Abstract
Microthrombosis after aneurysmal subarachnoid hemorrhage (aSAH) is considered to initiate neuroinflammation, vessel remodeling, and blood-brain barrier leakage. We aimed to verify the hypothesis that the intensity of thrombogenicity immediately after aSAH depends on the amount and distribution of extravasated blood. This observational cohort study included 37 consecutive aSAH patients admitted no longer than 24 h after ictus. Volumes of subarachnoid and intraventricular hemorrhages as well as the Subarachnoid Hemorrhage Early Brain Edema Scale (SEBES) score were calculated in each case. Platelet system status was described by platelet count (PLT), mean platelet volume (MPV), MPV to PLT ratio, and platelet-large cell ratio (P-LCR). Median hemorrhage volume amounted to 11.4 ml (interquartile range 2.8–26.8 ml). Patients with more severe hemorrhage had lower PLT and higher MPV to PLT ratio (ρ = − 0.49, p < .002; ρ = 0.50, p < .002, respectively). PLT decreased by 2.80 G/l per 1 ml of hemorrhage volume (95% CL 1.30–4.30, p < .001). Further analysis revealed that intraventricular hemorrhage volume was associated with P-LCR and MPV (ρ = 0.34, p < .039; ρ = 0.33, p < .048, respectively), whereas SAH volume with PLT and MPV:PLT ratio (ρ = − 0.40, p < .013; ρ = 0.41, p < .013, respectively). The odds of unfavorable neurological outcome increased 3.95 times per 1 fl of MPV (95% CI 1.19–13.12, p < .025). MPV was independently correlated with SEBES (ρ = 0.44, p < .006). This study demonstrated that the extent and distribution of aneurysmal subarachnoid hemorrhage are related to different types of acute platelet response, which may be interpreted as local and systemic thrombogenicity. Increased mean platelet volume measured in the acute phase of aSAH may identify patients at risk for unfavorable neurological outcomes and may serve as a marker of early brain injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite introducing nimodipine [1] and changing management strategies [2, 3], the morbidity and mortality after aneurysmal subarachnoid hemorrhage (aSAH) remains overwhelming [4]. Neuroscientists are currently evaluating novel therapeutic approaches targeting early brain injury and delayed cerebral ischemia; one of the widely discussed topics is microthrombosis [5, 6].
Microthrombosis merits special attention due to its critical consequences, namely vessel obstruction and brain hypoperfusion, initiation of neuroinflammation, vessel remodeling, and blood-brain barrier leakage [7, 8]. The proposed etiology of this phenomenon involves a decrease in nitric oxide (NO) concentration [9,10,11] and endothelial dysfunction; blood clots surrounding cerebral arteries contain hemoglobin, which scavenges NO and damages the endothelium [9, 12]. Therefore, an association between hemorrhage severity and platelet activity was proposed, but recent studies were either unsuccessful in proving the link [13] or used extrapolated data from consecutive days after aSAH [14]. Moreover, it appears that the distribution of hemorrhage should affect platelet activity: blood clots in subarachnoid cisterns interact with arteries, whereas intraventricular hemorrhage (IVH) may have an effect on circumventricular organs.
We decided to investigate whether postulated pathophysiological mechanisms can be detected in daily clinical practice. The main aims of the study were to confirm an association between aSAH severity and thrombogenicity in the first hours after SAH and investigate a relation between the distribution of hemorrhage and platelet activity. In addition, we intended to refer to the results of other studies and to test the prognostic value of thrombogenicity measured in the acute phase of aSAH.
Materials and methods
Study population and data collection
An observational cohort study was designed and conducted. All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was approved by The Ethics Committee of The Central Clinical Hospital of the Ministry of Interior and Administration, Warsaw, Poland (Number 109/2018).
From the hospital data system, we obtained a list of all patients admitted to the Clinical Department of Neurosurgery between January 1, 2016, and December 31, 2018, with the diagnosis of aneurysmal SAH. An inclusion criterion was the occurrence of aneurysmal SAH, while exclusion criteria were referral from another hospital, admission > 24 h after aneurysm rupture, and known platelet dysfunction (ongoing antiplatelet therapy, thrombocytosis > 450.000 G/l).
