Abstract
Postoperative ischemic complication results in neurological sequelae and longer hospitalization after unruptured middle cerebral artery (MCA) aneurysm clipping surgery. We evaluated the radiological and patient-related factors associated with ischemic complications after unruptured MCA aneurysm clipping surgery. Patient demographics, radiological findings, and intraoperative factors were compared between patients with and without postoperative ischemic complications. The clinical courses and outcomes of postoperative ischemic complications were compared according to the types of ischemic complication. Forty-two out of 2227 patients (1.9%) developed postoperative ischemic complications after MCA aneurysm clipping. Multivariate analysis revealed that diabetes mellitus (DM) was a patient-related factor. Intraarterial (IA) calcification of the distal internal carotid artery (ICA), preoperative M1 stenosis, and M1 aneurysm were radiological factors that increased the risk of postoperative ischemic complications. DM was significantly associated with divisional branch territory infarction (P = 0.04). The time to first presentation of ischemic complication was significantly longer in divisional branch territory infarction than perforator territory infarction (67.8 ± 75.9 h vs. 22 ± 20.7, P = 0.023). Twelve out of 42 patients with ischemic complications (28.6%) had unfavorable outcome (mRS > 3). Perforator territory infarction was significantly associated with an unfavorable outcome (mRS > 3, P = 0.019). IA calcification of the distal ICA, M1 stenosis and aneurysms, and DM were significantly associated with postoperative ischemic complications after unruptured MCA aneurysm clipping. Patients with DM should be closely monitored postoperatively to detect delayed occurrence of divisional branch infarction. Trial registration number: 2019-1002, Date of registration: January 1, 2005, “retrospectively registered”
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endovascular treatment is applied widely to treat middle cerebral artery (MCA) aneurysms [1,2,3]; however, clipping remains the preferred treatments because of surgical accessibility to MCA aneurysms, long-term durability, and favorable outcomes. The occurrence of major complications is < 3%, which is lower than endovascular treatment studies [4,5,6,7]. Postoperative ischemic complications from aneurysm clipping surgery remain a major concern because they can cause disabilities and lead to higher health care costs, even when patients reach a favorable functional outcome after long-term postoperative follow-up [8]. Previous studies have identified potential risk factors for ischemic complications after MCA aneurysm clipping that are associated with the architecture of aneurysms and the parent artery [4, 9]. In contrast, the clinical factors and features of postoperative ischemic complications following MCA aneurysm clipping have not been thoroughly investigated to date.
In this study, we evaluated the preoperative clinical factors associated with ischemic complication after MCA aneurysm clipping. In addition, we analyzed the clinical features of ischemic complication after MCA aneurysm clipping according to the territory of cerebral infarction.
Materials and methods
Patient data collection and preoperative evaluation
We retrospectively reviewed all patients who underwent surgical treatment for their unruptured MCA aneurysms at our institute between January 2005 and December 2018. The present study was approved by our institutional review board. All patients underwent a preoperative evaluation using computed tomography (CT) angiography and 4-vessel intraarterial digital subtraction angiography (DSA). Patient medical records were reviewed to identify demographic information, comorbid conditions, and previous medical history. Information regarding intraoperative factors, such as bypass with the obliteration of the aneurysm by parent artery trapping, intraoperative rupture, and any change in intraoperative monitoring, were gathered from the surgical records.
Preoperative radiological data included aneurysm location, size, and morphology; presence significant stenosis of the parent artery; and presence of intraarterial (IA) calcification of the distal internal carotid artery (ICA). Aneurysm size was defined as the maximal depth of the dome and classified as follows: small, < 10 mm; large, 10–25 mm; and giant, > 25 mm. IA calcification of distal ICA was defined as distal ICA with focal high-density of at least 90 Housefield units, which was identified on the 1-mm section image of preoperative CT angiograms [10, 11]. This is categorized as significant when the filling of the vascular lumen by calcification is > 50% of the intraluminal cross-sectional area (Fig. 1a). Significant M1 stenosis is defined as the stenosis is ≥ 50% of the nonstenotic portion of the proximal M1 (Fig. 1b).
a Significant intraarterial calcification is defined by a high-density of at least 90 Housefield units at the distal ICA on the 1-mm-thick section image of the CT angiogram, with > 50% filling of the vascular lumen of the intraluminal cross-sectional area. b Significant M1 stenosis is defined as ≥ 50% stenosis of the nonstenotic portion of the proximal M1
Patients diagnosed with infectious aneurysms and dissecting aneurysms secondary to head trauma were excluded.
