetic interactions between organisms are frequency-dependent, selection pressures on these interactions (e.g., disease, predation, recourse use, mating) tend to be frequency-dependent. Many central issues in evolutionary ecology invoke FDS, for example: game theory (Maynard-Smith 1982), the evolution of plant self-incompatibility (Joly and Schoen 2011), arms races in prey/host–predator/parasite systems (Barrett 1988; Haldane 1992; Chaboudez and Burdon 1995; Koskella and Lively 2009), eco-evolutionary dynamics (Yoshida et al. 2003), the evolution of sex ratio (Fisher 1958), adaptive dynamics (Friesen et al. 2004), and speciation (Higashi et al. 1999; Gavrilets and Waxman 2002). FDS is undoubtedly one of the most fundamental concepts in ecology and evolution. In “positive” frequency-dependent selection (PFDS), the survival/fitness advantage of a type increases as it becomes more common. PFDS will eventually lead to monomorphism for a trait (Thompson 1984; Endler 1988). Warning coloration and mimicry have commonly evolved through PFDS, mediated by the learning behavior of predators (Borer et al. 2010). On the other hand, in “negative” frequency-dependent selection (NFDS), rare types are favored over common ones. NFDS is at least theoretically the strongest form of balancing selection, and is essential for maintaining both species diversity in a community and genetic diversity in a species/population (Ayala 1972; Ishii and Shimada 2012).
Despite a long history of empirical investigation into NFDS, the mechanisms and processes contributing to the maintenance of genetic variation has remained largely unexplained in nature, probably due to the complexity associated with the wide range of contributing biological processes, such as plasticity, inter-individual interaction, temporal fluctuation in fitness, and evolutionary dynamics in gene frequency. As described below, fragmentary evidence is not sufficient to demonstrate the mechanisms underlying NFDS in nature. Here we present a general review of NFDS and point out some problems of previous works, in order to propose reasonable research strategies for testing NFDS. Then we focused on case study using the female-dimorphic damselfly Ischnura senegalensis, to fully confirm that NFDS can maintain genetic polymorphism in the wild. We also propose strategies for demonstrating frequency-dependent (FD) processes maintaining species diversity within a community, as well as intra-specific diversity.
Testing negative frequency-dependent selection
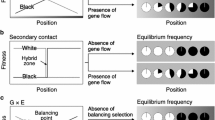
Empirical tests for NFDS in nature have traditionally concentrated on conspicuous, genetic polymorphisms within species, in which we can easily evaluate frequency dependence in the relative fitness of each type, and can observe the evolutionary changes in the frequency of each type within a relatively short time period without any molecular techniques. In general, NFDS maintaining such genetic polymorphisms results from prey–predator (trophic) interactions among species or from sexual/allelic interactions within species. In either process, we can identify four stages, as described below (Fig. 1).
The first stage is “the ecological/behavioral basis of interaction between morphs (or alleles)” as a trigger of NFDS. The relative fitness of each phenotype or allele is determined through either direct interaction between morphs with different strategies, or apparent interaction mediated by agents (e.g., predators, mates and resources). In the former, direct ecological/behavioral interactions between morphs with different strategies, or direct interactions between alleles at a locus, determine the relative fitness of each morph, depending on the relative frequency of each morph in a population. Color polymorphisms correlating with reproductive strategy in lizards (Sinervo and Lively 1996), marine isopods (Shuster and Wade 1991), odonates (Tsubaki et al. 1997), and snails (Asami et al. 1998; Schilthuizen et al. 2007) are thought to be maintained by NFDS with direct interaction (e.g., combat and competition) between morphs. Overdominant selection (heterosis), in which heterozygous individuals have a higher fitness than homozygous individuals, is a particular case of NFDS. Under this form of selection, the fitness of each allele is determined by the allele frequency in the population, via genetic interaction between each allele (e.g., MHC genes, self-incompatibility genes, and hemoglobin genes involved in sickle-cell anemia). In these cases, rare alleles are expected to have a fitness advantage because they tend to be heterozygous more often than common ones under random mating (Takahata and Nei 1990; Oliver et al. 2009; Joly and Schoen 2011). Typically, random encounters among individuals within a population results in frequency-dependent selection based on direct interactions between morphs/alleles. However, when interactions among morphs are apparent rather than direct, aggressive behavioral switching of agents in their preference for particular morphs, e.g., apostatic predation (Olendorf et al. 2006; Fitzpatrick et al. 2009) or learned mate choice (Hughes et al. 1999), is needed to achieve frequency-dependent inter-morph interactions. In general, frequency dependent apparent interaction mediated by agents may be relatively complicated compared to frequency dependent direct interaction.
Since apparent interaction mediated by agents is very common in nature (Allen 1988), the behavioral plasticity of agents in developing a varying preference for particular morphs has been well studied. This behavioral plasticity is known as search image formation (Bond 1983). A wide range of predators has been shown to select among morphs of preys in a frequency-dependent manner: they learn to prefer common morphs (Allen 1976; Sherratt and Harvey 1993). Blue jays often failed to detect rare morphs of virtual prey items, indicating that the short-term development of search images enhances the detection of common prey (Bond and Kamil 2002). Some parasitoid wasps also change their preferences with the relative proportions of each color morph in prey aphids (Langley et al. 2006). Mate choice decisions also change depending on prior mating experience (Farr 1977; Royle et al. 2008). For example, female guppies prefer to mate with rare morphs by avoiding the same male morphs with which they have mated, in an example of negatively frequency-dependent preference (Hughes et al. 1999). Such behavioral switching may be a trigger of frequency dependent selection mediated by apparent interactions. However, in some cases of apparent interactions mediated by resources, such as when morph-specific resource utilization is under disruptive selection, the abundance of alternative resources can result in frequency-dependent inter-morph interactions: density-dependent resource consumption within each morph with different resource use may contribute to frequency-dependent apparent interactions between morphs (Hori 1993).
