Abstract
Despite advancements in treatment modalities such as flow diverters, the optimal management of posterior communicating artery (PComA) aneurysms remains uncertain. While PComA aneurysms treated with the Pipeline Embolization Device (PED) has been reported, the characteristics and progression of incomplete occluded aneurysms remain unclear. Therefore, our study aims to investigate the occlusion status and recurrence rates of PComA aneurysms treated with PED. A retrospective review of consecutive PComA aneurysm patients treated with PED was conducted between January 2015 and December 2020. Only patients with radiological follow-up were included. PComA aneurysms were categorized into incomplete occlusion and complete occlusion group. The primary outcomes included the characteristics of incomplete occlusion at the follow-up angiography. Among 121 PComA aneurysms treated with PED at our institution, 80 aneurysms were eligible in our study. During the follow-up period, 19 (23.8%) aneurysms demonstrated incomplete occlusion. Notably, there were no instances of recurrence among the 80 followed-up cases. Baseline characteristics of patients and aneurysms were comparable between the groups with complete and incomplete occlusion. However, the incomplete occlusion group showed a lower rate of assisted coils embolization (21.2% vs. 55.7%, P = 0.017) and shorter median operative time (91.0 vs. 145.5 min, P = 0.039). Differences in functional outcomes, complications, and PComA occlusion status between the groups were not significant. Multivariate analysis revealed the use of coils was associated with lower odds of incomplete PComA aneurysm occlusion (OR 0.01, 95% CI 0.001–0.12; P = 0.001), while aneurysm size was associated with higher odds of incomplete occlusion (OR 1.25, 95% CI 1.10–1.46; P = 0.002). The treatment of PED for PComA aneurysm demonstrated favorable outcomes, with an acceptable rate of incomplete occlusion and no instances of recurrence observed. However, further research is needed to explore the optimal procedural strategy for large-sized PComA aneurysms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The posterior communicating artery (PComA) is a common location for intracranial aneurysms (IAs), accounting for approximately 25% of all cerebral aneurysms [1]. Despite its prevalence, the optimal treatment approach for PComA aneurysms remains uncertain. The landmark International Subarachnoid Aneurysm Trial (ISAT) compared neurosurgical clipping and endovascular coiling, finding that coiling had better safety and efficacy for ruptured IAs [2]. However, subsequent analyses from ISAT revealed higher rates of aneurysm recurrence and retreatment with endovascular therapy [3]. Given the anatomical variability and complex hemodynamics of PComA aneurysms, concerns persist regarding incomplete occlusion and recurrence with coil embolization [4,5,6]. In addition, the incompletely occluded aneurysms were also susceptible to mass effect caused by aneurysm growth, late rupture, and potential complications from additional treatments [7]. A comparative study had shown that simple coiling may lead to higher rates of recanalization, while flow diversion techniques offer better long-term radiological outcomes for PComA aneurysms [8].
The Pipeline Embolization Device (PED) has shown promising outcomes in terms of aneurysm occlusion and low rates of retreatment for IA [9, 10]. Given its success in treating IA, particularly with minimal recurrence, there is growing interest in its application to PComA aneurysms [11,12,13]. Previous studies reported the occlusion rates for PComA aneurysms treated with flow diverters (FD), comparable to those observed in overall aneurysm populations [14,15,16]. However, there remains limited and conflicting evidence regarding the risk factors associated with incomplete occlusion of PComA aneurysms treated with PED, as well as the incidence of aneurysm recurrence following PED treatment [14, 17, 18]. Therefore, our study aimed to explore the effectiveness and potential risk factors associated with PED treatment in achieving aneurysm occlusion and preventing recurrence in patients with PComA aneurysms.