Location of the MCA aneurysms
Aneurysm locations were separated into 3 categories. First, M1 aneurysms were defined when they occurred at the main MCA trunk (M1) between the bifurcation of internal carotid artery and main MCA trunk bifurcation. This included aneurysms arising at the origin of lenticulostriate arteries at the origin of early cortical and both early frontal and temporal branches [12, 13]. Second, MCA bifurcation (MCAb) aneurysms were defined as occurring at the main MCA trunk (M1) bifurcation. Third, M2 aneurysms or aneurysms distal to M2 were defined as those located distal to the main MCA bifurcations [12].
Surgical procedures of aneurysm clipping
We used a standard frontotemporal craniotomy for a pterional approach to the MCA aneurysms (Fig. 2a). Before clipping, MCA aneurysms were exposed after sylvian fissure dissection. The parent artery proximal to the neck of the aneurysm was identified for proximal control (Fig. 2b and c). Temporary clipping of the parent artery was used in selected cases when there was a high risk of aneurysmal rupture during aneurysm neck dissection, such as with large to giant aneurysms (Fig. 2c), and the duration of temporary clipping did not exceed 3 min. After aneurysmal neck clipping, the flow through the parent artery was confirmed by Doppler sonography or intraoperative indocyanine green (ICG) angiogram under a surgical microscope (Fig. 2d and e). Intraoperative evoked potential monitoring (IOM) was used after 2011. Significant IOM change was defined as a decrease in amplitude by ≥ 50% and/or a 10% delay in latency [14, 15].
a A standard frontotemporal craniotomy was performed for a pterional approach to the MCA aneurysm. b The Sylvian fissure was dissected preserving the Sylvian veins. c Temporary clipping was performed at the M1 proximal to the aneurysm. d and e Flow of the parent artery was confirmed using intraoperative Doppler sonography and ICG angiogram after permanent clipping of the aneurysm. Abbreviations: FL, Frontal lobe; TL, Temporal lobe; An, Aneurysm; ICG, Indocyanin green
Postoperative follow-up
CT angiography with perfusion was performed immediately after the surgery for all patients. All patients had postoperative management at the neurosurgical intensive care unit and were closely monitored neurologically and hemodynamically for at least postoperative day one. Patients who showed neurological deterioration during the postoperative period underwent brain CT without contrast enhancement and diffusion MRI to determine the presence of hemorrhagic or ischemic infarctions. Postoperative cerebral angiography was performed in selected patients who had complex aneurysms, any change in intraoperative EP monitoring, or findings of parent artery stenosis or compromise of any degree on the postoperative CT angiography.
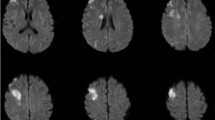
A postoperative ischemic complication was defined as an acute cerebral infarction identified by diffusion-weighted imaging (DWI) of MRI accompanied by symptoms relevant to the infarcted region documented in the medical record. Postoperative ischemic complications were radiologically classified as MCA perforator territory infarctions when DWI revealed a restriction lesion in the lentiform nucleus, caudate nucleus, and/or corona radiata [16] (Fig. 3a), or divisional branch territory infarctions when diffusion restriction in the cortical or subcortical area of the MCA territory was shown (Fig. 3b), secondary to the compromise of the MCA at or distal to MCAb [17].
Postoperative ischemic complications were identified on diffusion weighted magnetic resonance images. a MCA Perforator territory infarctions involving the lentiform neucleus which was recovered with favorable outcome. b and c MCA perforator territory infarction involving the posterior tip of the lentiform nucleus (arrow) and corona radiata (arrow heads) which caused side weakness and unfavorable outcome (patient No. 13, Table 4). d Divisional branch territory infarctions, such as cortical or subcortical infarctions of the MCA territory secondary to the compromise of the MCA at or distal to M2. e Divisional branch territory infarction secondary to the compromise of the inferior trunk of M2 which resulted in side weakness and aphasia (patient No. 25, Table 4)
Time to the first detection of ischemic complication was defined as the time from the initial postoperative CT angiography to the time of the detection of acute cerebral infarction on diffusion MRI. This was compared between patients with MCA perforator and divisional branch territory infarctions.