The second stage of NFDS is “frequency-dependent inter-morph interaction”, which can lead to a rare morph advantage. In a polymorphic system with direct interactions (Sinervo and Lively 1996) or with apparent interactions through resources (Hori 1993), frequency-dependent inter-morph interactions can be achieved without any aggressive behavioral changes. For instance, in side-blotched lizards (Uta stansburiana), each individual male experiences mating competitions with males of other morphs, depending on morph frequency (Sinervo and Lively 1996). The foraging success of lateral morphs in scale-eating fish depends on morph frequency. On the other hand, in cases of agents with aggressive behavioral switching, learned preferences by agents results in frequency-dependent apparent interaction. In many polymorphic systems, it has been reported that search image formation led to frequency-dependent predation risk in prey species (Shigemiya 2004). Learned preferences of pollinators for flower color results in frequency-dependent pollination in a rewardless orchid (Gigord et al. 2001). Mate choice depending on mating experience can also lead frequency-dependent mating interactions (Farr 1977; Hughes et al. 1999; Royle et al. 2008). Thus, search images based on foraging and mating experience may lead to frequency-dependent inter-morph interactions (Fitzpatrick et al. 2009).
The third stage is “rare morph (allele) advantage”, which is one of the strongest lines of evidence of NFDS. As a result of frequency-dependent direct/apparent interactions between morphs, rare morphs are expected to enjoy greater fitness. Rare morph advantage has been empirically tested in many polymorphic systems, both in the laboratory (Fitzpatrick et al. 2007; Koskella and Lively 2009) and in the field (Gigord et al. 2001; Olendorf et al. 2006; Fitzpatrick et al. 2009). In both situations, it has been found that rare morphs show higher survival rate (Olendorf et al. 2006) and/or reproductive success (Sinervo and Lively 1996) than common morphs. Rare allele fitness advantages under overdominant selection have been tested at MHC loci (Oliver et al. 2009), self-incompatibility loci (Joly and Schoen 2011) and hemoglobin loci (Clegg and Weatherall 1999). In some cases, rare morph advantages have not been detected (Watanabe and Taguchi 1990; Shuster and Wade 1991), because fitnesses, especially long-term fitnesses, are equivalent between morphs (alleles) as long as multiple morphs (alleles) are stably maintained in a population, proposing that we should measure temporal rare morph advantage as evidence of NFDS in populations where morph (allele) frequency shows temporal variation or populations where morph frequency is manipulated artificially.
The fourth stage of NFDS is “morph-frequency oscillation” as an eventual consequence of rare morph advantage. Theoretical studies suggest that rare morph advantage results in cyclical oscillations of morph frequency within a population (Nakajima et al. 2004; Takahashi and Hori 1998). Although morph frequencies may converge to a stable equilibrium state when the rare morph advantage is not strong (Arpin and Cushing 2008), the presence of morph frequency oscillations is distinctly suggestive evidence for NFDS. In laboratory experiments, the periodicity of morph frequency dynamics is repeatedly reported, and is often stable over time (e.g., Tyerman et al. 2005; Yoshida et al. 2007). Conversely, in nature, fewer clear cyclical oscillations have been reported, although longitudinal studies have revealed stable morph oscillations in several polymorphic systems (Sinervo and Lively 1996; Svensson et al. 2005). Clear morph-frequency oscillations over multiple generations are also reported in lateral dimorphism in cichlids (Hori 1993), but the frequency-dependent causes of the oscillation have not been sufficiently demonstrated.
Most of behavioral studies of NFDS have demonstrated only behavioral switching or frequency-dependent inter-morph interactions. In a few splendid cases, frequency-dependent morph–morph interactions, rare morph advantage and morph frequency oscillations have been fully demonstrated, e.g., trimorphic system in the side blotched lizards (Sinervo and Lively 1996; Sinervo 2001; Sinervo et al. 2001), in which NFDS was mainly induced by direct interaction between morphs. However, in cases with apparent inter-morph interaction mediated by agents, the behavioral basis of frequency-dependent interactions and clear morph frequency oscillations were not found, probably due to the complexity of the systems (cf. Svensson et al. 2005; Gosden et al. 2011) in spite of the fact that such cases are common in nature. Although each of the four stages of the NFDS process mentioned above has traditionally been separately reported as evidence of NFDS in different polymorphic systems, we advocate that each of these is not sufficient evidence of NFDS (Fig. 1). If encounter frequency among morphs or learning ability of agents is low, frequency-dependent interactions are not initiated in spite of a latent potential for behavioral switching revealed by laboratory investigations of search image formation. Decreased mating success or increased predation risks in common morphs may not always lead to rare morph advantage if the cost to common morphs is not strong enough to offset other fitness advantages experienced by common morphs under directional or stabilizing selection. Moreover, depending on the balance between selection pressure and random drift, a weak rare morph advantage may not result in the coexistence of multiple morphs. In some cases, although frequency-dependent costs may maintain prey polymorphism, it is not a sufficient condition for the polymorphism to persist (Merilaita 2006). In addition, polymorphism may result from other, non-frequency-dependent ecological process, such as environmental heterogeneity (Hedrick et al. 1976). Morph frequency oscillations may also arise from several causes, such as seasonal change (Heath 1974; Suiter et al. 2003), so oscillation itself is not proof of NFDS. Therefore, each of four components is not sufficient evidence of NFDS, and causal linkages among these four components (trigger of frequency-dependent interaction, frequency-dependent direct/apparent interaction, rare morph advantage and morph frequency dynamics) should be demonstrated in a “single” system in order to fully confirm that a polymorphic system is maintained under NFDS.