Methods
Patient selection
Consecutive patients diagnosed with IA and treated with the PED at a tertiary hospital between January 2015 and December 2020 were enrolled in this study. The research protocol was reviewed and approved by the local institutional review board, and all patients provided informed consent at the time of hospital admission for treatment. Inclusion criteria comprised the following: (1) PComA aneurysm (defined as an aneurysm originating from the internal carotid artery within the communicating segment adjacent to the PComA or aneurysms involving the PComA); (2) patients undergoing treatment with PED, with or without adjunctive coil embolization; and (3) first treated aneurysms. Exclusion criteria included: (1) patients with arteriovenous malformations or fistulas; (2) lack of baseline information or procedural images; and (3) loss of radiological follow-up outcomes due to unwillingness to reexam, missed follow-up or death during the first hospitalization. Aneurysms were categorized into two groups based on their occlusion status at the last follow-up: a group with complete PComA aneurysm occlusion and a group with incomplete PComA aneurysm occlusion showing persistent contrast filling.
Endovascular treatment
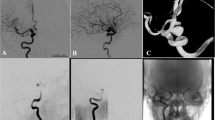
In this study, PED treatment strategies were selected based on comprehensive considerations, including the patient’s overall health condition, specific attributes of the aneurysm, and the preferences of both patients and their families. Adjunctive coiling was utilized for larger aneurysms or those assessed to have a heightened risk of rupture and recurrence, such as irregularly shaped aneurysms or those occurring in patients with a history of sentinel headache. The coils utilized in our study comprised Axiu (Medtronic, Dublin, Ireland), Jaspe (Achieva, Shanghai, China), Microplex (MicroVention, Aliso Viejo, CA), Orbit (Johnson & Johnson, New Brunswick, NJ), and Target (Stryker, Kalamazoo, MI). Coil embolization used in the PED treatment could be divided into loose packing and dense packing. Coil packing density was evaluated using the Raymond–Roy classification, where class III indicated loose packing, and class I/II indicated dense packing [19]. All procedures were conducted under general anesthesia with procedural heparinization, and stents and devices were implanted following the manufacturer’s recommended protocol. (Fig. 1)
After the initial operation, routine imaging follow-up was conducted at 3–6 months to assess the occlusion status of PComA aneurysms. This evaluation utilized computed tomography angiography, magnetic resonance angiography, and digital subtraction angiography. If incomplete occlusion was observed during these initial follow-ups, subsequent angiographic examinations at 12 months or longer post-operation were recommended to assess the long-term occlusion outcomes of the aneurysm.
Variables and outcomes
The study aimed to assess several outcomes: (1) the rate of incomplete occlusion of PComA aneurysms treated with the PED at each patient’s latest follow-up, along with the recurrence rate of PComA aneurysms; (2) identification of characteristics and treatment outcomes of PComA aneurysms with incomplete occlusion; and (3) analysis of risk factors for incomplete PComA aneurysm occlusion.
Incomplete occlusion of PComA aneurysms was defined as residual contrast filling of the aneurysm lumen on follow-up digital subtraction angiography or computed tomography angiography, evaluated using the O’Kelly-Marotta Scale [20]. Recurrence of aneurysms was defined as either new contrast filling after complete occlusion or increased contrast filling indicating aneurysm growth [5]. Additional treatment outcomes included unfavorable functional outcomes at follow-up (defined as a modified Rankin Scale [mRS] score > 2), intraoperative and postoperative complications, and the status of the PComA. In-stent thrombosis was identified via intraoperative digital subtraction angiography during PED deployment. Postoperative ischemic stroke included transient ischemic attack, mild ischemia, and severe ischemia, as defined by changes in the National Institutes of Health Stroke Scale scores. Stent stenosis was characterized by a reduction in vessel diameter of ≥ 50% compared to the maximum diameter of the parent artery, while PComA occlusion was defined as the absence of visualization of the PComA that was visible prior to stent release.
Baseline information on patient characteristics, aneurysm features, and procedural details was collected. Aneurysm size was defined as the maximum distance between any two points of the aneurysm, and neck width as the maximum distance between two points on the aneurysmal neck plane. Parent artery diameter was the mean artery diameter at the proximal and distal sides of the aneurysm neck. Fetal PComA was defined as a PComA originating from the internal carotid artery with no visible ipsilateral P1 segment or with a hypoplastic P1 segment [21]. Sidewall aneurysms originated from only one parent vessel or from a branch origin much smaller than the parent vessel. Hospitalization costs were converted to US dollars using the average exchange rate for 2023.
