Abstract
Outcomes of 37 patients of foramen magnum meningioma (FMM) were evaluated, and the related literature was reviewed to determine the efficacy of Gamma Knife radiosurgery (GKRS) for treating patients with FMM. We present the largest series reported from a single institution with the longest follow-up to date. The database of patients who underwent GKRS for FMM between 2007 and 2019 was evaluated retrospectively. A total of 37 patients with radiological and pathological features consistent with FMM were included in this series. Thirty-three patients were female, and 4 were male. The median age was 58 years (range, 23–74 years). The most common symptom at diagnosis was headache (64.9%). Twelve patients had a history of microsurgical resection. The median duration from the initial onset of symptoms to GKRS was 12 months (range 1–140 months). Among the 37 tumors, eight (21.6%) were located ventrally, 24 (64.9%) laterally, and five (13.5%) dorsally. The median target volume was 3.30 cm3 (range, 0.6–17.6 cm3). Thirty-five patients (95%) were treated with single fraction GKRS, and two patients (5%) were treated with hypofractionated GKRS. The median clinical follow-up was 80 months (range, 18–151 months), while the median radiological follow-up was 84 months (range, 18–144 months). At the last clinical follow-up after GKRS, 27 patients (73%) had improved symptoms, and none had worsened pre-GKRS symptoms. At the last radiological follow-up after GKRS, 23 tumors (62.2%) remained stable, 13 (35.1%) decreased in size, and 1 (2.7%) increased in size. Tumor control, including stable and regressed tumors, was achieved in 97.3% of patients. Our cohort demonstrates that GKRS is an effective and safe treatment for patients with either primary or recurrent/residual FMM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Meningiomas are the most common primary intracranial tumors [1, 2]. Foramen magnum meningiomas (FMMs) account for only 2–3% of all meningiomas but 75% of all benign extramedullary tumors at the foramen magnum [3,4,5,6,7]. Seventy percent of the meningiomas, even small ones, were shown to grow radiologically and become symptomatic [8]. Despite the rarity of FMMs, microsurgical resection is extremely challenging for neurosurgeons due to their intimate proximity to the critical structures, including the brainstem, lower cranial nerves, medulla, vertebral artery, and its branches.
Although advances in microsurgery and imaging techniques have provided relatively safe surgical resection, surgery of the FMMs continues to result in high rates of complication, morbidity, and recurrence [9,10,11,12]. Therefore, minimally invasive alternative treatment modalities have been considered to be advantageous in selected patients with FMMs. Gamma Knife radiosurgery (GKRS) has emerged as an efficacious primary or adjuvant therapy for intracranial meningiomas [13,14,15,16,17]. However, there is currently limited data regarding efficiency, outcome, and complications of the GKRS treatment for FMMs in the literature.
In the present study, long-term outcomes of 37 patients with FMMs were evaluated, and the related literature was reviewed to determine the efficacy of GKRS for patients with FMMs.
Material and methods
Patients
The medical records of cases that underwent GKRS between 2007 and 2019 were collected and evaluated retrospectively. A total of 37 patients were included in this study. Inclusion criteria were as follows:
-
1)
A histologically confirmed meningioma or magnetic resonance imaging (MRI) features consistent with meningioma
-
2)
A tumor located at the previously described regions [18]: (a) ventrally, the inferior third of the clivus and the superior edge of the C2 body; (b) laterally, the jugular tubercles and the superior border of the C2 lamina; and (c) dorsally, the anterior border of the squamous occipital bone and the C2 spinous process
-
3)
Patients who were not candidates for primary surgical treatment based on age and the projected operative risks due to medical comorbidities
- 4)
The median age was 58 years (range, 23–74 years), and most of the patients (89%) were female. Among the 37 tumors, eight (21.6%) were located ventrally, 24 (64.9%) laterally, and five (13.5%) dorsally. Fourteen patients had a history of microsurgical resection. GKRS was performed for a recurrent tumor in 11 patients (29.7%) and residual tumor in three patients (8.1%). On the other hand, GKRS was performed due to the high risk of surgery in six patients (16.2%) and patient preference in 17 patients (46%).