However, there are a few case studies of NFDS in which these four components have been demonstrated in a single system. Here we show a confirmed case of NFDS in nature using the female-dimorphic damselfly Ischnura senegalensis, where causal linkages among the behavioral basis of interaction, morph–morph direct/apparent interaction, rare morph advantage and morph frequency dynamics have been demonstrated in a series of studies (Takahashi and Watanabe 2008, 2009, 2010a, b; Takahashi et al. 2010). For data previously reported by Takahashi et al. (2010), new data points were added and re-analyzed in this study that provide further evidence for NFDS and reject alternative explanations for the patterns.
Female polymorphisms in damselflies
Sex-limited polymorphism is widely distributed among animal taxa, and is usually assumed to be maintained via sexual interactions (Svensson et al. 2008). Male-limited polymorphisms, which are observed in both vertebrates and invertebrates, are usually associated with different strategies for gaining access to females and for male–male combat (Gross 1996). On the other hand, female-limited polymorphisms are common in insects such as damselflies (Corbet 1999), butterflies (Turner 1978; Cook et al. 1994; Nielsen and Watt 2000) and beetles (Bergsten et al. 2001), and have been theoretically suggested to be an outcome of sexually antagonistic evolution (Gavrilets and Waxman 2002; Härdling and Bergsten 2006; Hayashi et al. 2007). Empirical studies suggest that male mating harassment concentrates on common female morphs in a frequency-dependent manner, resulting in rare morph advantages (Bergsten et al. 2001; Svensson et al. 2005). In many species of damselfly, females exhibit polymorphism in body color pattern (Corbet 1999; van Gossum and Sherratt 2008), in which one female morph resembles the conspecific male in color and pattern (the andromorph) whereas one or two other morph(s) is (are) distinctively different from the male (the gynomorph[s]). Female damselflies are well-studied polymorphic systems, and have been suggested to be maintained by NFDS (Fincke 1994; van Gossum et al. 2001; Svensson et al. 2005; Takahashi et al. 2011). Behavioral studies have suggested that frequency-dependent male harassment based on learned mate recognition lead to NFDS (Fincke 1994; Miller and Fincke 1999; van Gossum et al. 1999, 2001; Svensson et al. 2005). Though polymorphic systems with an unknown genetic basis or with a complicated genetic basis often complicate interpretation of mechanisms maintaining polymorphism, it has often been demonstrated that female polymorphism in damselflies is simply controlled by a few alleles at a single autosomal locus with sex-limited expression (Johnson 1964, 1966; Sánchez-Guillén et al. 2005). Damselfly systems have therefore been the target of many ecological and evolutionary studies of NFDS and polymorphism.
The common bluetail damselfly, Ischnura senegalensis, is a widespread species, distributed across Southern Africa, the Middle East, and Southern and Eastern Asia. Although males are monomorphic, females exhibit color dimorphism, consisting of an andromorph and a gynomorph (Fig. 2). The two female color morphs are controlled by only two alleles at a single autosomal locus with sex-limited expression. The andromorphic allele is recessive to the gynomorphic allele (Takahashi 2011), as in the case of other female-dimorphic damselflies (Johnson 1964, 1966). Female polymorphism in I. senegalensis is one of the simplest cases of genetic polymorphism.
Sexually mature males of I. senegalensis actively fly about searching for mates throughout the day. They attempt to mate with females they encounter without any courtship behavior (Takahashi and Watanabe 2009). Almost all females accept male mating attempts without any rejection in the early morning. Acceptance of copulation in females is restricted to the early morning; copulations begin at around 08:00 and terminate around noon. After that, daily oviposition and daily foraging activities are performed. Solitary females lay eggs into the leaves of floating plants or the live and withered stems of emergent plants from 12:30 to 16:30 with intermittent foraging flights. Although very few females accept mating attempts in the afternoon, males continuously attack females for the purpose of mating. After 16:30, both sexes roost on stems or blades of grass at the water’s edge without any reproductive behaviour.
Evidence 1: the formation of searching images for female morphs
The behavioral switching of an agent, such as a predator or the individuals of the opposite sex (mates), serves as a trigger of NFDS (Bond and Kamil 2002). In several damselfly species, males selectively search for common female morphs in a population (van Gossum et al. 1999; van Gossum and Sherratt 2008). Some experiments have shown that males of the damselfly genera Enallagma and Ischnura form a search image for female morphs based on their prior experience (Miller and Fincke 1999; van Gossum et al. 2001), although they lack an innate search image (Fincke et al. 2007). However, in-depth investigations into the proximate factors triggering behavioral switching, such as the stimulus for switching and the persistence of the search image, have not been undertaken.
As in other damselfly species, naïve males of I. senegalensis reared separately from females showed no specific preference for female morphotype in a binary choice experiment (Takahashi and Watanabe 2008, 2010a). They attempt to mate with either of the two female morphs at random, indicating that virgin males have no innate search image for a particular female morph (Fig. 3). On the other hand, exposure to a female affects the subsequent mate searching behavior of males. After being enclosed with a single female in a small cage, males that experienced copulation preferred the same morph with which they had copulated, indicating that males more likely to mate with females having same color as ex-partner (Takahashi and Watanabe 2010a). Contrarily, males that failed to copulate with the female during enclosure due to the rejection by female showed no preference for female morphs in subsequent choice experiments. These results indicate that males form search images for specific female morphs in accordance not with the local morph frequencies but rather with their prior copulation experience, which indirectly reflect local frequencies because males are more likely to encounter common morphs in their initial matings. However, such search images disappeared over a single night: mated males show no preference among female morphs on the next morning (Takahashi and Watanabe 2008), indicating that males can switch their search image depending on their day-by-day copulation experiences.