The choice of the exclusion criteria was dictated by the desire to study acute platelet response to aSAH. Our experience suggested that patients referred from other hospitals are frequently admitted later than 24 h after ictus. Moreover, patients’ clinical condition and comorbidities determine the priority of the transfer, which may lead to a significant selection bias. Furthermore, different laboratories use different hematologic systems providing different, unconvertible platelet volume indices; thus, including blood test results from other hospitals in our study would affect the accuracy of the results. Therefore, we analyzed a group of patients presented directly to the emergency department of our hospital.
Demographic data, past medical history, clinical features at onset, and clinical and radiological status at the time of diagnosis of SAH were taken into account. All patients were examined on admission and daily by a physician in charge. All patients were assessed using the Glasgow Coma Scale (GCS) [15] and the World Federation of Neurological Surgeons scale (WFNS) [16]. Data regarding 90-day mortality and neurological outcome 3 months after SAH (graded by the modified Rankin scale [mRS]) were collected during follow-up appointments in the outpatient neurosurgical clinic or via telephone interview, where data were missing.
Blood samples were routinely taken on admission with the use of ethylenediaminetetraacetic acid (EDTA) as anticoagulant and processed by a Sysmex XN-2000 analyzer within 120 min from the puncture of the peripheral vein, as recommended [17]. With the use of the impedance method, the hematology system provided platelet count (PLT) and platelet volume indices (PVI): mean platelet volume (MPV), platelet-large cell ratio (P-LCR).
Exposure and outcome measures
The results of computed tomography (CT) of the head on admission were assessed using Analyze 12.0 (AnalyzeDirect, Overland Park, KS, USA) and volumes of SAH and intraventricular hemorrhage (IVH) were calculated with the use of the region of interest (ROI) method [18, 19] (Fig. 1). The quantitative ROI method is characterized by a strong interobserver agreement; therefore, an assessment by a single observer was deemed satisfactory. Furthermore, all CT images were graded with modified Fisher [20] and Hijdra [21] scales. In order to calculate the Hijdra score, the amount of blood in ten cisterns (paired suprasellar, deep and superficial sylvian, ambient and unpaired quadrigeminal, and anterior interhemispheric cisterns) and four ventricles is graded on a scale from 0 to 4 and summed up. The observer was not informed of the clinical data and laboratory test results. Although ordinal scales (such as modified Fisher and Hijdra scales) have their disadvantages, we included them in our research in order to be able to relate to results of other studies. In order to investigate a relation between the distribution of hemorrhage and platelet activity, Hijdra scores were divided into two values: the sum of 10 scores representing cisternal bleeding (subarachnoid hemorrhage, range from 0 to 30) and the sum of 4 scores representing intraventricular hemorrhage (intraventricular hemorrhage, range from 0 to 12). The intensity of early brain injury was assessed by the Subarachnoid Hemorrhage Early Brain Edema Scale (SEBES) [22]. The MPV:PLT ratio was calculated in a similar manner as reported previously using the formula \( \frac{\mathrm{MPV}\ \left[ fl\right]}{\mathrm{PLT}\ \left[G/l\right]}\bullet 100 \) and as in the case of MPV and P-LCR considered as a marker of platelet activation and systemic thrombogenicity [13, 23,24,25,26].
Management protocol
Our institution follows the guidelines for the management of aneurysmal SAH [27]. The occurrence of delayed cerebral ischemia (DCI) was assessed routinely using a clinical definition introduced by Vergouwen et al. [28]: “The occurrence of focal neurological impairment (such as hemiparesis, aphasia, apraxia, hemianopia, or neglect), or a decrease of at least 2 points on the Glasgow Coma Scale (either on the total score or on one of its individual components [eye, motor on either side, verbal]). This should last for at least 1 hour, is not apparent immediately after aneurysm occlusion, and cannot be attributed to other causes by means of clinical assessment, CT or MRI scanning of the brain, and appropriate laboratory studies.”
Statistical analysis
Statistical analysis was performed using SAS® software, version 9.4 (SAS Institute Inc., Cary, North Carolina, USA). Quantitative variables were described using median and quartile values. Wilcoxon and Fisher tests were used to compare the groups of patients referred from other hospitals and admitted directly from the emergency department in terms of sex, age, WFNS and modified Fisher grades, time from ictus to admission, and presence of intracerebral and intraventricular hemorrhages. The Spearman rank correlation coefficients were used to assess the associations of platelet parameters and the following measures: total hemorrhage volume, subarachnoid hemorrhage volume, intraventricular hemorrhage volume, Hijdra score and its subdivisions representing subarachnoid and intraventricular hemorrhage, SEBES score. The correlations of platelet parameters with the total volume of hemorrhage, volumes of SAH and IVH and SEBES score were adjusted for the following parameters sequentially: age, sex, WFNS, history of smoking, hypertension, and diabetes mellitus. Linear regression models were calculated in order to describe the mean change in PLT for every milliliter of hemorrhage volume. Univariate logistic regression with analysis of maximum likelihood estimates and odds ratio estimates were computed in order to find predictors of DCI and long-term survival among platelet parameters.