Gamma Knife radiosurgery procedure
GKRS was performed as previously described [19]. In brief, the Leksell Gamma Knife® model 4C (2007–2012), Perfexion™ (2012–2017), and Icon™ (2017–2019) (Elekta Instrument AB, Stockholm, Sweden) were used for GKRS treatment. After administering local scalp anesthetic, a Leksell stereotactic coordinate frame was applied, and a contrast-enhanced MRI was obtained. Multiple-dose planning was performed using the GammaPlan. In case of hypofractionated GKRS, a thermoplastic mask molded over the patient’s face and reference cone-beam CT (CBCT) scan are obtained and registered with the planning MRI and CT using the registration algorithm of the Leksell GammaPlan (version 11.0.3 Elekta Instrument, Stockholm, Sweden). The target volume was delineated, and dose and fractionation regimens were chosen depending on the tumor size, location, and the projected risk to the adjacent structures. Maximal point spinal cord and brainstem doses were kept below 12 Gy when possible. Details of the GKRS parameters are demonstrated in Table 1.
The median target volume was 3.30 cm3 (range, 0.6–17.6 cm3). Thirty-five patients (95%) were treated with single fraction GKRS, and two patients (5%) were treated with hypofractionated GKRS. For single fraction GKRS, the median margin dose, maximum dose, and the isodose line were 12 Gy (range, 8–14 Gy), 24 Gy (range, 16–30 Gy), and 50% (range, 40–50%), respectively. For hypofractionated GKRS, the median margin dose, maximum dose, and the isodose line were 20 Gy in 5 fractions, 45 Gy (range, 40–50 Gy), and 45% (range, 40–50%), respectively.
Follow-up
All patients were followed up with serial contrast-enhanced MRIs at six-month intervals for the first year and annually thereafter. Follow-up MRIs of the patients were compared with MRIs obtained before GKRS, and tumor responses were defined as “progression” (increase in volume > 20%), “regression” (decrease in volume > 20%), and “stable” (maximum 20% change in volume) [13, 20]. Tumor volume was subsequently calculated as the sum of the surface areas multiplied by the slice thickness. Volume was calculated on the day of radiosurgery as a baseline and then on each follow-up MRI until the last available scan or until the patient underwent resection or repeat GKRS [21]. It was shown that the volume of a compact lesion could be calculated accurately with less than 10% error with a minimum of five slices through the region of interest [20]. Since 2017, Elements SmartBrush (Brainlab AG, Germany) was used for multi-planar 3D tumor contouring and volumetric measurement. Progression-free survival was defined as the time interval until progression or death and the last follow-up date. Clinical follow-up was performed by a neurosurgeon who assessed the patient’s symptoms or signs, neurological function, and Karnofsky performance scale score to determine whether further intervention was needed. Clinical complications were specified as new deficits on neurologic exam which were not present prior to GKRS. Adverse radiation events (AREs) were defined as new or worsening peritumoral edema that shows high intensity on T2-weighted or FLAIR MRI sequences after GKRS. Toxicity was ranked with the Common Terminology Criteria for Adverse Events version 5.
Statistical analyses
All analyses were conducted utilizing SPSS 26.0 (Statistical Package for Social Sciences, SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to define the study cohort. Kaplan-Meier survival plots were applied to estimate progression-free survival distributions. Log-rank test was used to assess predictive factors on clinical and radiological outcomes, which included age, gender, prior surgery, pretreatment KPS score, volume, marginal dose, and maximum dose. All tests were two-sided, and p < 0.05 was regarded as statistically significant.
Results
General characteristics
All patients presented with symptomatic tumors, and the most common symptom at diagnosis was headache (64.9%). The patients had a median of two symptoms or signs (range, 1–4). The tumor volume was not related with symptoms (p = 0.685). The median duration from the initial onset of symptoms to GKRS was 12 months (range 1–140 months). The tumors showed progression in 27 patients (73%) from the initial diagnosis to the time of GKRS. The median Karnofsky performance score before treatment was 90 (range, 40–90). A summary of the baseline and treatment features of this study is shown in Table 1.