Schematic illustration of experience-based mating preferences (search image formation) in males of Ischnura senegalensis. Although naïve males reared separately from females showed no specific preferences among female morphs, prior exposure to females affects the subsequent mate searching behavior of males. Males that had experienced copulation preferred the same morph with which they had copulated, indicating that males more likely to mate with females having similar coloration to their previous partner
Evidence 2: frequency-dependent interaction
Because in I. senegalensis the daily mating activity is restricted to the morning, during which almost all females accept male mating attempts independent of morph (Sawada 1999; Takahashi and Watanabe 2009), every male is expected to reconstruct its searching image on a daily basis in response to copulation experiences each morning. Since almost males mate with females of each morph at random without any preference in the morning, learned mate preference depending on copulation experience in the morning results in frequency dependence of the average male search image formation at a population level. That is, the proportion of males that had succeeded in copulating with each morph in the morning must coincide with the proportion of morphs in the female population, and consequently the majority of males will form search images for the common morphs in the population. Individual experience-based behavioral switching of males is suggested to result in a population-level frequency-dependent searching images for female morphs by the afternoon.
In binary choice experiments in the field, males of I. senegalensis equally attempted to mate with both female morphs in the morning, providing additional support for our prediction that males have no search image before the daily mating activity. Females consequently experienced copulation during the morning irrespective of their morph (Takahashi and Watanabe 2009). On the other hand, as predicted by individual-experience based behavioral switching, population-level search image was frequency-dependent in the afternoon, which is the time for ovipositing and foraging activity. Males selectively attempted to mate with females of the common morph in each local population in our binary choice experiment. The harassment risk of common morphs is therefore higher than that of rare morphs. Indeed, field observation revealed that females of the common morph suffered from male harassment more often than rare morph females (Takahashi and Watanabe 2010b). These results suggest that the behavioral switching of males mediates frequency-dependent apparent interaction between female morphs in this system.
Evidence 3: rare morph advantage
Male harassment prevents females from oviposition and foraging (Takahashi and Watanabe 2010b). Common morphs excreted fewer faeces than rare morphs, indicating that preferential harassment of common morphs decreased their food intake. They also produced fewer eggs than rare morphs, and laid fewer eggs than rare morphs, suggesting that male harassment indeed hinders females from oviposition and foraging activities and consequently reduces female reproductive success. Hence it is expected that the reproductive success of one morph decreases when its frequency becomes higher than the other in a population, because males attempt to mate with female morphs in a frequency dependent manner during the afternoon (Takahashi and Watanabe 2009, 2010b). Indeed, rare morphs showed higher fitness than common ones in natural populations, although the trend was based on only four data points (Takahashi et al. 2010). In order to confirm the negative frequency-dependence of female fitness, in this review, we added some new points to the outcome of Takahashi et al. (2010). New data were corrected in a similar way to Takahashi et al. (2010) in five populations (26°05′11″N, 127°41′18″E; 36°13′46″N, 136°10′47″E; 36°27′44″N, 140°35′39″E; 36°14′40″N, 140°19′11″E; 36°09′29″N, 140°03′47″E) during May–June in 2011. Figure 4 shows the relative reproductive success of each morph plotted against the frequency of andromorphs, and indicates that the fitness of the each female morph was reduced as its frequency increased, as shown in Takahashi et al. (2010). This clearly shows a rare morph advantage, and provides the conditions necessary for NFDS in I. senegalensis. Frequency-dependent apparent interaction mediated by males (harassment) results in a rare morph advantage experienced by females in this system.
Relative reproductive success of andromorph (R A; open symbols) and gynomorph (R G; filled symbols) females as a function of andromorph frequency. R A and R G were respectively calculated as R A = I A − F A and R G = I G − F G, where I is the mean number of eggs remaining in the abdomen when oviposition was inhibited and F is the mean number of eggs remaining in the abdomen after oviposition, when oviposition was allowed to occur (after Takahashi et al. 2010). Five data points (square symbols) collected during 2011 were added to data from a previous study (Takahashi et al. 2010). Regression line for andromorphs and gynomorphs is Y = −1.7418X + 0.8412 (t = −8.656, R 2 = 0.915, P < 0.0001) and Y = 1.7418X − 0.8412 (t = −8.656, R 2 = 0.915, P < 0.0001), respectively
Evidence 4: morph frequency oscillations
The two regression lines in Fig. 4 are overall symmetrical at their intersection. The equilibrium frequency, at which both morphs have the same fitness, is nearly 1:1. Based on symmetric frequency-dependence in fitness, Takahashi et al. (2010) built a simple mathematical model to predict morph frequency dynamics under rare morph advantage. This model assumes a simple genetic basis of color morphs, with one autosomal locus having two alleles (Takahashi 2011), and that the fitness reduction of each morph is a function of its frequency. This model predicts that a strong rare morph advantage will lead to the maintenance of polymorphism, with clear morph frequency oscillations, and a weak rare morph advantage will lead to a stable equilibrium frequency or sometimes monomorphism, as in the case of lateral dimorphism in cichlids (Arpin and Cushing 2008). Demographic noise that becomes larger in small populations is predicted to enlarge the amplitude of frequency oscillations (Takahashi et al. 2010).