Statistical analysis
Normally distributed continuous variables were expressed as mean with standard deviation (SD) and compared using the unpaired t-test. Non-normally distributed continuous variables were presented as median with interquartile range (IQR) and analyzed using the Mann-Whitney U test. Categorical variables were presented as numbers with percentages and analyzed using the χ2 test or Fisher’s exact test. Logistic regression analysis was employed to identify factors associated with incomplete aneurysm occlusion. Covariates showing a significance level of P < 0.20 in the univariate logistic regression analyses, along with relevant expansion covariates, were included in a multivariate logistic regression analysis. A significance level of P < 0.05 was considered statistically significant. The statistical analyses were performed using SPSS version 22 (SPSS, Chicago, USA).
Results
Descriptive overview of incomplete occlusion and recurrence
A total of 898 consecutive aneurysms treated with the PED were initially identified in our institution. Among them, 121 first-treated PComA aneurysms with PED were initially included in this study. However, after applying the inclusion criteria, 15 patients with concurrent other cerebrovascular diseases, five patients with missing baseline characteristics, and 21 patients without radiological follow-up outcomes were excluded. Consequently, 80 PComA aneurysms treated with PED, which underwent follow-up imaging, were included in the final cohort.
Assessed by the angiographic follow-up outcomes (median follow-up period 8.0 [5.0–15.0] months), 61 PComA aneurysms (76.3%) were completely occluded, while 19 PComA aneurysms (23.8%) exhibited persistent incomplete occlusion. Among the incompletely occluded PComA aneurysms, all were initially incompletely occluded at postoperative angiography and did not show increased contrast filling during follow-up. Consequently, the recurrence rate of PComA aneurysm treated with PED in our study was 0.0% (0/80). (Tables 1 and 2)
Baseline characteristics
In Table 1, the characteristics of PComA aneurysms with incomplete occlusion are presented. All the identified cases were unruptured aneurysms, and patients presented with a favorable condition (mRS score ≤ 2) upon admission. The mean age of these patients was 54.63 ± 12.25 years, with a majority being female (68.4%). The median aneurysm size and aneurysmal neck measured 11.40 [6.80, 13.90] mm and 6.80 [5.25, 10.45] mm, respectively. Notably, four fetal PComAs were identified among the incorporated PComAs. Among the incompletely occluded PComA aneurysms, 17 were saccular (89.5%), one was dissecting (5.3%), and one was fusiform (5.3%). Comparing incompletely occluded with completely occluded aneurysms, we observed significantly lower rates of coil usage (21.1% vs. 55.7%, P = 0.017), a lower rate of coil packing (loose packing rate 21.1% vs. 24.6%, dense packing rate 0.0% vs. 31.1%; P = 0.009), and shorter operative times (91.00 [76.25, 147.25] min vs. 145.50 [102.75, 180.00] min, P = 0.039) in the former group. However, other characteristics between the two groups did not reach statistical significance.
Treatment outcomes
No instances of aneurysm recurrence were observed among the incomplete PComA aneurysm occlusions. Among these cases, the rate of unfavorable functional outcomes was 10.5% (2/19). Specifically, one patient experienced a transient ischemic attack during the postoperative period, while another patient suffered from subarachnoid hemorrhage (SAH). The stent stenosis rate for incomplete PComA aneurysms occlusion was also 10.5% (2/19). Upon deployment of the PED, intraoperative angiographic assessment revealed that the PComA was covered in 18 patients (94.7%), with complete occlusion observed in one patient (5.6%). Subsequent postoperative follow-up imaging showed a PComA occlusion rate of 11.1% (2/18), and notably, none of the fetal PComAs were occluded during PED treatment. Furthermore, when comparing treatment outcomes between the incomplete PComA aneurysm occlusion group and the complete PComA aneurysm occlusion group, no significant differences were observed. Detailed characteristics of treatment outcomes between these groups are provided in Table 2.