Patient functional outcomes were recorded using the modified Rankin scale (mRS). These were determined on clinical follow-up at 6 months after discharge date.
Statistical analysis
SPSS version 22.0 (IBM, Armonk, NY, USA) was used for all statistical analyses. Data normality was assessed using the Kolmogorov-Smirnov test. The independent t test was used to evaluate differences between continuous variables. The chi-square test was performed to determine differences between categorical variables. P values < 0.05 were considered to be statistically significant. We used a multiple-logistic-regression model with significant variables from the univariate analyses to find independent predictors of postoperative ischemic complications. Significant variables from the univariate analysis were entered into a backward stepwise logistic-regression model to identify variables independently associated with ischemic complication. Odds ratios (OR) with 95% confidence intervals (CIs) are reported. The goodness of fit of the multiple-logistic regression model was tested using the Hosmer-Lemeshow test.
Results
Patient characteristics
From January 2005 to December 2018, 2227 patients (1585 females and 642 males) underwent surgical treatment for 2274 unruptured MCA aneurysms. The mean age was 58.31 ± 8.9 years (range, 20–83). The most common location of the MCA aneurysm was MCAb (86.8%), followed by M1 (12.9%) and M2 or distal MCA (2%). The most aneurysms were classified as small, with the largest depth ≤ 10 mm (small 2186/2274 [96.1%], large 82/2274 [3.61%], giant 6/2274 [2.64%]). There were 9 (0.4%) recurrent cases with a history of treatment by either surgical clipping or endovascular coiling. Parent artery trapping with MCA to superficial temporal artery (STA) bypass was performed in 19 patients for 6 cases of small aneurysms, 10 cases of large aneurysms, and 3 cases of giant aneurysms.
Factors associated with ischemic complications
Ischemic complications occurred in 42 out of 2227 patients (1.9%) during the postoperative follow-up period. The occurrence of ischemic complications was significantly higher in patients with DM (13/189 patients [6.9%] vs. 42/2227 patients [1.9%], P < 0.001). None of the 23 patients with chronic kidney disease (CKD) including autosomal polycystic kidney disease (ADPKD) had postoperative ischemic complications. History of previous ischemic stroke had no significant association (P = 0.15). IA calcification of the distal ICA and M1 stenosis were significantly associated with postoperative ischemic complications (P < 0.001 and P = 0.001, respectively). In addition, IOM change during clipping surgery was significantly associated with ischemic complications (P < 0.001), and the proportion of patients without ischemic complication was significantly higher when intraoperative ICG was used (P = 0.04) (Table 1).
Multivariate analysis identified DM, significant IA calcification and M1 stenosis on preoperative angiogram, M1 aneurysms, and IOM change as significant risk factors for postoperative ischemic complications (Table 2).
Type of ischemic complications
Twenty-five and 17 cases showed infarction of the perforator artery territory and divisional branch territory infarctions, respectively. The proportion of DM patients was higher in the divisional branch territory infarction group than in the perforator territory infarction group (47.4% vs. 17.4%, P = 0.04; Table 3). The time to first detection of the postoperative ischemic complications by diffusion MRI was significantly longer in the divisional branch territory infarction group when compared with the perforator territory infarction (P = 0.023). The proportions of preoperative M1 stenosis and M1 aneurysm were higher in patients who exhibited perforator territory infarction when compared with the patients with MCA divisional branch territory infarction (17.4% vs. 5.3% and 43.5% vs. 26.3%, respectively); however, this did not reach significance. IOM change was detected in 4 patients with postoperative MCA perforator territory infarctions. No patients with MCA divisional branch infarction had IOM changes during clipping surgery (Table 3).
Table 4 summarizes the presenting symptoms of ischemic complications, areas of infarction, and treatment for the ischemic complication. Four out of 42 patients with postoperative ischemic complications had clip reposition on the same day of the MCA aneurysm clipping, 1 started dual antiplatelet therapy on postoperative day 4, and 37 patients had conservative treatment, including hydration. Three patients were readmitted due to ischemic symptoms in MCA territories, which were confirmed as MCA divisional branch infarction. There were significantly more patients with postoperative 6 month mRS ≥ 3 in the MCA perforator territory infarction group when compared with the MCA divisional branch territory infarction group (10 [43.5%] vs. 2 [10.5%], P = 0.019; Tables 3 and 4).