Results
Two hundred and ninety-six patients were admitted to the Clinical Department of Neurosurgery between January 1, 2016, and December 31, 2018, with a diagnosis of aneurysmal SAH. A total of 249 patients were transferred from other hospitals; 10 other patients met the remaining exclusion criteria. One in three referred patients was admitted later than 24 h after ictus; the percentage of late admission was significantly higher among referred patients (12.8% vs. 31.7%, p < 0.008). Patients referred from other hospitals and admitted directly from the emergency department did not differ in terms of sex, age, WFNS, and modified Fisher grades, nor the presence of intracerebral and intraventricular hemorrhages. Among six patients admitted beyond 24 h after SAH, the median of presentation time amounted to 4.5 days (range 2–10 days), five patients were scored 15 points in GCS, and one patient 14 points. Eventually, 37 patients were enrolled (Fig. 2). Table 1 shows demographic and clinical characteristics of all patients.
There were no missing data on radiological studies, blood test results, and follow-up. All radiological and laboratory studies were performed no later than 24 h after ictus. None of the patients was administered catecholamines or intensive fluid resuscitation before obtaining blood samples. Table 2 summarizes exposure and outcome parameters. Data were obtained in the second quarter of 2019.
Severity of aSAH and platelet activity
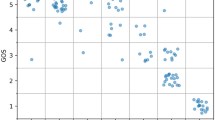
Both PLT and MPV:PLT ratio were correlated with the volume of hemorrhage (ρ = − 0.49, p < .002; ρ = 0.50, p < .002; respectively) and Hijdra score (ρ = − 0.36, p < .026; ρ = 0.37, p < .025; respectively). The associations could still be observed, when adjusted sequentially for age, sex, WFNS, history of smoking, hypertension, and diabetes mellitus. Patients with more severe hemorrhage (modified Fisher grades 3–4 vs. 1–2) had lower PLT (median 234.0, IQR 182.0–290.5 vs. median 328.0, IQR 261.0–372.0, p < .007) and higher MPV:PLT ratios (median 4.52, IQR 3.53–5.81 vs. median 3.37, IQR 2.79–3.99; p < .008; Fig. 3). A simple linear regression was calculated to predict PLT based on the volume of hemorrhage. A significant regression model was found (F(1,34) = 14.49, p < .001) with an R2 of .30. Mean platelet count decreased by 2.80 G/l (95% CL: 1.30–4.30) for every milliliter of hemorrhage volume (Fig. 3).
a Linear regression model predicting PLT in terms of the volume of aneurysmal hemorrhage. Volumes of extravasated blood were calculated with ROI method. b, c Correlations of hemorrhage volume with P-LCR and MPV:PLT ratio. d Differences in MPV:PLT ratios between patients with different SAH severity (modified Fisher grades 1–2 vs. 3–4). IVH intraventricular hemorrhage, MPV mean platelet volume, PLT platelet count, P-LCR platelet-large cell ratio, ROI region of interest, SAH subarachnoid hemorrhage
Distribution of aSAH and platelet activity
The increasing volume of SAH was related to a decrease in PLT (ρ = − 0.40, p < .013) and an increase in MPV:PLT ratio (ρ = 0.41, p < .013). The associations could still be observed, when adjusted sequentially for age, sex, WFNS, history of smoking, hypertension, and diabetes mellitus. Similar correlations were calculated using the Hijdra subscore, receiving ρ = − 0.33, p < .045 and ρ = − 0.33, p < .050 for correlations of PLT and MPV:PLT ratio with IVH volume, respectively. A simple linear regression was calculated in order to predict PLT based on the volume of subarachnoid hemorrhage. A significant regression model was found (F(1,34) = 9.37, p < .004) with an R2 of .22. Mean platelet count decreased by 2.61 G/l (95% CL: 0.88—4.34) for every milliliter of subarachnoid hemorrhage volume (Fig. 4).