The temporal dynamics of andromorph frequency were reported in two natural populations of I. senegalensis with different population sizes (Takahashi et al. 2010). The demographic effects on the observed frequency dynamics in the two populations agree well with the predictions of the model: the oscillations are larger in amplitude in the smaller population, and morph frequencies were relatively more constant over time in a larger population. The good fit between our model predictions based on NFDS and our empirical data indicate that these oscillations observed in natural populations likely result from NFDS and rare morph advantage (Takahashi et al. 2010). However, such oscillations in natural populations could be caused by other mechanisms, such as seasonal changes in natural selection, because this species has two generations per year (a spring and an autumn generation), and these generations may be exposed to different biotic and abiotic environments during both larval and adult stages. To reject such an explanation, we added new data on morph frequency from populations that were used in Takahashi et al. (2010). The data were collected in a similar way to Takahashi et al. (2010) in 2010 and 2011. In one of two populations, we found a reversal of the oscillation cycle after a second generation in 2009. This result, including new data points, shows that the phase of oscillation did not correspond to season, and therefore that these oscillations do not arise from seasonal changes (Fig. 5). This means that in the absence of other competing explanations, a rare morph advantage in this system is likely responsible for the maintenance and dynamics of the genetic polymorphism in females.
Frequency dynamics in natural populations. Frequencies oscillated with approximately a two-generation period. The amplitude of oscillation was larger in a small population (filled symbols) and smaller in a large population (open symbols) (after Takahashi et al. 2010). Data points collected in 2010 and 2011 (square symbols) were added to data from a previous study by Takahashi et al. (2010). Lower average frequency of andromorph in a large population than that in a small population may due to difference in potential fitness in relation to temperature (Takahashi et al. 2011) and/or difference in life-history strategies (Takahashi and Watanabe 2010c)
Conclusion
Conservation of ecosystems is one of the most important issues faced by ecologists and policymakers today. Although conservation biology has traditionally focused on species diversity within communities and ecosystems, the focus of conservation has recently expanded to encompass genetic diversity (Frankham and Briscoe 2002). Most species exhibit intraspecific variation in phenotypes and genotypes, and variation within a population contributes to population dynamics (Sinervo et al. 2000; Yoshida et al. 2007), evolution (Forsman et al. 2008), and the viability of a population and its potential for future adaptation in new, changing environments (Wennersten and Forsman 2012). Recently, genetic variation within populations has been suggested to affect community structure and ecosystem functioning (Harmon et al. 2009; Jousset et al. 2011). Therefore, the maintenance of genetic diversity within species/populations, in addition to species diversity within a community, is emerging as one of most important issues of conservation biology and evolutionary biology. In this paper, we presented a general review of NFDS and proposed reasonable research strategies for testing whether this form of selection provides a sufficient mechanism for maintaining genetic variation within natural populations.
Genetic polymorphisms supposedly maintained by NFDS have been widely reported (Punzalan et al. 2005; Svensson et al. 2005; Sinervo and Calsbeek 2006). Some studies have demonstrated direct interaction, e.g., frequency-dependent inter-morph competition (Watanabe and Taguchi 1990; Sinervo and Lively 1996; Svensson et al. 2005), or apparent inter-morph interaction, e.g., frequency-dependent predation (Bond and Kamil 1998; Svensson et al. 2005; Langley et al. 2006). Other works showed experimentally that the fitness of each morph is negatively frequency-dependent (Gigord et al. 2001; Olendorf et al. 2006; Sinervo and Calsbeek 2006; Fitzpatrick et al. 2007; Arpin and Cushing 2008; Takahashi et al. 2010). A few studies have reported cyclical oscillations in natural populations (Hori 1993). Several elegant studies have provided evidence for inter-morph interaction, NFD fitness and morph frequency dynamics. The widely recognized, long-term studies of side-blotched lizards (Sinervo and Lively 1996; Sinervo and Calsbeek 2006) and blue-tailed damselflies (van Gossum et al. 2001; Svensson et al. 2005) have demonstrated several of these components. Especially in side-blotched lizards with direct inter-morph interactions, clear oscillation with around 5-years period was reported as well as frequency-dependent interactions and rare morph advantage (Sinervo and Lively 1996; Sinervo 2001; Sinervo et al. 2001). However, morph frequency oscillation and/or detailed mechanisms underlying behavioral switching remains unclear in systems with apparent inter-morph interactions mediated by agents, probably due to the complexity of these systems that are affected by various other factors. The lack of one or more of these four components may lead the misinterpretation or overvaluation of NFDS, since frequency-dependence in behavioral switching does not always lead NFDS and other ecological and evolutionary processes (e.g., spatiotemporal environmental heterogeneity) can lead to the same consequences as NFDS (e.g., rare morph advantage and morph frequency oscillation). Conversely, in the current example of I. senegalensis, the linkage of four components (trigger of frequency-dependent interaction, frequency-dependent direct/apparent interaction, rare morph advantage and morph frequency dynamics) was fully demonstrated. To our knowledge, this system is a first confirmed case of NFDS in nature. Recently, comparisons between population differentiation of neutral loci and loci presumed to be subject to selection have been used to detect NFDS in some systems (McKay and Latta 2002; Runemark et al. 2010; Sánchez-Guillén et al. 2011), for instance by comparing population-pairwise F ST values for neutral loci with F ST values for loci suspected to be subject to selection (Lynch and Walsh 1998). Future studies with these molecular approaches may provide additional, indirect evidence for NFDS in the I. senegalensis system.