Discussion
Radiological and patient-related factors for postoperative ischemic complications
The definitions of ischemic complication vary and reported complications range from 0.8 to 3.1% after surgical treatment for unruptured MCA aneurysms [4, 5, 14, 18]. In the present study, 1.9% of patients experienced symptomatic postoperative ischemic complications after the surgical clipping of unruptured MCA aneurysms, which is consistent with recent studies [4, 5, 14].
Preoperative radiological factors, including M1 aneurysm and significant M1 stenosis, were significantly associated with the postoperative ischemic complications in all patients in the present study. Yeon et al. [9] have reported a higher risk of perforator injury in patients with proximal MCA aneurysms located close to the ICA bifurcation. In line with this, the present study found a higher risk of ischemic complications in patients with M1 aneurysms than in other MCA locations. Patients with M1 aneurysms may have a higher chance of lenticulostriate artery injury because most of the lenticulostriate arteries arise from the M1 segment [9, 19]. We sought to evaluate the association with postoperative ischemic complications after MCA aneurysm clipping by focusing on patient-related factors. The proportion of patients with coexisting DM and unruptured MCA aneurysms in the present study was 8.5% (189/2227). This was within the range of 6.3–14.2%, which has been reported from the previous studies [4, 20,21,22]. Furthermore, higher rates of postoperative morbidity and major complications in patients with DM have been reported after the surgical treatment of unruptured intracranial aneurysms [20, 21]. Michalak et al. [23] have shown higher postoperative complication rates in patients with insulin dependent DM when compared with noninsulin dependent DM in all cerebrovascular surgeries. The present study identified DM as an independent risk factor for postoperative ischemic complications. Interestingly, no significant risk of postoperative ischemic complications in patients with DM was found in recent two studies by Byoun et al. [14] and Chung et al. [4], which included 411 and 416 patients, respectively. However, a comparison between these findings and the results of the present study is limited by the different number of DM patients included in each dataset.
Twenty-three patients (1%) had chronic kidney disease at the diagnosis of unruptured MCA aneurysms in the present study and 13 (0.6%) out of 2227 patients were diagnosed with ADPKD. The incidence of coexisting ADPKD was lower in the present study comparing to the previous cohort study by Nurmonen et al. [24] which reported 1.7% of ADPKD among patients with MCA aneurysms [24]. We assume that relatively more patients with ADPKD were referred to the neurointerventionists considering the multiplicity of intracranial aneurysms.
Unlike the higher rate of CKD associated stroke event reported by Chillon et al. [25], none of the patients with CKD had neither postoperative ischemic nor hemorrhagic complications. However, we could not elucidate the association of CKD with postoperative ischemic complication due to the limited number of patients. Instead, we assume that there might have been more concern in intraoperative procedures and postoperative management.
Previous stroke occurrence was not associated with postoperative ischemic complications in this study, which is in contrast to Byoun et al. [14], which identified a history of stroke as an independent risk factor for ischemic complications after MCA aneurysm clipping. Our findings may be attributed to the additional preventive measures that patients with a history of ischemic stroke received, including antiplatelet medication and more aggressive DM and hypertension control. For example, all patients with a history of ischemic stroke had been prescribed at least one type of antiplatelet medication.
IA calcification of the ICA has been suggested as significant risk for the occurrence of ischemic stroke [26,27,28]. Correspondingly, significant IA calcification of the distal ICA was an independent risk factor for postoperative ischemic complication in the present study. This suggests that IA calcification of the distal ICA and DM may reflect an association between the burden of atherosclerosis and the occurrence of ischemic complication after aneurysm clipping surgery.
Types of postoperative ischemic complications and follow-up
There was a significantly higher association between MCA divisional branch territory infarction and DM when compared with perforator territory infarction (47.4% vs. 17.4%, P = 0.04; Table 3). Furthermore, the proportion of M1 stenosis and aneurysm was higher in the MCA perforator territory infarction group than the MCA divisional branch territory infarction group; however, this did not reach significance. Therefore, MCA perforator infarction after MCA aneurysm clipping may be more associated with preoperative radiological factors, such as the architecture of MCA aneurysms and the parent artery.
Four cases of IOM change during MCA aneurysm clipping were associated with perforator territory infarctions. In contrast, no cases with divisional branch territory infarctions exhibited IOM change. This suggests that IOM change is indicative of the occurrence of postoperative MCA perforator territory infarction. However, divisional branch territory infarction is not predictable with IOM change.
