Abstract
While intracerebral hemorrhage (ICH) scoring systems provide mortality and morbidity prediction, the actual mortality rates seem to be lower than those predicted by scoring systems in our clinical impression. To assess the validity of the ICH score and the Surgical Swedish ICH (SwICH) score, we retrospectively reviewed surgically treated ICH patients between 2012 and 2019. Uni- and multivariate analyses were performed to identify variables in predicting 30-day mortality. We identified 203 patients (mean ICH score 2.7; mean SwICH score 2.0). The actual 30-day mortality was 7%, which was significantly lower than those predicted by the ICH and the SwICH scores (55% and 16%, respectively; p < 0.001). Both scores were strongly correlated with the modified Rankin scale (mRS) at discharge (correlation coefficient 0.97 and 0.98; critical value 0.81). The only significant prognostic factors for the 30-day mortality by multivariate analysis were anisocoria (p = 0.03) and preoperative Glasgow Coma Scale (p = 0.03). These two factors also predicted mRS at discharge (p < 0.001). After discharge, 15% of patients improved regarding mRS and 29% of wheelchair-bound patients gained the ability to ambulate. No significant relationship existed between the degree of recovery after discharge and preoperative ICH score (p = 0.25). The ICH and SwICH scores were more valid in predicting morbidity, rather than mortality after surgical intervention for ICH. Anisocoria and Glasgow Coma Scale < 7 were the only two factors that predicted 30-day mortality and morbidity at discharge.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intracerebral hemorrhage (ICH) is one of the most fatal types of stroke accounting for 15–30% of all cerebrovascular accidents [1]. ICH scoring systems are well-known for predicting 30-day mortality and 12-month functional outcome [1,2,3,4]. Moreover, neurosurgeons from Sweden recently published the Surgical Swedish ICH (SwICH) score, reflecting their hypothesis that surgical hematoma evacuation provides a survival benefit [2]. However, the authors’ clinical impression amid the care of ICH patients was that the actual mortality was much lower than the predicted scores. In response to that, the authors aimed to evaluate the external validity of the ICH and SwICH scores and summarize the outcome of ICH patients over the past years to create our own prognostic scheme.
Methods
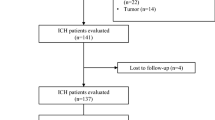
We retrospectively reviewed patients who underwent surgical intervention for ICH between January 2012 and March 2020. Our institutional review board did not require informed consent for the study participation because this study relied on information obtained as part of routine clinical practice. The exclusion criteria were traumatic causes and ICH secondary to aneurysmal subarachnoid hemorrhage. The following variables were collected from their medical charts: age, sex, the date and style of surgery, date of diagnosis of ICH, initial Glasgow Coma Scale (GCS), presence of anisocoria, hematoma sites, hematoma volume at the initial computed tomography (CT), the degree of midline shift in case of supratentorial ICH, the degree of prepontine cistern obscuration in case of infratentorial ICH, presence of acute hydrocephalus, presence of intraventricular hematoma (IVH), subsequent shunt surgery, presence of subarachnoid hemorrhage (in putaminal hemorrhage cases), past medical history (hypertension, diabetes mellitus, myocardial infarction), medications (antiplatelets or anticoagulants), preoperative laboratory data (hemoglobin A1c, lactate dehydrogenase, white blood cell count, platelet count, prothrombin time international normalized ratio, d-dimer), pathology from surgical specimen, and outcome (modified Rankin scale (mRS) at discharge and the latest follow-up, 30-day mortality, length of stay, and mortality at the time of the last follow-up). Hematoma volume was calculated using the ABC/2 method, where A was the largest diameter in the axial image, B the diameter perpendicular to A, and C the height of the hematoma measured by coronal image or, if the coronal image was not available, the number of axial slices of hemorrhage multiplied by slice thickness.
Based on these variables, the ICH score [3] and SwICH score [2] were calculated to assess their validity in our population. The ICH score factors into the GCS score (2 points for 3–4, 1 point for 5–12, 0 for 13–15), ICH volume (1 point for 30 mL or more), IVH (1 point for the presence of IVH), infratentorial origin (1 point for infratentorial hemorrhage), and age (1 point for 80 years old or older) and ICH is stratified based on the calculated score of 0–6 [3]. The SwICH score factors into GCS, age, ICH volume, type 2 diabetes, and prior myocardial infarction making a total score range of 0–6 [2]. We also analyzed prognostic factors for 30-day mortality. The latest status of our patients after discharge was also assessed.
Statistical analysis
Statistical analyses were carried out using Microsoft Excel, IBM SPSS Statistics 25, and KyPlot 6.0. A p value of 0.05 or less was considered statistically significant. Categorical and continuous variables were analyzed using the Chi-squared test and Student’s t test, respectively. Analysis of variance was used for comparisons of means between more than two groups. The regression lines of actual and predicted 30-day mortality were drawn and analyzed using a parallelism test. For prognostic factor analysis, univariate regression analysis was performed first, and multivariate logistic regression and Cox regression analyses were performed by including only those whose p values were less than 0.1 in the univariate analysis.