NFDS often results from prey–predator (trophic) interactions between species, or from intersexual or competitive interactions within species. Although the triggers and processes of NFDS vary among systems, frequency-dependent behavioral/ecological/genetic interactions between morphs, rare morph advantage and morph frequency dynamics must form the basis of all systems maintained under NFDS. NFDS occurring between species within a community, which acts to maintain alpha diversity, likely also comprises the same four stages (Chesson 2000; Ishii and Shimada 2012). Therefore, causal linkages among the trigger of frequency-dependent interaction, inter-morph (or inter-species) interactions, rare morph (or species) advantage and frequency oscillations should be ideally tested in each system, although frequency oscillations are not always observed, depending on strength of selection and the effect of stochastic events (Arpin and Cushing 2008; Takahashi et al. 2010). In addition to genetic diversity within species/populations, mechanisms maintaining species diversity within a community via FD processes should be addressed through the comprehensive approach that we present here (Ishii and Shimada 2012). Discovering the mechanisms maintaining diversity is currently a fundamental issue in ecology and evolutionary biology.
References
Allen J (1976) Further evidence for apostatic selection by wild passerine birds: training experiments. Heredity 36:173–180
Allen JA (1988) Frequency-dependent selection by predators. Philos Trans R Soc Lond B Biol Sci 319:485–503
Arpin SL, Cushing JM (2008) Modeling frequency-dependent selection with an application to cichlid fish. Math Biosci Eng 5:889–903
Asami T, Cowie RH, Ohbayashi K (1998) Evolution of mirror images by sexually asymmetric mating behavior in hermaphroditic snails. Am Nat 152:225–236
Ayala FJ (1972) Frequency-dependent mating advantage in Drosophila. Behav Genet 2:85–91
Barrett J (1988) Frequency-dependent selection in plant fungal interactions. Philos Trans R Soc Lond B Biol Sci 319:473–483
Bergsten J, Töyrä A, Nilsson AN (2001) Intraspecific variation and intersexual correlation in secondary sexual characters of three diving beetles (Coleoptera: Dytiscidae). Biol J Linn Soc 73:221–232
Bond A (1983) Visual search and selection of natural stimuli in the pigeon: the attention threshold hypothesis. J Exp Psychol 9:292–306
Bond A, Kamil A (1998) Apostatic selection by blue jays produces balanced polymorphism in virtual prey. Nature 395:594–596
Bond AB, Kamil AC (2002) Visual predators select for crypticity and polymorphism in virtual prey. Nature 415:609–613
Borer M, Van Noort T, Rahier M, Naisbit RE (2010) Positive frequency-dependent selection on warning color in alpine leaf beetles. Evolution 64:3629–3633
Chaboudez P, Burdon J (1995) Frequency-dependent selection in a wild plant–pathogen system. Oecologia 102:490–493
Chesson P (2000) Mechanisms of maintenance of species diversity. Ann Rev Ecol Syst 31:343–366
Clegg JB, Weatherall DJ (1999) Thalassemia and malaria: new insights into an old problem. Proc Assoc Am Physicians 111:278–282
Cook S, Vernon J, Bateson M, Guilford T (1994) Mate choice in the polymorphic African swallowtail butterfly, Papilio dardanus: male-like females may avoid sexual harassment. Anim Behav 47:389–397
Corbet PS (1999) Dragonflies: behaviour and ecology of Odonata. Harley Books, Colchester
Endler JA (1988) Frequency-dependent predation, crypsis and aposematic coloration. Philos Trans R Soc Lond B Biol Sci 319:505–523
Farr J (1977) Male rarity or novelty, female choice behavior, and sexual selection in guppy, Poecilia reticulata Peters (Pisces: Poeciliidae). Evolution 31:162–168
Fincke O (1994) Female colour polymorphism in damselflies: failure to reject the null hypothesis. Anim Behav 47:1249–1266
Fincke OM, Fargevieille A, Schultz TD (2007) Lack of innate preference for morph and species identity in mate-searching Enallagma damselflies. Behav Ecol Sociobiol 61:1121–1131
Fisher R (1958) Polymorphism and natural selection. J Ecol 46:289–293
Fitzpatrick MJ, Feder E, Rowe L, Sokolowski MB (2007) Maintaining a behaviour polymorphism by frequency-dependent selection on a single gene. Nature 447:210–212
Fitzpatrick BM, Shook K, Izally R (2009) Frequency-dependent selection by wild birds promotes polymorphism in model salamanders. BMC Ecol 9:12
Forsman A, Ahnesjo J, Caesar S, Karlsson M (2008) A model of ecological and evolutionary consequences of color polymorphism. Ecology 89:34–40
Frankham R, Briscoe D (2002) Introduction to conservation genetics. Cambridge University Press, Cambridge
Friesen ML, Saxer G, Travisano M, Doebeli M (2004) Experimental evidence for sympatric ecological diversification due to frequency-dependent competition in Escherichia coli. Evolution 58:245–260
Gavrilets S, Waxman D (2002) Sympatric speciation by sexual conflict. Proc Natl Acad Sci USA 99:10533–10538
Gigord L, Macnair M, Smithson A (2001) Negative frequency-dependent selection maintains a dramatic flower color polymorphism in the rewardless orchid Dactylorhiza sambucina (L.) Soò. Proc Natl Acad Sci USA 98:6253–6255
Gosden TP, Stoks R, Svensson EI (2011) Range limits, large-scale biogeographic variation, and localized evolutionary dynamics in a polymorphic damselfly. Biol J Linn Soc 102:775–785