The time to first detection of ischemic symptoms was significantly longer in MCA divisional branch territory infarction than perforator territory infarction. Unfavorable outcome (mRS ≥ 3) was significantly more common in patients with MCA perforator territory infarction than in MCA divisional branch territory infarction (10/23, 43.5% vs. 2/19, 10.5%, P = 0.019) in the present study. Although the extent of infarction is small, MCA perforator territory infarction may result in unfavorable outcome as shown in Fig. 3b and c when involving the upper part of the posterior limb of the internal capsule [29]. Delayed occurrence and more favorable outcome in patients with MCA divisional branch territory infarction can be explained by vascular flow from leptomeningeal collaterals [30,31,32]. In contrast, compromise of MCA perforators such as lenticulostriate artery injury could be more associated with the postoperative ischemic complication of early onset because of the relatively lower collateral flow when compared with compromised divisional branches after M1 bifurcation [9, 33].
Therefore, the occurrence of perforator territory infarction should be carefully monitored postoperatively in the patients with M1 stenosis or M1 aneurysm particularly when IOM change was shown during clipping MCA aneurysms. Also, careful attention should be paid on the surgical procedure such as M1 manipulation for proximal control or M1 temporary clipping for those high risk patients.
On the other hands, we highlight the requirement to remain in contact with patients with DM longer during perioperative follow-up than those without DM considering higher association of DM with MCA divisional branch territory infarction in the present study.
Favorable outcomes (mRS ≤ 2) were achieved by conservative management in 27 out of 37 patients without clip reposition or antiplatelet treatments. However, we cannot conclude that there is a benefit of clip reposition after the detection of postoperative ischemic complications to prevent further deterioration in the present study due to the limited number of reported cases.
Future perspectives
Higher postoperative complication rates among the patients with DM is concerning for other surgeries; therefore, clinical recommendations for perioperative glycemic control have been suggested [34,35,36]. However, no recommendations have suggested regarding preoperative long-term control of DM to reduce risk of postoperative ischemic complications in cerebrovascular surgery. Our findings are confined to unruptured MCA aneurysms and elective clipping surgeries; however, the presence of DM should be applied to future study when evaluating ischemic complications after unruptured aneurysm clipping surgeries of other intracranial arteries [22, 37]. Moreover, optimal levels of long-term glycemic control using hemoglobin A1c before surgical treatment should be investigated in unruptured intracranial aneurysm surgery in future research to reduce ischemic complications and improve clinical outcomes.
Study limitations
In the present study, besides the patients’ underlying diseases such as DM, ischemic heart disease or the history of stroke, smoking history as a patient related factor was not investigated due to the insufficient data. Also, we only included patients with unruptured MCA aneurysms because ischemic complications in the MCA are better correlated with the relevant symptoms than the territories of other intracranial vessels. Furthermore, postoperative ischemic complications can be detected more easily. Future studies should use the patient-related risk factors that have been identified in this study as well as the history of CKD and smoking to evaluate other ischemic complications in unruptured aneurysm clipping surgeries from other intracranial arteries.
The present study included a large number of patients with unruptured MCA aneurysms that were treated by surgical clipping; however, the subgroup analysis of patients with DM may be less reliable due to the limited number of patients who developed ischemic complications (13 out of 189). Further investigations should include a prospective analysis of the treatment outcome of patients with DM and intracranial aneurysms after strict preoperative DM control.
Conclusion
We found that IA calcification of the distal ICA, M1 stenosis, M1 aneurysms, and DM were risk factors for postoperative ischemic complications after unruptured MCA aneurysm clipping. Patients with MCA perforator territory infarction showed more unfavorable outcome compared to those with MCA divisional branch territory infarction. DM was significantly associated with divisional branch territory infarction and time to first presentation of ischemic complication was longer in the case of divisional branch territory infarction than in perforator territory infarction. Therefore, patients with DM should be monitored closely to assess for the delayed occurrence of divisional branch infarction after clipping surgery for unruptured MCA aneurysms.
Data availability
The authors confirm that the data supporting the findings of this study are available within its supplementary materials.