Associations between SAH distribution and platelet volume indices. a Linear regression model predicting PLT in terms of subarachnoid hemorrhage volume. b, c Correlations of IVH with P-LCR and MPV:PLT ratio. Volumes of subarachnoid and intraventricular hemorrhage were calculated with the ROI method. d Association between the Subarachnoid Hemorrhage Early Brain Edema Scale (SEBES) and MPV. Spearman’s rank correlation coefficients were calculated and presented. IVH intraventricular hemorrhage, MPV mean platelet volume, P-LCR platelet-large cell ratio, PLT platelet count, ROI region of interest, SAH subarachnoid hemorrhage
The extent of IVH was in relation with MPV and P-LCR (ρ = − 0.33, p < .046, ρ = − 0.34, p < .039, respectively); however, it became insignificant, when adjusted for WFNS (ρ = 0.23, p < .17, ρ = 0.25, p < .14 for partial correlations of MPV and P-LCR with IVH volume, respectively). Patients with greater IVH volume tended to present MPV above the reference range (median 0.16, IQR 0.0 – 0.38 vs. median 0.89, IQR 0.12 – 11.62, p < .063). Due to different anatomical distribution of hemorrhage from the rupture of anterior and posterior cerebral circulation aneurysms, we performed a sensitivity analysis for anterior circulation aneurysms; the correlations between the volume of IVH and platelet volume indices in this group were as follows: ρ = 0.53, p < .003, ρ = 0.50, p < .006 for correlations of MPV and P-LCR with IVH volume, respectively.
Both platelet volume indices and MPV:PLT ratio are markers of platelet activity. We observed a relation between MPV:PLT ratio and the extent of hemorrhage, but we did not observe a relation between platelet volume indices (PVI) and the extent of hemorrhage. Therefore, we decided to test whether the correlation between MPV:PLT ratio and the volume of hemorrhage was caused by that of PLT with hemorrhage volume. We calculated logarithms to the base of 10 of PLT and MPV:PLT ratio and adjusted correlation between MPV:PLT ratio and hemorrhage volume receiving ρ = 0.11, p < .51.
Platelet activity and clinical course
Eight patients were diagnosed with DCI, whereas eleven patients died within 90 days from aSAH. Logistic regression analysis revealed predictors of unfavorable outcome—MPV, P-LCR, and MPV:PLT ratio (Table 3); for a 1 fl change in MPV, the odds of unfavorable neurological outcome (modified Rankin scale 3–6 vs. 0–2) are expected to change 3.95 times (95% CI 1.19—13.12). The platelet parameters were not predictive of the occurrence of DCI.
The SEBES scores were correlated with PLT (ρ = − 0.32, p < .051), MPV:PLT ratio (ρ = 0.37, p < .023), MPV (ρ = 0.44, p < .006) and P-LCR (ρ = 0.45, p < .005). The associations that could still be observed after adjusting sequentially for volumes of SAH and IVH, age, sex, WFNS, history of smoking, hypertension, and diabetes mellitus were correlations with MPV and P-LCR (ρ = 0.42, p < .011; ρ = 0.45, p < .007, respectively, controlled for the volume of SAH).
Discussion
Platelet response to severity of aSAH
The main objective of our study was to verify the hypothesis of increasing thrombogenicity with increasing aSAH severity. We found that with the increase in hemorrhage volume, the platelet count decreases and the MPV:PLT ratio increases in the first 24 h after aneurysmal bleeding. The associations were independent from patients’ clinical characteristics. According to our results, we support the thesis that aneurysmal bleeding initiates acute platelet activation in a dose-dependent manner.
The volumes of aneurysmal hemorrhage usually do not exceed 25 ml, which is less than 0.5% of the total blood volume, and do not lead to substantial loss of platelets. None of the patients were administered fluid resuscitation before blood tests; therefore, PLT did not decrease by hemodilution. Lower platelet count among patients with greater extent of subarachnoid hemorrhage may result from thrombogenicity limited to cerebral circulation. Blood clots located within subarachnoid space interact with arterial walls leading to endothelial dysfunction and scavenging of NO by hemoglobin [6, 9,10,11,12]. Damage of the endothelial cells causes an increase in the expression of adhesion molecules and a decrease in the production of antiaggregant factors such as nitric oxide and prostacyclin [29, 30]. As a consequence, thrombocytes activate and form clots, which is reflected in blood tests as a reduction in platelet count.
The study meets several of Bradford Hill’s criteria (strength, specificity, temporality, biological gradient, plausibility, and coherence) which allows for drawing causal interfaces about subarachnoid hemorrhage volume and thrombogenicity. Our findings indirectly confirm the hypothesis that microthrombosis is dependent on the interaction of blood clots with walls of arteries.