Clinical and radiological outcomes
The median clinical follow-up was 80 months (range, 18–151 months), while the median radiological follow-up was 84 months (range, 18–144 months). Four foreign patients were lost to follow-up following a median clinical follow-up was 19.5 months (range, 18–22 months) and a median radiological follow-up was 18 months (range, 18–18 months).
At the last clinical follow-up after GKRS, 27 patients (73%) had improved symptoms, and none had worsened pre-GKRS symptoms. The improved pre-GKRS symptoms or signs were as follows: headache in 83.3% (20 of 24 patients), ataxia in 53.3% (8 of 15 patients), numbness in 63.6% (7 of 11 patients), cranial nerve deficits in 20% (2 of 10 patients), neck pain 83.3% (5 of 6 patients), and weakness and dizziness in 20% (1 of 5 patients, each). Pre-GKRS variables were analyzed with univariate analyses to determine their effect on clinical improvement, and none of the tested variables were found to be effective (Table 2). Thirty patients (81.1%) were still alive at the time of the final follow-up. Three patients (8.1%) were dead; however, none of the patients died from complications or progression associated with FMM. Two patients died from heart attack 12.5 and 6.6 years after GKRS treatment, and one died from lung cancer 7.5 years after GKRS treatment. As stated before, although all four patients (10.8%) were alive at the last follow-up, the final statuses of the patients who were lost to follow-up were unknown.
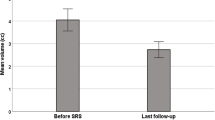
At the last radiological follow-up after GKRS, 23 tumors (62.2%) remained stable, 13 (35.1%) decreased in size, and 1 (2.7%) increased in size. The median reduction in tumor volume was −16% (range, −67 to 120%) to reach a median final tumor volume of 2.4 cm3 (range, 0.5–18.9 cm3) that differed significantly from baseline (p < 0.0001). Volume change was not related to marginal or maximum dose (p = 0.465 and 0.357, respectively). Tumor control, including stable and regressed tumors, was achieved in 97.3% of patients. A sample patient with tumoral regression is shown in Fig. 1. By Kaplan-Meier analysis (Fig. 2), progression-free survival at 2, 5, and 10 years was 100, 100, and 95.2%, respectively. Assuming that the patients who were lost to follow-up showed progression, progression-free survival at 2, 5, and 10 years would be 89.2, 89.2, and 84.9%, respectively. Pre-GKRS variables were analyzed with univariate analyses to determine their effect on tumor progression; none of the tested variables predicted a worse response (Table 2). The median time to tumor regression was 22 months (range 17–34 months). One patient with a tumor resection history showed tumor volume progression 80 months after GKRS treatment with 8 Gy delivered marginal dose. The patient underwent repeat tumor resection at a different center and is still alive.
Toxicity
GKRS was generally well tolerated. Treatment-related clinical toxicity was not observed in any patient.
Discussion
FMMs remain challenging tumors to treat for neurosurgeons due to high morbidity, mortality, and recurrence rates. Our study analyzed 37 patients with FMMs who underwent GKRS treatment. Findings from our series revealed that GKRS provided excellent tumor control with a rate of 97.3% during a median radiological follow-up of 7 years. Notably, this is the largest single-center FMM patient series that underwent GKRS with the longest follow-up period in the literature.