Predictors of incomplete aneurysm occlusion
In the univariate analysis, the use of coils was significantly associated with incomplete occlusion of PComA aneurysms, with an odds ratio (OR) of 0.21 (95% confidence interval [CI] 0.06–0.66; p = 0.012). Additionally, there was a trend towards a higher odds ratio of ischemic stroke history for incomplete PComA aneurysm occlusion (OR 7.06, 95% CI 0.64-157.41; p = 0.119). However, factors such as aneurysm size (OR 1.02, 95% CI 0.95–1.10; p = 0.574), age (OR 0.99, 95% CI 0.95–1.05; p = 0.814), and fetal PComA (OR 0.69, 95% CI 0.18–2.23; p = 0.557) showed no significant association with aneurysm occlusion status. In multivariate analysis, use of coils (OR 0.01, 95% CI 0.001–0.12; p = 0.001) were associated with lower odds of incomplete PComA aneurysm occlusion. And aneurysm size (OR 1.25, 95% CI 1.10–1.46; p = 0.002) were associated with higher odds of incomplete aneurysm occlusion. (Table 3)
Discussion
In our study of PComA aneurysms treated with the PED, several key findings emerged: (1) a favorable complete occlusion rate, (2) no instances of aneurysm recurrence during the follow-up period, and (3) associations between incomplete PComA aneurysm occlusion and the use of coils and aneurysm size.
PComA aneurysms with complete occlusion constituted 76.3% of all treated cases with follow-up angiography. This outcome aligns with previous studies on PED treatment for PComA aneurysms, which reported complete occlusion rates ranging from 69.4 to 87.2% [15, 17, 18]. Importantly, these outcomes are comparable to those observed for aneurysms located in the internal carotid artery and other sites, [22, 23] underscoring the favorable effectiveness of the PED for PComA aneurysms. Regarding alternative treatments, studies have reported incomplete aneurysm rates of approximately 32.0–33.1% for PComA aneurysms treated with simple coiling [4, 8]. While direct comparisons between PED and coiling outcomes are lacking, our data suggest that the angiographic outcomes of PED are at least equivalent to those of coiling procedures. In addition to the PED, the Surpass Streamline device was also utilized in the treatment of PComA aneurysms. Computational fluid dynamics studies have shown that Surpass has effective outcomes for fetal-type PComA aneurysms compared to other FD [24]. Previous research has suggested complete occlusion rates ranging from 73.7 to 77.8% for PComA aneurysms treated with Surpass, consistent with the findings of our study [25, 26]. However, it is important to acknowledge the impact of inconsistent baseline characteristics and confounding factors in different research studies, which may affect the comparison of treatment outcomes with FD. Therefore, as experience with upgraded FD grows, further exploration of optimal treatment strategies for complex PComA aneurysms is warranted.
In our study, no instances of aneurysm recurrence were observed in PComA aneurysms treated with the PED. Unlike immediate occlusion achieved through surgical clipping or coiling embolization, the mechanism of aneurysm occlusion with the PED involves gradual endothelial cell growth at the aneurysm neck and thrombosis within the sac. This process leads to eventual aneurysm occlusion, even in cases initially presenting with incomplete occlusion at PED deployment [27]. The efficacy of the PED in achieving aneurysm occlusion without the need for unnecessary repeated angiography has been demonstrated in previous studies on IAs [11]. Thus, in PComA aneurysms characterized by complex hemodynamic profiles, the PED has shown satisfactory angiographic outcomes in aneurysm recurrence. Studies on PComA aneurysm recurrence have indicated that treatment modality plays a significant role, with aneurysms treated with coiling being more prone to recanalization [8, 28]. Factors such as aneurysm size, presence of a fetal PComA, immediate aneurysm occlusion, use of assisted stents, and degree of coil packing have been reported to be associated with recurrence in PComA aneurysms treated with coiling embolization [5, 6, 29]. However, there was no prior research describing the recurrence of PComA aneurysm treated with PED. Despite the different mechanism of coiling and PED as well as the low concern for aneurysm recurrence in the treatment of PED, further research is needed to investigate the possible impact of the above factors in PED treatment and elucidate the detailed mechanisms underlying aneurysm occurrence.