Results
Baseline characteristics
Patient characteristics are shown in Table 1. A total of 203 patients were identified and their mean age at diagnosis was 66 years. The mean ICH and SwICH scores were 2.7 (range, 0–6) and 2.0 (range, 0–5), respectively. The putamen was the most common site of hemorrhage (39%), followed by subcortical hemorrhage (30%). Among the 80 putaminal hemorrhages, 21 (27%) showed subarachnoid hemorrhage (SAH) inside the Sylvian cistern. The average hematoma volume between those with Sylvian cistern SAH and those without were 87 mL and 71 mL, respectively, which was statistically significant (p < 0.05) (not shown in a table). IVH was found in 146 patients, most of which were secondary extensions from ICH (97%), and primary IVH accounted for 3%. Of 146 IVH cases, 14 (10%) resulted in subsequent shunt placement. One patient had already been shunted for idiopathic normal pressure hydrocephalus and one underwent endoscopic third ventriculostomy.
Mortality and its validation of ICH scores
The 30-day mortality rate was 7% (15 of 203). Three patients died after 30 days, and five patients died after discharge during the average follow-up of 318 days. The survival curve is shown in Fig. 1 and the validity of both the ICH and SwICH scores is shown in Fig. 2a, b. A large gap existed between the actual 30-day mortality and predicted 30-day mortality. The gap was smaller between the actual 30-day mortality and 30-day mortality predicted by the SwICH score than by the ICH score. The two lines (actual mortality and predicted mortality) were significantly different from each other (p < 0.001).
Morbidity and mortality of ICH and SwICH scores in our cohort. The actual mortality was significantly lower than predicted by both ICH (a) and SwICH (b) scores. Modified Rankin Scale (mRS) at discharge correlated well with both scores (c). SwICH score of 6 did not exist in our cohort and mRS corresponding to SwICH score of 6 was not marked
Morbidity and its validation of ICH scores
Contrary to the mortality described above, both scores were significantly correlated with mRS at discharge. The correlation coefficient of mRS at discharge and the ICH score was 0.97 (critical value 0.81), while the correlation coefficient of mRS at discharge and the SwICH score was 0.98 (critical value 0.81). As seen in Table 1, the average length of stay was 45 days.
The status of mRS after discharge is shown in Table 2. Of the 80 patients (39%) able to gain the latest mRS after discharge, 38% (30 of 80) improved overall with physical medicine and rehabilitation using an appropriate device. Of note, 29% (16 of 56) of those unable to walk at the time of transfer to the rehabilitation hospital gained the ability to ambulate independently with orthoses or walking aids. Although 50% (40 of 80) remained unchanged with respect to mRS, their functional independence measure improved by rehabilitation. For those whose mRS worsened (10 patients), the causes were aspiration pneumonia (three patients), preexisting malignancy progression (one patient had glioblastoma, the other two patients had cancers outside the brain), airway bleeding secondary to chronic kidney disease (one patient), another ICH (one patient), senescence (one patient), and unknown (one patient). Of all, four patients (aspiration in 3 and another ICH in 1) were associated with the insult caused by ICH. Finally, there were no significant differences in ICH scores between the improved, unchanged, and worsened groups (p = 0.25).
Prognostic factors in our cohort
The relationship between factors and the 30-day mortality is shown in Table 3. Anisocoria, preoperative GCS, white blood cell count, and residual hematoma were statistically significant prognostic factors by univariate analysis. Age, hematoma volume, IVH, and history of diabetes mellitus or myocardial infarction were not significant factors for 30-day mortality. On multivariate analysis, anisocoria and preoperative GCS were found to be significant (both p = 0.03).
Based on the preoperative status of pupils and GCS, the patients could be classified into four groups (Table 4). Both mortality and mRS were significantly correlated with these two preoperative factors (p < 0.001). It should be noted that none of those with isocoria and GCS > 7 preoperatively (group 1) died by day 30. On the other hand, 23% of anisocoric patients with GCS ≤ 7 (group 4) died by day 30, and when they survived, most of them ended up bedridden at the time of discharge.
Discussion
This study showed that the 30-day mortality of surgically treated ICH patients was significantly lower than that predicted by the ICH and SwICH scores. Anisocoria and preoperative GCS were found to be the only two significant prognostic factors for 30-day mortality and morbidity at discharge.