Taking into account several papers concerning microthrombosis, we decided to calculate MPV:PLT ratio. However, the association of MPV:PLT ratio with the volume of SAH became insignificant when adjusted for PLT. Moreover, we did not observe a relation between PVI and the volume of hemorrhage. As opposed to MPV:PLT ratio, platelet volume indices are verified markers of platelet activity [24, 31,32,33,34], which suggests an absence of systemic thrombogenicity and the need for further studying MPV:PLT ratio as its marker. MPV is known to rise in several conditions such as hyperdestructive thrombocytopenia, metastatic colon cancer, myocardial infarction, ischemic stroke, and inflammatory bowel diseases [24, 35, 36]; however, all these states are characterized as prothrombotic or proinflammatory [32]. The main advantages of MPV over more specific methods such as thromboelastography and vasodilator-stimulated phosphoprotein are its accessibility and widespread use.
The relation described above has not been observed beforehand. Thrombogenicity after aSAH gradually rises during the first days after ictus, reaches its maximum value in 3–5 days and then gradually decreases [13, 14]. Previous studies on SAH pathophysiology enrolled patients admitted 48 or 72 h after ictus, which makes the analysis of acute platelet response impossible. In opposition to all other studies on this topic, we excluded all patients admitted later than 24 h after bleeding.
Platelet activity and distribution of aneurysmal hemorrhage
The second objective of the study was to investigate an impact of hemorrhage distribution on platelet activity. We anticipated the difference in platelet response to intraventricular and subarachnoid hemorrhage. Indeed, the volume of subarachnoid hemorrhage turned out to be independently associated with PLT and MPV:PLT ratio, whereas the volume of IVH was associated with MPV and P-LCR. We discussed the former relation above, because of its inseparability from the association of total hemorrhage volume with platelet parameters.
The second trend may result from the irritation of circumventricular organs by extravasated blood (Fig. 5). Proinflammatory substances present in the cerebrospinal fluid after aSAH [37,38,39,40] can easily pass into the brain through the regions lacking a blood-brain barrier and a cerebrospinal fluid-brain barrier [41] such as the vascular organ of lamina terminalis, the subfornical organ, and the area postrema. These organs control the hypothalamo-pituitary-adrenal axis [41] and the sympathetic nervous system as well as contribute to sickness behavior [42]. Sympathetic hyperactivity and elevated levels of catecholamines are well-known phenomena after aSAH [43,44,45,46], which can activate platelets [47, 48] and stimulate thrombopoesis [49, 50]. For this reason, we ensured that none of the patients was given catecholamines before obtaining blood tests. Furthermore, MPV was found to reflect sympathetic overactivity [51]. On the basis of these facts and our results, we believe that intraventricular hemorrhage stimulates circumventricular organs, which leads to sympathetic overactivity and an increase of MPV.
Proposed model of interactions of severity and distribution of aneurysmal bleeding with platelet activity. SAH is responsible for local thrombogenicity, which may be observed as a decrease in PLT. IVH may cause circumventricular organs dysfunction and in result systemic thrombotic state. CVOs circumventricular organs, IVH intraventricular hemorrhage, MPV mean platelet volume, P-LCR platelet-large cell ratio, PLT platelet count, subarachnoid hemorrhage.
Aneurysms of posterior circulation usually rupture in the proximity to the pons and medulla oblongata, and thus, blood clots irritate these areas and distort the normal activity of cardiovascular centers. Therefore, we performed a sensitivity analysis in the subgroup of anterior circulation aneurysms, which confirmed our theses. We also observed an inverse relationship of WFNS grade with MPV; it may result from the mentioned activity of circumventricular organs.
Platelet activity and clinical course
We found that MPV, P-LCR, and MPV:PLT ratio measured in the acute phase of aSAH are predictors of neurological outcome, but not delayed cerebral ischemia. As discussed above, blood clots in ventricles might alter the function of the autonomic nervous system and hypothalamus, which explains why MPV and P-LCR may predict neurological outcomes. The role of mean platelet volume as a marker of sympathetic dysregulation should be further studied. As suggested by the results, the proposed mechanism is unconnected with DCI.
The intensity of early brain injury measured by the SEBES score was independently correlated with MPV and P-LCR, which suggests that the parameters are markers of early brain injury and provides another possible explanation for the predictive value of MPV and P-LCR.