The baseline information of PComA aneurysms showed that the incomplete occlusion group showed shorter median operative time and a lower rate of assisted coils embolization. This difference in operative time may be attributed to the lower proportion of aneurysms treated with adjunctive coil embolization in the incomplete occlusion group. The use of adjunctive coil embolization tends to prolong procedure times due to the additional application of coiling. The analysis of predictor factors for occlusion of PComA aneurysms in our study revealed that the use of coils was associated with complete occlusion, while aneurysm size was associated with incomplete occlusion. These findings align with prior studies investigating the use of the PED in IAs, which have consistently demonstrated the influence of coils and aneurysm size on occlusion outcomes for PComA aneurysm. Specifically, a previous study focusing on PComA aneurysms treated with PED also highlighted the association of large size and incorporated vessels with angiographic outcomes [17]. Given the complexity of large aneurysms with more complex hemodynamic characteristics, diligent attention during the follow-up period is warranted. However, the impact of fetal-type PComA on aneurysm occlusion remains contentious. While some studies of FD treatment for PComA aneurysms have suggested an association between fetal PComA and incomplete occlusion, [14, 16, 18] others have found no significant correlation [15, 17]. In our study, we found no association between fetal-type PComA and incomplete occlusion. Despite the characteristic larger vessel diameter and increased blood flow associated with fetal PComA, the mechanism of PED in achieving aneurysm occlusion involves the reduction of flow through the aneurysm sac via endothelization of the aneurysm neck, which appears effective even in higher hemodynamic circumstances. And the discrepancies in these findings may be attributed to variations in baseline information, definitions of fetal PComA, treatment outcomes, and differences in stent usage across studies. Furthermore, the coverage and occlusion of PComA or fetal PComA do not appear to be associated with aneurysm occlusion. As suggested by several studies of meta-analysis, favorable outcomes including aneurysm obliteration and acceptable complications were reported in IA treatment with FD involving covered branch vessels [30, 31]. Although fetal-type PComA is a common anatomical variation with potential theoretical implications for clinical and angiographic outcomes, its precise effect on PComA aneurysms treated with endovascular therapy remains unclear. Further investigation is necessary to determine whether PComA aneurysms with large size or other potential risk factors may benefit from alternative treatment modalities.
Limitations
This study has several limitations that should be acknowledged. Firstly, its retrospective, single-center, descriptive design and the existing loss of angiographic follow-up outcomes restricted the generalizability of our findings. Our analysis primarily focused on the incomplete occlusion rate and recurrence rate of PComA aneurysms treated with PED at our center. Secondly, the median angiographic follow-up time was less than one year, potentially impacting the assessment of PED’s long-term effectiveness. Thirdly, despite being one of the largest cohorts of PComA aneurysm with PED, the sample size of our study may have restricted the inclusion of a comprehensive set of covariates in the multivariate logistic regression analysis. Additionally, PComA aneurysms treated with alternative endovascular or surgical interventions during the study period were not included in our analysis, which could introduce bias. Further studies comparing the PED with other treatment modalities are imperative to determine the optimal approach for managing PComA aneurysms.