Baseline characteristics
When the population of the original study of ICH score was compared with our patient’s characteristics, age, sex, initial GCS, and site of hemorrhage were similar [3]. The presumed etiology was similar to that reported in previous studies, with hypertension being the primary cause, followed by cerebral amyloid angiopathy, arteriovenous malformation ruptures, and other causes [5, 6]. However, the mean hematoma volume was higher than that of other studies [3, 7, 8]. This may be due to our study design, where only surgical intervention was performed. The proportion of patients on antiplatelet or anticoagulant drugs (25%) was similar to that reported in a previous study [2]. Overall, our patient cohort was not much deviated in its baseline characteristics from previous studies except for higher hematoma volume (Table 1).
Mortality
The 30-day mortality predicted by either the ICH or SwICH scores did not match the actual mortality in our cohort (Fig. 2). The dissociation between the actual and predicted mortality may be attributed to (1) the proportion of the surgical intervention group, (2) difference in the style of acute phase management, and (3) other reasons. In the original ICH score article, the surgical hematoma evacuation arm consisted of only 13% of the cohort [3]. In other studies that verified the ICH score mortality, the surgical arm consisted of only 7% [9]. Furthermore, the mortality predicted by SwICH, exclusively applied to the open surgery group [2], was closer to the actual mortality (Fig. 2b). Another possibility could be that the degrees of medical, nursing, and rehabilitation care were different. Due to the national medical insurance system in our country, practically all patients could receive the same quality of treatment unless their families or relatives wished otherwise. This may partially explain the difference in the acute care management of ICH. Another potential reason for the dissociation could be that the ICH score, calculated 24 h from the presentation, may be a better predictor [10]. Unfortunately, since the 24-h ICH score was not available in all patients, we were not able to assess this possibility.
Morbidity
In contrast to mortality, both the ICH and SwICH scores showed a correlation with the mRS at discharge, in line with previous literature [4]. Both the ICH and SwICH scores were valid for predicting morbidity. A statistically significant correlation was observed in our study (Fig. 2c). Thus, these scores may be useful for predicting morbidity, rather than mortality, independent of where medicine is practiced. Mortality may differ between countries depending on the social environment surrounding the medical practice.
Of the 80 patients followed-up after discharge, 38% improved in mRS. Up to 29% of patients unable to walk independently were ambulatory after going through the rehabilitation hospitals. These findings were similar to those of a previous study [4, 11]. No significant tendency existed in the mRS status change based on the ICH score (Table 2) and this was similar for the four groups (Table 4), where improved patients existed regardless of preoperative severity. Hence, every patient can improve with help and we should not give up on them no matter how severe is their preoperative neurological function. Of the 10 (13%) worsened patients, the most common reasons were repeated aspiration pneumonia (30%) and malignancy (30%). Aspiration pneumonia may be due to ICH. Forty percent of the deterioration could be attributed to ICH, but not all patients died because of ICH. Although the medical cost is not negligible [12], we should not readily give up on radiologically devastating ICH. Even the worst prognostic group 4 patients (Table 4) had a good chance of survival and room for improvement after discharge.
Prognostic factors in our cohort
The only significant prognostic factors for 30-day mortality were anisocoria and preoperative GCS (Table 3). This was consistent with our initial impression when we encountered patients with ICH. However, contrary to our expectations, the size of the hematoma, hematoma extension to the ventricle (IVH), or age were not statistically significant. The reasons for this may be due to the three possibilities described above. Regardless of the hematoma’s largeness, timely management of intracranial pressure may decrease the patient’s mortality, although not necessarily morbidity. The same principle may apply to the status of IVH by placing endoscopic ventricular drainage or endoscopically removing the hematoma. The degree of midline shift (supratentorial hematoma) or radiological obscuration of the prepontine cistern (cerebellar hemorrhage) were not significant factors. These findings are similar to those of a previous study, where the midline shift of more than 5 mm was not significant in the multivariate analysis [6]. The status of anticoagulants, antiplatelets, or platelet count was not a significant factor. This suggests that although these factors may be negative in the surgical intervention, once appropriate reversal agents were administered and meticulous hemostasis achieved intraoperatively, mortality was not affected. Regarding the effects of anticoagulants or antiplatelets on the prognosis, some studies implied that they were not significant factors [2, 3] similar to our findings, whereas others reported that they were [13, 14].
Based on our findings, we proposed to stratify the patients into four groups (Table 4). This stratification was simple and could be performed quickly, requiring only physical examinations. Since this grouping method was significantly correlated with mortality and morbidity, prospective analyses are warranted to further validate this classification.