References
Bracard S, Abdel-Kerim A, Thuillier L, Klein O, Anxionnat R, Finitsis S, Lebedinsky A, de Freitas CM, Pinheiro N, de Andrade GC, Picard L (2010) Endovascular coil occlusion of 152 middle cerebral artery aneurysms: initial and midterm angiographic and clinical results. Journal of neurosurgery 112:703–708. https://doi.org/10.3171/2009.6.JNS09483
Doerfler A, Wanke I, Goericke SL, Wiedemayer H, Engelhorn T, Gizewski ER, Stolke D, Forsting M (2006) Endovascular treatment of middle cerebral artery aneurysms with electrolytically detachable coils. AJNR American journal of neuroradiology 27:513–520
Vendrell JF, Menjot N, Costalat V, Hoa D, Moritz J, Brunel H, Bonafe A (2009) Endovascular treatment of 174 middle cerebral artery aneurysms: clinical outcome and radiologic results at long-term follow-up. Radiology 253:191–198. https://doi.org/10.1148/radiol.2531082092
Chung J, Hong CK, Shim YS, Joo JY, Lim YC, Shin YS, Kim YB (2015) Microsurgical clipping of unruptured middle cerebral artery bifurcation aneurysms: incidence of and risk factors for procedure-related complications. World neurosurgery 83:666–672. https://doi.org/10.1016/j.wneu.2015.01.023
Nussbaum ES, Madison MT, Goddard JK, Lassig JP, Kallmes KM, Nussbaum LA (2018) Microsurgical treatment of unruptured middle cerebral artery aneurysms: a large, contemporary experience. Journal of neurosurgery:1-7. doi:https://doi.org/10.3171/2018.1.jns172466
Nussbaum ES, Madison MT, Myers ME, Goddard J (2007) Microsurgical treatment of unruptured intracranial aneurysms. A consecutive surgical experience consisting of 450 aneurysms treated in the endovascular era. Surgical neurology 67:457–464; discussion 464-456. https://doi.org/10.1016/j.surneu.2006.08.069
Smith TR, Cote DJ, Dasenbrock HH, Hamade YJ, Zammar SG, El Tecle NE, Batjer HH, Bendok BR (2015) Comparison of the efficacy and safety of endovascular coiling versus microsurgical clipping for unruptured middle cerebral artery aneurysms: a systematic review and meta-analysis. World neurosurgery 84:942–953. https://doi.org/10.1016/j.wneu.2015.05.073
Alshekhlee A, Mehta S, Edgell RC, Vora N, Feen E, Mohammadi A, Kale SP, Cruz-Flores S (2010) Hospital mortality and complications of electively clipped or coiled unruptured intracranial aneurysm. Stroke 41:1471–1476. https://doi.org/10.1161/STROKEAHA.110.580647
Yeon JY, Kim JS, Hong SC (2011) Angiographic characteristics of unruptured middle cerebral artery aneurysms predicting perforator injuries. British journal of neurosurgery 25:497–502. https://doi.org/10.3109/02688697.2010.535924
Quiney B, Ying SM, Hippe DS, Balu N, Urdaneta-Moncada AR, Mossa-Basha M (2017) The association of intracranial vascular calcification and stenosis with acute ischemic cerebrovascular events. Journal of computer assisted tomography 41:849–853. https://doi.org/10.1097/rct.0000000000000629
Zhang J, Li Y, Wang Y, Niu W, Zhang Y, Gao P, Zhang L, Lin H, Chen K, Zhu D (2011) Arterial stiffness and asymptomatic intracranial large arterial stenosis and calcification in hypertensive chinese. American journal of hypertension 24:304–309. https://doi.org/10.1038/ajh.2010.246
Elsharkawy A, Lehecka M, Niemela M, Billon-Grand R, Lehto H, Kivisaari R, Hernesniemi J (2013) A new, more accurate classification of middle cerebral artery aneurysms: computed tomography angiographic study of 1,009 consecutive cases with 1,309 middle cerebral artery aneurysms. Neurosurgery 73:94–102; discussion 102. https://doi.org/10.1227/01.neu.0000429842.61213.d5
Tanriover N, Kawashima M, Rhoton AL Jr, Ulm AJ, Mericle RA (2003) Microsurgical anatomy of the early branches of the middle cerebral artery: morphometric analysis and classification with angiographic correlation. Journal of neurosurgery 98:1277–1290. https://doi.org/10.3171/jns.2003.98.6.1277
Byoun HS, Bang JS, Oh CW, Kwon OK, Hwang G, Han JH, Kim T, Lee SU, Jo SR, Kim DG, Park KS (2016) The incidence of and risk factors for ischemic complications after microsurgical clipping of unruptured middle cerebral artery aneurysms and the efficacy of intraoperative monitoring of somatosensory evoked potentials: a retrospective study. Clinical neurology and neurosurgery 151:128–135. https://doi.org/10.1016/j.clineuro.2016.10.008