Conclusions
In our study, PComA aneurysms treated with the PED demonstrated a favorable complete occlusion rate, with no instances of recurrence observed during follow-up. Additionally, we found that aneurysm size and the use of coils may influence the likelihood of incomplete occlusion. These findings suggest that the PED is an effective treatment for PComA aneurysms. However, further research is needed to explore the optimal procedural strategy, particularly for managing large PComA aneurysms.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- PComA:
-
Posterior Communicating Artery
- IA:
-
Intracranial Aneurysm
- ISAT:
-
International Subarachnoid Aneurysm Trial
- PED:
-
Pipeline Embolization Device
- FD:
-
Flow Diverters
- mRS:
-
Modified Rankin Scale
- SD:
-
Standard Deviation
- IQR:
-
Interquartile Range
- SAH:
-
Subarachnoid Hemorrhage
- OR:
-
Odds Ratio
- CI:
-
Confidence Interval
References
Molyneux A, Kerr R, Stratton I et al (2002) International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet Lond Engl 360:1267–1274
Molyneux AJ, Kerr RSC, Yu L-M et al (2005) International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet Lond Engl 366:809–817
Campi A, Ramzi N, Molyneux AJ et al (2007) Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT). Stroke 38:1538–1544
Choi HH, Cho YD, Yoo DH et al (2020) Impact of fetal-type posterior cerebral artery on recanalization of posterior communicating artery aneurysms after coil embolization: matched-pair case-control study. J Neurointerventional Surg 12:783–787
Kim MJ, Chung J, Park KY et al (2021) Recurrence and risk factors of posterior communicating artery aneurysms after endovascular treatment. Acta Neurochir (Wien) 163:2319–2326
Yasuda R, Miura Y, Suzuki Y et al (2022) Posterior communicating artery-incorporated internal carotid-posterior communicating artery aneurysms Prone to Recur after Coil Embolization. World Neurosurg 162:e546–e552
Ferns SP, Majoie CBLM, Sluzewski M et al (2010) Late adverse events in coiled ruptured aneurysms with incomplete occlusion at 6-Month Angiographic Follow-Up. Am J Neuroradiol 31:464–469
Scerrati A, Trevisi G, Sturiale CL et al (2021) Radiological outcomes for endovascular treatment of posterior communicating artery aneurysms: a retrospective multicenter study of the occlusion rate. J Integr Neurosci 20:919–931
Chalouhi N, Tjoumakaris S, Starke RM et al (2013) Comparison of flow diversion and coiling in large unruptured intracranial saccular aneurysms. Stroke 44:2150–2154
Park MS, Nanaszko M, Sanborn MR et al (2016) Re-treatment rates after treatment with the Pipeline Embolization device alone versus Pipeline and coil embolization of cerebral aneurysms: a single-center experience. J Neurosurg 125:137–144
Chalouhi N, Patel PD, Atallah E et al (2018) Low yield of cerebral angiography in adequately occluded aneurysms after Flow Diversion. Neurosurgery 83:1294
Daou B, Atallah E, Chalouhi N et al (2018) Aneurysms with persistent filling after failed treatment with the Pipeline embolization device. J Neurosurg 130:1376–1382
Trivelato FP, Ulhôa AC, Rezende MT et al (2018) Recurrence of a totally occluded aneurysm after treatment with a pipeline embolization device. Case Rep 2018:bcr
Roy AK, Howard BM, Haussen DC et al (2018) Reduced efficacy of the Pipeline Embolization device in the treatment of posterior communicating region aneurysms with fetal posterior cerebral artery configuration. Neurosurgery 82:695–700
Enriquez-Marulanda A, Ravindran K, Salem MM et al (2019) Evaluation of Radiological features of the posterior communicating artery and their impact on efficacy of Saccular Aneurysm Treatment with the Pipeline Embolization device: a Case Series Study. World Neurosurg 125:e998–1007
Vivanco-Suarez J, Rodriguez-Calienes A, Kan PT et al (2023) Flow Diverter performance in Aneurysms arising from the posterior communicating artery: a systematic review and Meta-analysis. Neurosurgery 93:764–772
Moubark M, Allah AE-KA, Yosef H et al (2020) Flow diverter devices in the treatment of posterior communicating artery aneurysms: mid-term clinical and radiological outcomes. Egypt J Radiol Nucl Med 51:118