Gross M (1996) Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol Evol 11:92–98
Haldane J (1992) Disease and evolution. Curr Sci 63:599–604
Härdling R, Bergsten J (2006) Nonrandom mating preserves intrasexual polymorphism and stops population differentiation in sexual conflict. Am Nat 167:401–409
Harmon LJ, Matthews B, Des Roches S, Chase JM, Shurin JB, Schluter D (2009) Evolutionary diversification in stickleback affects ecosystem functioning. Nature 458:1167–1170
Hayashi TI, Vose M, Gavrilets S (2007) Genetic differentiation by sexual conflict. Evolution 61:516–529
Heath D (1974) Seasonal changes in frequency of the “yellow” morph of the isopod Sphaeroma rugicauda. Heredity 32:299–307
Hedrick P, Ginevan M, Ewing E (1976) Genetic polymorphism in heterogeneous environments. Ann Rev Ecol Syst 7:1–32
Higashi M, Takimoto G, Yamamura N (1999) Sympatric speciation by sexual selection. Nature 402:523–526
Hori M (1993) Frequency-dependent natural selection in the handedness of scale-eating cichlid fish. Science 260:216–219
Hughes KA, Du L, Rodd F, Reznick DN (1999) Familiarity leads to female mate preference for novel males in the guppy, Poecilia reticulata. Anim Behav 58:907–916
Ishii Y, Shimada M (2012) Learning predator promotes coexistence of prey species in host-parasitoid systems. Proc Natl Acad Sci USA 109:5116–5120
Johnson C (1964) The inheritance of female dimorphism in the damselfly, Ischnura damula. Genetics 49:513–519
Johnson C (1966) Genetics of female dimorphism in Ischnura demorsa. Heredity 21:453–459
Joly S, Schoen DJ (2011) Migration rates, frequency-dependent selection and the self-incompatibility locus in Leavenworthia (Brassicaceae). Evolution 65:2357–2369
Jousset A, Schmid B, Scheu S, Eisenhauer N (2011) Genotypic richness and dissimilarity opposingly affect ecosystem functioning. Ecol Lett 14:537–545
Koskella B, Lively C (2009) Evidence for negative frequency-dependent selection during experimental coevolution of a freshwater snail and a sterilizing trematode. Evolution 63:2213–2221
Langley SA, Tilmon KJ, Cardinale BJ, Ives AR (2006) Learning by the parasitoid wasp, Aphidius ervi (Hymenoptera: Braconidae), alters individual fixed preferences for pea aphid color morphs. Oecologia 150:172–179
Lynch M, Walsh B (1998) Genetics and analysis of quantitative traits. Sinauer Associates, Sunderland
Maynard-Smith J (1982) Evolution and the theory of games. Cambridge University Press, Cambridge
McKay JK, Latta RG (2002) Adaptive population divergence: markers, QTL and traits. Trends Ecol Evol 17:285–291
Merilaita S (2006) Frequency-dependent predation and maintenance of prey polymorphism. J Evol Biol 19:2022–2030
Miller M, Fincke O (1999) Cues for mate recognition and the effect of prior experience on mate recognition in Enallagma damselflies. J Insect Behav 12:801–814
Nakajima M, Matsuda H, Hori M (2004) Persistence and fluctuation of lateral dimorphism in fishes. Am Nat 163:692–698
Nielsen M, Watt W (2000) Interference competition and sexual selection promote polymorphism in Colias (Lepidoptera, Pieridae). Funct Ecol 14:718–730
Olendorf R, Rodd FH, Punzalan D, Houde AE, Hurt C, Reznick DN, Hughes KA (2006) Frequency-dependent survival in natural guppy populations. Nature 441:633–636
Oliver MK, Telfer S, Piertney SB (2009) Major histocompatibility complex (MHC) heterozygote superiority to natural multi-parasite infections in the water vole (Arvicola terrestris). Proc Roy Soc Lond B Biol Sci 276:1119–1128
Punzalan D, Rodd F, Hughes K (2005) Perceptual processes and the maintenance of polymorphism through frequency-dependent predation. Evol Ecol 19:303–320
Royle NJ, Lindstrom J, Metcalfe NB (2008) Context-dependent mate choice in relation to social composition in green swordtails Xiphophorus helleri. Behav Ecol 19:998–1005
Runemark A, Hansson B, Pafilis P, Valakos ED, Svensson EI (2010) Island biology and morphological divergence of the Skyros wall lizard Podarcis gaigeae: a combined role for local selection and genetic drift on color morph frequency divergence? BMC Evol Biol 10:269
Sánchez-Guillén R, van Gossum H, Rivera A (2005) Hybridization and the inheritance of female colour polymorphism in two ischnurid damselflies (Odonata: Coenagrionidae). Biol J Linn Soc 85:471–481
Sánchez-Guillén RA, Hansson B, Wellenreuther M, Svensson EI, Cordero-Rivera A (2011) The influence of stochastic and selective forces in the population divergence of female colour polymorphism in damselflies of the genus Ischnura. Heredity 107:513–522
Sawada K (1999) Female sexual receptivity and male copula guarding during prolonged copulations in the damselfly Ischnura senegalensis (Odonata: Coenagrionidae). J Ethol 17:25–31
Schilthuizen M, Craze PG, Cabanban AS, Davison A, Stone J, Gittenberger E, Scott BJ (2007) Sexual selection maintains whole-body chiral dimorphism in snails. J Evol Biol 20:1941–1949
Sherratt T, Harvey I (1993) Frequency-dependent food selection by arthropods: a review. Biol J Linn Soc 48:167–186
Shigemiya Y (2004) Reversible frequency-dependent predation of a puffer, Takifugu niphobles (Pisces: Tetraodontidae), related to spatial distribution of colour-polymorphic prey. Biol J Linn Soc 81:197–202
Shuster S, Wade M (1991) Equal mating success among male reproductive strategies in a marine isopod. Nature 350:608–610