Limitations
The retrospective nature and the different medical practice customs, as well as the national insurance system were the major limitations to our study. However, it is worth noting that the ICH mortality rate is better than what is predicted by the current scoring systems in some countries. Due to its retrospective nature, there was some missing information about the variables. Another drawback was the different patient populations. It may be possible that more severe cases were not included in our cohort, potentially inducing a selection bias. The mean SwICH scores of the original cohort and ours were 2.4 [2] and 2.0, respectively (p < 0.001). The mean of the ICH scores of the original cohort and ours were 2.2 [3] and 2.7 respectively (p < 0.001). Consequently, it may be possible that the SwICH score was not properly evaluated by our cohort. Finally, evaluating morbidity based on mRS may not be sufficient in some cases, since the status of paralysis (or paresis) strongly affects mRS (3 or 4).
Conclusions
The mortality of ICH in this study was better than what was expected from the ICH score or the SwICH score. Anisocoria and GCS < 7 were the two predictive factors for 30-day mortality and morbidity.
Data availability
Data transparency was confirmed.
References
Greenberg MS (2016) Handbook of neurosurgery, 9th edn. Thieme, Germany
Fahlström A, Nittby Redebrandt H, Zeberg H, Bartek J Jr, Bartley A, Tobieson L, Erkki M, Hessington A, Troberg E, Mirza S, Tsitsopoulos PP, Marklund N (2019) A grading scale for surgically treated patients with spontaneous supratentorial intracerebral hemorrhage: the Surgical Swedish ICH Score. J Neurosurg:1–8. https://doi.org/10.3171/2019.5.JNS19622
Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC (2001) The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 32:891–897. https://doi.org/10.1161/01.str.32.4.891
Hemphill JC 3rd, Farrant M, Neill TA Jr (2009) Prospective validation of the ICH score for 12-month functional outcome. Neurology 73:1088–1094. https://doi.org/10.1212/WNL.0b013e3181b8b332
Appelboom G, Hwang BY, Bruce SS, Piazza MA, Kellner CP, Meyers PM, Connolly ES (2012) Predicting outcome after arteriovenous malformation-associated intracerebral hemorrhage with the original ICH score. World Neurosurg 78:646–650. https://doi.org/10.1016/j.wneu.2011.12.001
Cheung RT, Zou LY (2003) Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke 34:1717–1722. https://doi.org/10.1161/01.Str.0000078657.22835.B9
Rost NS, Smith EE, Chang Y, Snider RW, Chanderraj R, Schwab K, FitzMaurice E, Wendell L, Goldstein JN, Greenberg SM, Rosand J (2008) Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke 39:2304–2309. https://doi.org/10.1161/strokeaha.107.512202
Schmidt FA, Liotta EM, Prabhakaran S, Naidech AM, Maas MB (2018) Assessment and comparison of the max-ICH score and ICH score by external validation. Neurology 91:e939–e946. https://doi.org/10.1212/wnl.0000000000006117
Clarke JL, Johnston SC, Farrant M, Bernstein R, Tong D, Hemphill JC 3rd (2004) External validation of the ICH score. Neurocrit Care 1:53–60. https://doi.org/10.1385/ncc:1:1:53
Aysenne AM, Albright KC, Mathias T, Chang TR, Boehme AK, Beasley TM, Martin-Schild S (2013) 24-hour ICH score is a better predictor of outcome than admission ICH score. ISRN stroke 2013:605286. https://doi.org/10.1155/2013/605286
Ojha P, Sardana V, Maheshwari D, Bhushan B, Kamble S (2019) Clinical profile of patients with acute Intracerebral hemorrhage and ICH score as an outcome predictor on discharge, 30 days and 60 days follow-up. J Assoc Physicians India 67:14–18
Feigin VL, Lawes CM, Bennett DA, Anderson CS (2003) Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol 2:43–53. https://doi.org/10.1016/s1474-4422(03)00266-7
Houben R, Schreuder F, Bekelaar KJ, Claessens D, van Oostenbrugge RJ, Staals J (2018) Predicting prognosis of Intracerebral hemorrhage (ICH): performance of ICH score is not improved by adding oral anticoagulant use. Front Neurol 9:100. https://doi.org/10.3389/fneur.2018.00100
Zis P, Leivadeas P, Michas D, Kravaritis D, Angelidakis P, Tavernarakis A (2014) Predicting 30-day case fatality of primary inoperable intracerebral hemorrhage based on findings at the emergency department. J Stroke Cerebrovasc Dis 23:1928–1933. https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.02.006
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. SH made a study design, collected patient data, drafted and revised the manuscript. KM was a supervisor and made a substantial contribution to revising the original draft. AN, TH, MN, and YS were the supervisors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Code availability
Not applicable.
Ethical approval
This study was done under our institutional review board approval and did not require patient consent.
Consent to participate
Our institutional review board did not require informed consent for study participation because this study relied on information obtained as part of routine clinical practice.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hong, S., Maruyama, K., Noguchi, A. et al. Is using intracerebral hemorrhage scoring systems valid for mortality prediction in surgically treated patients?. Neurosurg Rev 44, 2747–2753 (2021). https://doi.org/10.1007/s10143-020-01451-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01451-8