Rinaldo L, Brinjikji W, Cloft H et al (2019) Effect of fetal posterior circulation on efficacy of Flow Diversion for treatment of posterior communicating artery aneurysms: a multi-institutional study. World Neurosurg 127:e1232–e1236
Raymond J, Guilbert F, Weill A et al (2003) Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 34:1398–1403
Joshi MD, O’Kelly CJ, Krings T et al (2013) Observer variability of an angiographic grading scale used for the assessment of intracranial aneurysms treated with flow-diverting stents. AJNR Am J Neuroradiol 34:1589–1592
Capone S, Shah N, George-St Bernard RR (2019) A fetal-type variant posterior communicating artery and its clinical significance. Cureus 11:e5064
Hanel RA, Cortez GM, Lopes DK et al (2023) Prospective study on embolization of intracranial aneurysms with the pipeline device (PREMIER study): 3-year results with the application of a flow diverter specific occlusion classification. J Neurointerventional Surg 15:248–254
Fiorella D, Gache L, Frame D et al (2020) How safe and effective are flow diverters for the treatment of unruptured small/medium intracranial aneurysms of the internal carotid artery? Meta-analysis for evidence-based performance goals. J Neurointerventional Surg 12:869–873
Fu Y, Bian X, Zou R et al (2023) Hemodynamic alterations of flow diverters on aneurysms at the fetal posterior communicating artery: A simulation study using CFD to compare the surpass streamline, pipeline flex, and tubridge devices. J Neuroradiol J Neuroradiol. ;S0150-9861(23)00221-3
Ten Brinck MFM, Rigante L, Shimanskaya VE et al (2021) Limitations of Flow diverters in posterior communicating artery aneurysms. Brain Sci 11:349
Kan P, Mohanty A, Meyers PM et al (2023) Treatment of large and giant posterior communicating artery aneurysms with the surpass streamline flow diverter: results from the SCENT trial. J Neurointerventional Surg 15:679–683
Ravindran K, Casabella AM, Cebral J et al (2020) Mechanism of Action and Biology of Flow diverters in the treatment of intracranial aneurysms. Neurosurgery 86:S13–S19
Lee HJ, Choi JH, Shin YS et al (2021) Risk factors for the recurrence of posterior communicating artery aneurysm: the significance of fetal-type posterior cerebral artery. J Stroke Cerebrovasc Dis 30:105821
Wu D, Sheng B, Fang X et al (2022) Risk factors of recurrence after endovascular embolization of posterior communicating artery aneurysms. Interv Neuroradiol 28:562–567
Liu J, Cao F, Zhenmei N et al (2023) Flow-Diverter stents in intracranial aneurysm treatment: impact on covered cerebral artery branches. Int J Surg Lond Engl Published Online First: 18 Oct. https://doi.org/10.1097/JS9.0000000000000762
Touzé R, Gravellier B, Rolla-Bigliani C et al (2020) Occlusion rate and visual complications with Flow-Diverter Stent placed across the Ophthalmic artery’s origin for Carotid-Ophthalmic aneurysms: a Meta-analysis. Neurosurgery 86:455–463
Acknowledgements
Not applicable.
Funding
1、Natural Science Foundation of China (82171290).
2、Beijing Nova Program (20220484167).
Author information
Authors and Affiliations
Contributions
XX: study design, data interpretation, statistical analysis, manuscript drafting, and critical revision of the manuscript. LX: data collection, data analysis, and data interpretation. YM, MS and HL: data collection and critical revision of the manuscript. XT and AL: study design, study supervision, and critical revision of the manuscript. AL: the author responsible for the overall content as guarantor. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study obtained ethics approval: Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University KY 2021-038-02. And participants gave informed consent to participate in the study before taking part.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 1.
Conflict of interest
No conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xue, X., Liang, X., Miao, Y. et al. The aneurysm occlusion and recurrence of posterior communicating artery aneurysms following the treatment with the pipeline embolization device. Neurosurg Rev 47, 330 (2024). https://doi.org/10.1007/s10143-024-02580-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-024-02580-0