Sinervo B (2001) Runaway social games, genetic cycles driven by alternative male and female strategies, and the origin of morphs. Genetica 112:417–434
Sinervo B, Calsbeek R (2006) The developmental, physiological, neural, and genetical causes and consequences of frequency-dependent selection in the wild. Annu Rev Ecol Evol Syst 37:581–610
Sinervo B, Lively C (1996) The rock-paper-scissors game and the evolution of alternative male strategies. Nature 380:240–243
Sinervo B, Svensson E, Comendant T (2000) Density cycles and an offspring quantity and quality game driven by natural selection. Nature 406:985–988
Sinervo B, Bleay C, Adamopoulou C (2001) Social causes of correlational selection and the resolution of a heritable throat color polymorphism in a lizard. Evolution 55:2040–2052
Suiter AM, Bänziger O, Dean AM (2003) Fitness consequences of a regulatory polymorphism in a seasonal environment. Proc Natl Acad Sci USA 100:12782–12786
Svensson EI, Abbott J, Härdling R (2005) Female polymorphism, frequency dependence, and rapid evolutionary dynamics in natural populations. Am Nat 165:567–576
Svensson E, Abbott J, Gosden T, Coreau A (2008) Female polymorphisms, sexual conflict and limits to speciation processes in animals. Evol Ecol 23:93–108
Takahashi Y (2011) Testing negative frequency-dependent selection: linking behavioral plasticity and evolutionary dynamics. Bull Kanto Branch Ecol Soc Jpn 59:8–14
Takahashi S, Hori M (1998) Oscillation maintains polymorphisms—a model of lateral asymmetry in two competing scale-eating cichlids. J Theor Biol 195:1–12
Takahashi Y, Watanabe M (2008) Male mate preference depending on the mating experience in the damselfly, Ischnura senegalensis (Rambur) (Odonata: Coenagrionidae). Kontyu 11:13–17 (in Japanese with English summary)
Takahashi Y, Watanabe M (2009) Diurnal changes and frequency dependence in male mating preference for female morphs in the damselfly Ischnura senegalensis (Rambur) (Odonata: Coenagrionidae). Entomol Sci 12:219–226
Takahashi Y, Watanabe M (2010a) Mating experience affecting male discrimination between sexes and female morphs in Ischnura senegaleasis (Rambur) (Zygoptera: Coenagrionidae). Odonatologica 39:47–56
Takahashi Y, Watanabe M (2010b) Female reproductive success is affected by selective male harassment in the damselfly Ischnura senegalensis. Anim Behav 79:211–216
Takahashi Y, Watanabe M (2010c) Morph Morph-specific fecundity and egg size in the female-dimorphic damselfly Ischnura senegalensis. Zool Sci 27:325–329
Takahashi Y, Yoshimura J, Morita S, Watanabe M (2010) Negative frequency-dependent selection in female color polymorphism of a damselfly. Evolution 64:3620–3628
Takahashi Y, Morita S, Yoshimura J, Watanabe M (2011) A geographic cline induced by negative frequency-dependent selection. BMC Evol Biol 11:25
Takahata N, Nei M (1990) Allelic genealogy under overdominant and frequency-dependent selection and polymorphism of major histocompatibility complex loci. Genetics 124:967–978
Thompson V (1984) Polymorphism under apostatic and aposematic selection. Heredity 53:677–686
Tsubaki Y, Hooper R, Siva-Jothy M (1997) Differences in adult and reproductive lifespan in the two male forms of Mnais pruinosa costalis Selys (Odonata: Calopterygidae). Res Popul Ecol 39:149–155
Turner J (1978) Why male butterflies are non-mimetic—natural selection, sexual selection, group selection, modification and sieving. Biol J Linn Soc 10:385–432
Tyerman JG, Havard N, Saxer G, Travisano M, Doebeli M (2005) Unparallel diversification in bacterial microcosms. Proc R Soc Lond B 272:1393–1398
van Gossum H, Sherratt T (2008) The evolution of sex-limited colour polymorphisms. Oxford University Press, New York, pp 219–229
van Gossum H, Stoks R, Matthysen E, Valck F, De bruyn L (1999) Male choice for female colour morphs in Ischnura elegans (Odonata, Coenagrionidae): testing the hypotheses. Anim Behav 57:1229–1232
van Gossum H, Stoks R, De Bruyn L (2001) Reversible frequency-dependent switches in male mate choice. Proc Roy Soc B Biol Sci 268:83–85
Watanabe M, Taguchi M (1990) Mating tactics and male wing dimorphism in the damselfly, Mnais pruinosa costalis Selys (Odonata: Calopterygidae). J Ethol 8:129–137
Wennersten L, Forsman A (2012) Population-level consequences of polymorphism, plasticity and randomized phenotype switching: a review of predictions. Biol Rev 87:756–767
Yoshida T, Jones L, Ellner S, Fussmann G (2003) Rapid evolution drives ecological dynamics in a predator–prey system. Nature 424:303–306
Yoshida T, Ellner SP, Jones LE, Bohannan BJM, Lenski RE, Hairston NG Jr (2007) Cryptic population dynamics: rapid evolution masks trophic interactions. PLoS Biol 5:e235–e235
Acknowledgments
We thank M. Watanabe, J. Yoshimura and S. Morita for logical support, and L. Lancaster for grammatical correction of the manuscript. We also thank two anonymous referees, whose advice greatly improved the manuscript. This study was supported in part by the Research Fellowship of the Japan Society for the Promotion of Science (JSPS) for Young Scientists (20-104 and 23-2212) to YT and Ecosystem Adaptability GCOE Program of Tohoku University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takahashi, Y., Kawata, M. A comprehensive test for negative frequency-dependent selection. Popul Ecol 55, 499–509 (2013). https://doi.org/10.1007/s10144-013-0372-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-013-0372-7