Recent studies described several platelet parameters (including time trends in PLT [52] and MPV:PLT ratio [13]) to be predictive of DCI after aSAH [14, 53, 54]. We did not detect predictors of DCI. As mentioned above, restrictive exclusion criteria allowed us to analyze acute platelet response to aSAH. The studies cited above included patients admitted even 72 h after ictus, which resulted in a heterogenous group of patients—some patients with initial thrombogenicity and some already developing neuroinflammation. Our study allowed the analysis of the influence of initial platelet response on the development of delayed cerebral ischemia. We hypothesize that it may be the thrombogenicity that develops on consecutive days after aSAH rather than initial thrombogenicity that is predictive of delayed cerebral ischemia. The thrombogenicity that was detected by the cited studies and develops on consecutive days after aSAH may be secondary to the neuroinflammation—one of the fundamentals of DCI pathophysiology.
Clinical implications and future research directions
Thrombocytes play a very important role in the pathogenesis of the early brain injury and the delayed cerebral ischemia—they not only form microthrombi, which reduce cerebral blood flow, but also interact with various types of cells such as endothelial cells, neutrophils, and monocytes. In the light of their pleiotropic activity, attempts are being made to target platelet activity after aSAH as a mode of action of promising therapies such as antiaggregants [55, 56] or unfractionated heparins (known widely as anticoagulants, but believed to reduce microthrombosis) [57].
Our study implies that platelets respond to subarachnoid hemorrhage with a few hours. This leads to the question whether therapies targeting their activity should be started as soon as possible after ictus (preventing from initiating neuroinflammation etc.). However, the impairment of hemostasis in the acute phase of aSAH may lead to rebleeding, enlargement of intracerebral hemorrhage, and hemorrhagic expansion of ischemic regions. One can prevent only the former consequence (by securing the aneurysm). Therefore, future research should focus on developing therapies interfering with platelets’ ability to initialize the inflammation without disruption of hemostasis.
Our study confirms previous findings that it may be the thrombogenicity that develops on consecutive days after aSAH rather than initial thrombogenicity that is predictive of delayed cerebral ischemia. Firstly, it provides one more reason to start therapies targeted on early brain injury as soon as possible. Secondly, it suggests that early brain injury is not a homogenous phenomenon, but involves various processes developing at different paces. According to the regression analysis model, we found that acute thrombogenicity is related to aSAH severity, but the clinical factors modulating acute platelet response and leading to thrombogenicity occurring on consecutive days still remain unknown. Further studies on microthrombosis should focus on identifying these factors, which will contribute to the development of new therapeutic strategies.
The clinical value of MPV:PLT ratio as a marker of thrombogenicity also needs to be studied. Our study shows that it should always be analyzed in the context of PLT and MPV. It is particularly important in the case of SAH, where time trends of PLT [52] may have an impact on both the MPV:PLT ratio and the percentage of active platelets.
Strengths and limitations of the study
The main strength of our study is enrolling a homogenous group of patients in terms of admission time, applied diagnostics, and management, which allowed the study of the acute phase of aSAH. Other strengths include calculating the exact volume of SAH and IVH in each case and no missing data regarding exposure and outcome measures. Although the sample size was limited, on the basis of the analysis described above, we are of the opinion that the selected group was representative. Recruiting patients to a study on the acute phase of SAH is difficult due to the rarity of aSAH and delays in the initial diagnosis, management, and transfer to the reference neurosurgical center. On the other hand, the longer recruitment time period may be affected by the cohort effect.
Our cohort study has some limitations. Due to the limited sample size, we were unable to perform multivariate analysis. For obvious reasons, we did not draw blood for analysis just before aneurysms rupture, and therefore, we can only hypothesize that lower platelet counts among patients with more extensive SAH arise from platelet consumption. Noteworthy, all patients had platelet counts greater than 100 G/l, which is thought to provide accurate hemostasis [58, 59] and does not prolong the bleeding time [60]. We used platelet parameters of known relevance for analyses; however, the relation of the number of microthrombi with PVI and PLT remains unknown.