Although meningiomas are characteristically slow-growing tumors, a recent review by Nakasu et al. [8] showed that only 30% of incidental meningiomas did not grow radiologically. They also found that if the treatment had been withheld for smaller tumors for several years, the majority of them would have enlarged to become symptomatic. Therefore, the progression of FFMs can result in a high morbidity rate due to brainstem and vessel compression and/or lower CN deficits [9,10,11,12, 22]. Given the rarity of FMMs, outcomes after various treatments and appropriate treatment paradigms are not defined clearly. Historically, microsurgical resection has been considered the primary treatment for a long time. Although reports with large series from highly experienced centers revealed that total resection was achieved in 63–96% of patients (8–11), the surgical complication risks can be significantly high, with a postoperative morbidity rate up to 42%, including lower CN deterioration leading to aspiration and pneumonia, hydrocephalus, weakness, numbness, and ambulatory difficulty [9,10,11,12, 22]. Furthermore, the postoperative mortality rate was reported as up to 7.5% in these studies (8–11).
Radiosurgery is a beneficial addition to the armamentarium of treatment options for intracranial meningiomas. Numerous large series with satisfactory long-term tumor control and outcome results for intracranial meningiomas underwent GKRS were presented in the literature. These meningioma series at various intracranial regions have revealed 5-year tumor control rates of 86.2 to 98.5% and 10-year rates of 73 to 97%. In a recently published paper by Lippitz et al. [23], they retrospectively analyzed 86 patients with 130 meningiomas that were treated using GKRS. They reported a local tumor control of 87.8% after a median follow-up period of 10 years. Eighty-six percent of patients were neurologically unchanged or improved in their study. Cohen-Inbar et al. [24] also stated that stereotactic radiosurgery is an important treatment option in managing meningiomas and can be used as an upfront or adjuvant treatment modality. They reported that SRS seemed to afford a high and durable tumor control rate with a very low complication rate and a high safety profile. Therefore, the pendulum has swung away from aggressive surgical resection to GKRS for these tumors [13, 25,26,27,28].
Up to date, only two series with a maximum radiological follow-up period of 47 months reported FMM cases treated by GKRS with detailed data [29, 30]. Both the small number of cases and the lack of long-term follow-up have limited the outcomes and results of GKRS for FMMs. Therefore, there is a significant gap in the literature about the safety and efficiency of the GKRS for FMMs. Zenonos et al. revealed the results of 24 patients with FMMs who underwent GKRS [30]. In their series, the median tumor volume was 4.1 cm3 (range, 0.7–15.9 cm3), and the median radiation dose was 13 Gy (range, 11–15 Gy). They observed 100% tumor control at a median follow-up of 47 months (range, 3–128 months). In their international multicenter study, Mehta et al. included 57 patients with a median tumor volume of 2.9 cm3 (range, 0.4–17.0 cm3) [29]. The median radiological follow-up was 36 months (range, 6–196 months). They observed 93% tumor volume control at a median follow-up of 36 months (range, 6–196 months) with a median dose of 12.5 Gy (range, 10–16 Gy). In the present study, 37 patients were included, and a 97.3% tumor volume control was achieved at a median radiological follow-up of 84 months (range, 18–144 months). Notably, this is the longest post-GKRS follow-up period for FMMs in the literature. The median target volume before GKRS was 3.30 cm3 (range, 0.6–17.6 cm3). The median marginal dose was 12 Gy (range, 8–20 Gy). The median marginal dose used in the present study was lower compared to those presented in the literature. This finding revealed that excellent tumor volume control could be achieved with lower radiation doses.
While 13% of the patients showed tumor volume regression in the present study, the tumor volume regression rates were 44 and 47% in two major series [29, 30]. The main explanation for this finding may be the lower radiation doses in the present study, as Mehta et al. revealed that tumor volume regression was associated with a higher radiation dose [29]. However, we could not detect any association between volume regression and marginal or maximum dose (p = 0.969 and 0.490, respectively). One patient showed tumor volume progression in our study and underwent repeat microsurgical resection at a different center. The marginal dose was 8 Gy due to severe brain stem compression by the tumor. In Mehta et al.’s study, PFS at 2, 5, 8, and 10 years was 100, 92, 92, and 92%, respectively [29]. In our study, PFS at 2, 5, 8, and 10 years was 100, 100, 95.2, and 95.2%, respectively.