Chung J, Park W, Hong SH, Park JC, Ahn JS, Kwun BD, Lee SA, Kim SH, Jeon JY (2018) Intraoperative use of transcranial motor/sensory evoked potential monitoring in the clipping of intracranial aneurysms: evaluation of false-positive and false-negative cases. Journal of neurosurgery:1-13. doi:https://doi.org/10.3171/2017.8.Jns17791
Donzelli R, Marinkovic S, Brigante L, de Divitiis O, Nikodijevic I, Schonauer C, Maiuri F (1998) Territories of the perforating (lenticulostriate) branches of the middle cerebral artery. Surg Radiol Anat 20:393–398. https://doi.org/10.1007/BF01653128
Kim JS, Caplan LR (2016) Clinical stroke syndromes. Frontiers of neurology and neuroscience 40:72–92. https://doi.org/10.1159/000448303
Rodriguez-Hernandez A, Sughrue ME, Akhavan S, Habdank-Kolaczkowski J, Lawton MT (2013) Current management of middle cerebral artery aneurysms: surgical results with a “clip first” policy. Neurosurgery 72:415–427. https://doi.org/10.1227/NEU.0b013e3182804aa2
Rosner SS, Rhoton AL Jr, Ono M, Barry M (1984) Microsurgical anatomy of the anterior perforating arteries. Journal of neurosurgery 61:468–485. https://doi.org/10.3171/jns.1984.61.3.0468
Kerezoudis P, McCutcheon BA, Murphy M, Rayan T, Gilder H, Rinaldo L, Shepherd D, Maloney PR, Hirshman BR, Carter BS, Bydon M, Meyer F, Lanzino G (2016) Predictors of 30-day perioperative morbidity and mortality of unruptured intracranial aneurysm surgery. Clinical neurology and neurosurgery 149:75–80. https://doi.org/10.1016/j.clineuro.2016.07.027
Kim JE, Lim DJ, Hong CK, Joo SP, Yoon SM, Kim BT (2010) Treatment of unruptured intracranial aneurysms in South Korea in 2006 : a nationwide multicenter survey from the korean society of cerebrovascular surgery. Journal of Korean Neurosurgical Society 47:112–118. https://doi.org/10.3340/jkns.2010.47.2.112
Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, Nakayama T, Sakai M, Teramoto A, Tominari S, Yoshimoto T (2012) The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 366:2474–2482. https://doi.org/10.1056/NEJMoa1113260
Michalak SM, Rolston JD, Lawton MT (2016) Incidence and predictors of complications and mortality in cerebrovascular surgery: national trends from 2007 to 2012. Neurosurgery 79:182–193. https://doi.org/10.1227/neu.0000000000001251
Nurmonen HJ, Huttunen T, Huttunen J, Kurki MI, Helin K, Koivisto T, von Und Zu Fraunberg M, Jaaskelainen JE, Lindgren AE (2017) Polycystic kidney disease among 4,436 intracranial aneurysm patients from a defined population. Neurology 89:1852–1859. https://doi.org/10.1212/WNL.0000000000004597
Chillon JM, Massy ZA, Stengel B (2016) Neurological complications in chronic kidney disease patients. Nephrol Dial Transplant 31:1606–1614. https://doi.org/10.1093/ndt/gfv315
Kim JM, Park KY, Bae JH, Han SH, Jeong HB, Jeong D (2019) Intracranial arterial calcificationes can reflect cerebral atherosclerosis burden. Journal of clinical neurology (Seoul, Korea) 15:38-45. doi:https://doi.org/10.3988/jcn.2019.15.1.38
Koton S, Tashlykov V, Schwammenthal Y, Molshatzki N, Merzeliak O, Tsabari R, Tanne D (2012) Cerebral artery calcification in patients with acute cerebrovascular diseases: determinants and long-term clinical outcome. European journal of neurology 19:739–745. https://doi.org/10.1111/j.1468-1331.2011.03620.x
Yilmaz A, Akpinar E, Topcuoglu MA, Arsava EM (2015) Clinical and imaging features associated with intracranial internal carotid artery calcifications in patients with ischemic stroke. Neuroradiology 57:501–506. https://doi.org/10.1007/s00234-015-1494-8
Ohara T, Yamamoto Y, Tamura A, Ishii R, Murai T (2010) The infarct location predicts progressive motor deficits in patients with acute lacunar infarction in the lenticulostriate artery territory. J Neurol Sci 293:87–91. https://doi.org/10.1016/j.jns.2010.02.027
Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP (2005) Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR American journal of neuroradiology 26:1789–1797