Conclusions
Our research confirms that acute platelet response to aneurysmal subarachnoid hemorrhage depends on the severity and distribution of bleeding. We found that the volume of subarachnoid hemorrhage results in a decrease in PLT, whereas the volume of intraventricular hemorrhage results in an increase in MPV and P-LCR, which may be interpreted as local and systemic thrombogenicity, respectively. We also found that an increase in MPV and P-LCR measured in the first 24 h after aSAH may identify patients at risk for unfavorable neurological outcomes and is connected with early brain injury; their role as markers of autonomic dysregulation needs further studies. Our results confirm the hypothesis formulated by experimental studies and suggest a time window for introducing therapies targeted at the activity of thrombocytes and underline the need for further research on the role of platelets in the pathophysiology of early brain injury and the necessity of verifying the accuracy of MPV:PLT ratio as a marker of thrombogenicity.
Data availability
The data that support the findings of this study are available in a public repository (https://doi.org/10.6084/m9.figshare.12771287.v1).
References
Barker FG, Ogilvy CS (1996) Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. J Neurosurg 84:405–414. https://doi.org/10.3171/jns.1996.84.3.0405
Cesarini KG, Hårdemark H-G, Persson L (1999) Improved survival after aneurysmal subarachnoid hemorrhage: review of management during a 12-year period. J Neurosurg 90:664–672. https://doi.org/10.3171/jns.1999.90.4.0664
Lovelock CE, Rinkel GJE, Rothwell PM (2010) Time trends in outcome of subarachnoid hemorrhage: population-based study and systematic review. Neurology 74:1494–1501. https://doi.org/10.1212/WNL.0b013e3181dd42b3
van Gijn J, Kerr RS, Rinkel GJ (2007) Subarachnoid haemorrhage. Lancet 369:306–318. https://doi.org/10.1016/S0140-6736(07)60153-6
Macdonald RL (2014) Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol 10:44–58. https://doi.org/10.1038/nrneurol.2013.246
Sehba FA, Hou J, Pluta RM, Zhang JH (2012) The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol 97:14–37. https://doi.org/10.1016/j.pneurobio.2012.02.003
Friedrich V, Flores R, Muller A, Sehba FA (2010) Luminal platelet aggregates in functional deficits in parenchymal vessels after subarachnoid hemorrhage. Brain Res 1354:179–187. https://doi.org/10.1016/j.brainres.2010.07.040
Sehba FA, Mostafa G, Friedrich V, Bederson JB (2005) Acute microvascular platelet aggregation after subarachnoid hemorrhage. J Neurosurg 102:1094–1100. https://doi.org/10.3171/jns.2005.102.6.1094
Bulters D, Gaastra B, Zolnourian A, Alexander S, Ren D, Blackburn SL, Borsody M, Doré S, Galea J, Iihara K, Nyquist P, Galea I (2018) Haemoglobin scavenging in intracranial bleeding: biology and clinical implications. Nat Rev Neurol 14:416–432. https://doi.org/10.1038/s41582-018-0020-0
Sehba FA, Schwartz AY, Chereshnev I, Bederson JB (2000) Acute decrease in cerebral nitric oxide levels after subarachnoid hemorrhage. J Cereb Blood Flow Metab 20:604–611. https://doi.org/10.1097/00004647-200003000-00018
Voetsch B, Jin RC, Loscalzo J (2004) Nitric oxide insufficiency and atherothrombosis. Histochem Cell Biol 122:353–367. https://doi.org/10.1007/s00418-004-0675-z
Sabri M, Ai J, Knight B, Tariq A, Jeon H, Shang X, Marsden PA, Macdonald RL (2011) Uncoupling of endothelial nitric oxide synthase after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab 31:190–199. https://doi.org/10.1038/jcbfm.2010.76
Ray B, Tinsley L, Ford L, Thompson DM, Sidorov EV, Bohnstedt BN (2018) Trends of platelet volume index predicts delayed cerebral ischemia after subarachnoid hemorrhage. World Neurosurg 111:e624–e631. https://doi.org/10.1016/j.wneu.2017.12.131
Ray B, Pandav VM, Mathews EA, Thompson DM, Ford L, Yearout LK, Bohnstedt BN, Chaudhary S, Dale GL, Prodan CI (2018) Coated-platelet trends predict short-term clinical outcome after subarachnoid hemorrhage. Transl Stroke Res 9:459–470. https://doi.org/10.1007/s12975-017-0594-7
Teasdale G, Jennett B (1974) ASSESSMENT OF COMA AND IMPAIRED CONSCIOUSNESS: a practical scale. Lancet 304:81–84. https://doi.org/10.1016/S0140-6736(74)91639-0