Mehta et al. revealed that 52% of their patients showed an improvement in their symptoms at a median of 36 months after GKRS [29]. Likewise, Zenonos et al. revealed that 58% of their patients showed an improvement in their symptoms at a median of 62 months after GKRS [30]. Zenonos et al. revealed a significant correlation between mean tumor volumes at presentation and symptom improvement [30]. In the present study, 73% of the patient symptoms improved at a median of 80 months after GKRS. We could not observe any significant correlation between tumor volume at presentation and symptom improvement (p = 0.515). The main explanation for this may be the significantly higher follow-up period of our study than those previous series.
Adverse radiation effects are rare for FMMs following GKRS. Zenonos et al. did not report any AREs [30]. Mehta et al. demonstrated that only 2% of patients (n = 1) showed AREs, including worsened hearing loss and left side extremity numbness following GKRS for FMM [29]. In concordance with the literature, none of our patients experienced AREs.
Limitations
While the results of this study are encouraging, our analysis was limited by the retrospective study design and small sample size. Also, we did not test for differences between the patients who did not meet the inclusion criteria and those who were not included due to the absence of repeat MRI and/or follow-up, potentially introducing a selection bias. We recognize that while the clinically meaningful data were delineated and reviewed, potential long-term differences in clinical outcomes were not reviewed, given the variability of the long-term follow-up in this cohort. Additional prospective studies with a larger sample size investigating the correlation with clinical outcome changes are warranted to analyze further the role of GKRS in the management of FMMs. Finally, we cannot emphasize enough that this is a highly selected group of patients; strict criteria were maintained during case selection, and these cases were performed in a setting with vast experience in GKRS. Moreover, until larger prospective studies substantiate the safety and efficacy of GKRS in FMMs, caution and judicious patient selection should be exercised in selecting this approach. Despite the limitations above, one of the key strengths of our study is that it is the largest single-center series of patients with FMM who were treated with GKRS. Besides, although having two patients, the current study does address the use of hypofractionated GKRS and its potential role in the management of FMM. Another strength of our study is that the long-term clinical and radiological follow-ups (median = 80 and 84 months, respectively) were sufficiently long to allow for informative monitoring of tumor progression and patient outcomes following GKRS.
Conclusion
Tumor volume control was achieved in most patients with lower radiation doses at a long-term follow-up period. The majority of the patients showed improvement in their neurological symptoms without any new neurological function worsening. Our findings revealed that excellent tumor volume control might be achieved with lower radiation doses. Finally, given its low-risk profile, GKRS is an effective and safe treatment for patients with FMM in a long-term follow-up period.
References
Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2016) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro-Oncology 18:v1–v75. https://doi.org/10.1093/neuonc/now207
Rogers L, Barani I, Chamberlain M, Kaley TJ, McDermott M, Raizer J, Schiff D, Weber DC, Wen PY, Vogelbaum MA (2015) Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review J Neurosurg 122:4–23. https://doi.org/10.3171/2014.7.JNS131644