Mohammad YM, Christoforidis GA, Bourekas EC, Slivka AP (2008) Qureshi grading scheme predicts subsequent volume of brain infarction following intra-arterial thrombolysis in patients with acute anterior circulation ischemic stroke. J Neuroimaging 18:262–267. https://doi.org/10.1111/j.1552-6569.2007.00233.x
Qureshi AI, El-Gengaihi A, Hussein HM, Suri MF, Liebeskind DS (2008) Occurence and variability in acute formation of leptomeningeal collaterals in proximal middle cerebral artery occlusion. J Vasc Interv Neurol 1:70–72
Decavel P, Vuillier F, Moulin T (2012) Lenticulostriate infarction. Frontiers of neurology and neuroscience 30:115–119. https://doi.org/10.1159/000333606
Arthur CPS, Mejia OAV, Lapenna GA, Brandao CMA, Lisboa LAF, Dias RR, Dallan LAO, Pomerantzeff PMA, Jatene FB (2018) Perioperative management of the diabetic patient referred to cardiac surgery. Brazilian journal of cardiovascular surgery 33:618-625. doi:10.21470/1678-9741-2018-0147
Mendes-Braz M, Martins JO (2018) Diabetes mellitus and liver surgery: the effect of diabetes on oxidative stress and inflammation. Mediators of inflammation 2018:2456579–2456511. https://doi.org/10.1155/2018/2456579
Peters A, Kerner W (1995) Perioperative management of the diabetic patient. Exp Clin Endocrinol Diabetes 103:213–218. https://doi.org/10.1055/s-0029-1211353
Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, Forbes GS, Thielen K, Nichols D, O'Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC, International Study of Unruptured Intracranial Aneurysms I (2003) Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362:103–110
Acknowledgment
The authors are thankful to all staff members of neurosurgical department and the patients of the study group whose contributions made this work possible.
Author information
Authors and Affiliations
Contributions
Heui Seung Lee M.D. (first author) conceived and designed the analysis, collected the data, contributed data or analysis tools, performed the analysis, and wrote the paper, and other contribution. Moinay Kim M.D conceived and designed the analysis, collected the data, contributed data or analysis tools, performed the analysis, wrote the paper, and thoroughly reviewed the manuscript and made corrections. Jung Cheol Park M.D conceived and designed the analysis, collected the data, contributed data or analysis tools, performed the analysis, wrote the paper, and thoroughly reviewed the manuscript and made corrections. Jae Sung Ahn, M.D, Ph.D conceived and designed the analysis, collected the data, contributed data or analysis tools, performed the analysis, wrote the paper, and other contribution. Seungjoo Lee, M.D, Ph.D conceived and designed the analysis, collected the data, contributed data or analysis tools, performed the analysis, wrote the paper, and other contribution. Wonhyoung Park, M.D., Ph.D. (corresponding author) conceived and designed the analysis, collected the data, contributed data or analysis tools, performed the analysis, wrote the paper, and thoroughly reviewed the manuscript and made corrections.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (name of institute/committee) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate (include appropriate statements)
For this type of study, retrospective formal consent from patients is not required.
Consent for publication (include appropriate statements)
For this type of retrospective study, formal consent is not required.
Code availability (software application or custom code)
SPSS version 22.0 (IBM, Armonk, NY, USA) was used for all statistical analyses
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(CSV 95.4 kb)
Rights and permissions
About this article
Cite this article
Lee, H.S., Kim, M., Park, J.C. et al. Clinical features of ischemic complications after unruptured middle cerebral artery aneurysm clipping: patients and radiologically related factors. Neurosurg Rev 44, 2819–2829 (2021). https://doi.org/10.1007/s10143-021-01475-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-021-01475-8