Arnautovic KI, Al-Mefty O, Husain M (2000) Ventral foramen magnum meningiomas. J Neurosurg 92:71–80. https://doi.org/10.3171/spi.2000.92.1.0071
Dodge HW Jr, Gottlieb CM, Love JG (1956) Benign tumors at the foramen magnum; surgical considerations. J Neurosurg 13:603–617. https://doi.org/10.3171/jns.1956.13.6.0603
Meyer FB, Ebersold MJ, Reese DF (1984) Benign tumors of the foramen magnum. J Neurosurg 61:136–142. https://doi.org/10.3171/jns.1984.61.1.0136
Stein BM, Leeds NE, Taveras JM, Pool JL (1963) Meningiomas of the foramen magnum. J Neurosurg 20:740–751. https://doi.org/10.3171/jns.1963.20.9.0740
Yasuoka S, Okazaki H, Daube JR, MacCarty CS (1978) Foramen magnum tumors. Analysis of 57 cases of benign extramedullary tumors. J Neurosurg 49:828–838. https://doi.org/10.3171/jns.1978.49.6.0828
Nakasu S, Nakasu Y (2020) Natural history of meningiomas: review with meta-analyses. Neurol Med Chir (Tokyo) 60:109–120. https://doi.org/10.2176/nmc.ra.2019-0213
Bassiouni H, Ntoukas V, Asgari S, Sandalcioglu EI, Stolke D, Seifert V (2006) Foramen magnum meningiomas: clinical outcome after microsurgical resection via a posterolateral suboccipital retrocondylar approach. Neurosurgery 59:1177–1185; discussion 1185-1177. https://doi.org/10.1227/01.NEU.0000245629.77968.37
George B, Lot G, Boissonnet H (1997) Meningioma of the foramen magnum: a series of 40 cases. Surg Neurol 47:371–379. https://doi.org/10.1016/s0090-3019(96)00204-2
Pamir MN, Kilic T, Ozduman K, Ture U (2004) Experience of a single institution treating foramen magnum meningiomas. J Clin Neurosci 11:863–867. https://doi.org/10.1016/j.jocn.2004.02.007
Samii M, Klekamp J, Carvalho G (1996) Surgical results for meningiomas of the craniocervical junction. Neurosurgery 39:1086–1094; discussion 1094-1085. https://doi.org/10.1097/00006123-199612000-00003
Akyoldas G, Hergunsel OB, Yilmaz M, Sengoz M, Peker S (2020) Gamma Knife radiosurgery for anterior clinoid process meningiomas: a series of 61 consecutive patients. World Neurosurg 133:e529–e534. https://doi.org/10.1016/j.wneu.2019.09.089
Lee JY, Niranjan A, McInerney J, Kondziolka D, Flickinger JC, Lunsford LD (2002) Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg 97:65–72. https://doi.org/10.3171/jns.2002.97.1.0065
Pollock BE, Stafford SL (2005) Results of stereotactic radiosurgery for patients with imaging defined cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys 62:1427–1431. https://doi.org/10.1016/j.ijrobp.2004.12.067
Santacroce A, Walier M, Regis J, Liscak R, Motti E, Lindquist C, Kemeny A, Kitz K, Lippitz B, Martinez Alvarez R, Pedersen PH, Yomo S, Lupidi F, Dominikus K, Blackburn P, Mindermann T, Bundschuh O, van Eck AT, Fimmers R, Horstmann GA (2012) Long-term tumor control of benign intracranial meningiomas after radiosurgery in a series of 4565 patients. Neurosurgery 70:32–39; discussion 39. https://doi.org/10.1227/NEU.0b013e31822d408a
Talacchi A, Hasanbelliu A, D'Amico A, Regge Gianas N, Locatelli F, Pasqualin A, Longhi M, Nicolato A (2020) Long-term follow-up after surgical removal of meningioma of the inner third of the sphenoidal wing: outcome determinants and different strategies. Neurosurg Rev 43:109–117. https://doi.org/10.1007/s10143-018-1018-1
Bruneau M, George B (2008) Foramen magnum meningiomas: detailed surgical approaches and technical aspects at Lariboisiere Hospital and review of the literature. Neurosurg Rev 31:19–32; discussion 32-13. https://doi.org/10.1007/s10143-007-0097-1
Akyoldas G, Hergunsel OB, Ozdemir IE, Sengoz M, Peker S (2019) Gamma Knife radiosurgery for pituitary spindle cell oncocytomas. Clin Neurol Neurosurg 187:105560. https://doi.org/10.1016/j.clineuro.2019.105560
Snell JW, Sheehan J, Stroila M, Steiner L (2006) Assessment of imaging studies used with radiosurgery: a volumetric algorithm and an estimation of its error. Technical note J Neurosurg 104:157–162. https://doi.org/10.3171/jns.2006.104.1.157
Harrison G, Kano H, Lunsford LD, Flickinger JC, Kondziolka D (2016) Quantitative tumor volumetric responses after Gamma Knife radiosurgery for meningiomas. J Neurosurg 124:146–154. https://doi.org/10.3171/2014.12.JNS141341
Magill ST, Shahin MN, Lucas CG, Yen AJ, Lee DS, Raleigh DR, Aghi MK, Theodosopoulos PV, McDermott MW (2019) Surgical outcomes, complications, and management strategies for foramen magnum meningiomas. J Neurol Surg B Skull Base 80:1–9. https://doi.org/10.1055/s-0038-1654702
Lippitz BE, Bartek J Jr, Mathiesen T, Forander P (2020) Ten-year follow-up after Gamma Knife radiosurgery of meningioma and review of the literature. Acta Neurochir 162:2183–2196. https://doi.org/10.1007/s00701-020-04350-5
Cohen-Inbar O, Lee CC, Sheehan JP (2016) The contemporary role of stereotactic radiosurgery in the treatment of meningiomas. Neurosurg Clin N Am 27:215–228. https://doi.org/10.1016/j.nec.2015.11.006
Iwai Y, Yamanaka K, Ikeda H (2008) Gamma Knife radiosurgery for skull base meningioma: long-term results of low-dose treatment. J Neurosurg 109:804–810. https://doi.org/10.3171/JNS/2008/109/11/0804
Kreil W, Luggin J, Fuchs I, Weigl V, Eustacchio S, Papaefthymiou G (2005) Long term experience of gamma knife radiosurgery for benign skull base meningiomas. J Neurol Neurosurg Psychiatry 76:1425–1430. https://doi.org/10.1136/jnnp.2004.049213
Sheehan JP, Starke RM, Kano H, Kaufmann AM, Mathieu D, Zeiler FA, West M, Chao ST, Varma G, Chiang VL, Yu JB, McBride HL, Nakaji P, Youssef E, Honea N, Rush S, Kondziolka D, Lee JY, Bailey RL, Kunwar S, Petti P, Lunsford LD (2014) Gamma Knife radiosurgery for sellar and parasellar meningiomas: a multicenter study. J Neurosurg 120:1268–1277. https://doi.org/10.3171/2014.2.JNS13139
Starke RM, Williams BJ, Hiles C, Nguyen JH, Elsharkawy MY, Sheehan JP (2012) Gamma Knife surgery for skull base meningiomas. J Neurosurg 116:588–597. https://doi.org/10.3171/2011.11.JNS11530
Mehta GU, Zenonos G, Patibandla MR, Lin CJ, Wolf A, Grills I, Mathieu D, McShane B, Lee JY, Blas K, Kondziolka D, Lee CC, Lunsford LD, Sheehan JP (2018) Outcomes of stereotactic radiosurgery for foramen magnum meningiomas: an international multicenter study. J Neurosurg 129:383–389. https://doi.org/10.3171/2017.3.JNS163008
Zenonos G, Kondziolka D, Flickinger JC, Gardner P, Lunsford LD (2012) Gamma Knife surgery in the treatment paradigm for foramen magnum meningiomas. J Neurosurg 117:864–873. https://doi.org/10.3171/2012.8.JNS111554
Author information
Authors and Affiliations
Contributions
Conception and design: G.A., S.P., M.Ş. Acquisition of data: G.A., M.Y.S. Analysis and interpretation of data: G.A., M.Y., M.Y.S. Drafting the article: G.A., M.Y.S. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: G.A. Study supervision: S.P., M.Ş.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Research Ethics Committee of Koç University (No. 2020.190.IRB1.058).
Consent to participate
Informed consent was provided before intervention by each patient and his/her nearest family member.
Consent for publication
Patient consent was also obtained for publication of images or other clinical information of the patient in a medical publication, journal, website, medical book, or other forms of publication with maximum efforts to conceal his/her identity. All authors of this manuscript also consent to the publication of the manuscript in Neurosurgical Review journal.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akyoldaş, G., Samancı, Y., Yılmaz, M. et al. Long-term results of gamma knife radiosurgery for foramen magnum meningiomas. Neurosurg Rev 44, 2667–2673 (2021). https://doi.org/10.1007/s10143-020-01446-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01446-5