Teasdale GM, Drake CG, Hunt W, Kassell N, Sano K, Pertuiset B, De Villiers JC (1988) A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry 51:1457
Lancé MD, van Oerle R, Henskens YMC, Marcus MAE (2010) Do we need time adjusted mean platelet volume measurements? Lab Hematol Off Publ Int Soc Lab Hematol 16:28–31. https://doi.org/10.1532/LH96.10011
Jiménez-Roldán L, Alén JF, Gómez PA, Lobato RD, Ramos A, Munarriz PM, Lagares A (2013) Volumetric analysis of subarachnoid hemorrhage: assessment of the reliability of two computerized methods and their comparison with other radiographic scales. J Neurosurg 118:84–93. https://doi.org/10.3171/2012.8.JNS12100
Lagares A, Jiménez-Roldán L, Gomez PA, Munarriz PM, Castaño-León AM, Cepeda S, Alén JF (2015) Prognostic value of the amount of bleeding after aneurysmal subarachnoid hemorrhage: a quantitative volumetric study. Neurosurgery 77:898–907. https://doi.org/10.1227/NEU.0000000000000927
Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES, Macdonald RL, Mayer SA (2006) Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified Fisher scale. Neurosurgery 59:21–27. https://doi.org/10.1227/01.neu.0000243277.86222.6c
Hijdra A, Brouwers PJ, Vermeulen M, van Gijn J (1990) Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke 21:1156–1161. https://doi.org/10.1161/01.STR.21.8.1156
Ahn S-H, Savarraj JP, Pervez M, Jones W, Park J, Jeon S-B, Kwon SU, Chang TR, Lee K, Kim DH, Day AL, Choi HA (2018) The subarachnoid hemorrhage early brain edema score predicts delayed cerebral ischemia and clinical outcomes. Neurosurgery 83:137–145. https://doi.org/10.1093/neuros/nyx364
Azab B, Torbey E, Singh J, Akerman M, Khoueiry G, McGinn JT, Widmann WD, Lafferty J (2011) Mean platelet volume/platelet count ratio as a predictor of long-term mortality after non-ST-elevation myocardial infarction. Platelets 22:557–566. https://doi.org/10.3109/09537104.2011.584086
Leader A, Pereg D, Lishner M (2012) Are platelet volume indices of clinical use? A multidisciplinary review. Ann Med 44:805–816. https://doi.org/10.3109/07853890.2011.653391
Oh GH, Chung SP, Park YS, Hong JH, Lee HS, Chung HS, You JS, Park JW, Park I (2017) Mean platelet volume to platelet count ratio as a promising predictor of early mortality in severe sepsis. Shock 47:323–330. https://doi.org/10.1097/SHK.0000000000000718
Quan W, Chen Z, Yang X, Li J, Li X, Weng Y, Li Y, Zhang X (2017) Mean platelet volume/platelet count ratio as a predictor of 90-day outcome in large artery atherosclerosis stroke patients. Int J Neurosci 127:1019–1027. https://doi.org/10.1080/00207454.2017.1296438
Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P, American Heart Association Stroke Council, Council on Cardiovascular Radiology and Intervention, Council on Cardiovascular Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Clinical Cardiology (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 43:1711–1737. https://doi.org/10.1161/STR.0b013e3182587839
Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas H, Terbrugge KG, Macdonald RL, Diringer MN, Broderick JP, Dreier JP, Roos YBWEM (2010) Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 41:2391–2395. https://doi.org/10.1161/STROKEAHA.110.589275
Chen Y-C, Huang AL, Kyaw TS, Bobik A, Peter K (2016) Atherosclerotic plaque rupture: identifying the straw that breaks the camel’s back. Arterioscler Thromb Vasc Biol 36:e63–e72. https://doi.org/10.1161/ATVBAHA.116.307993
Kaplan Z, Jackson S (2011) The role of platelets in atherothrombosis. Hematol Educ Program Am Soc Hematol Am Soc Hematol Educ Program 2011:51–61. https://doi.org/10.1182/asheducation-2011.1.51
Jakubowski JA, Thompson CB, Vaillancourt R, Valeri CR, Deykin D (1983) Arachidonic acid metabolism by platelets of differing size. Br J Haematol 53:503–511. https://doi.org/10.1111/j.1365-2141.1983.tb02052.x
Korniluk A, Koper-Lenkiewicz OM, Kamińska J, Kemona H, Dymicka-Piekarska V (2019) Mean platelet volume (MPV): new perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediat Inflamm 2019:9213074–9213014. https://doi.org/10.1155/2019/9213074
Martin JF, Trowbridge EA, Salmon G, Plumb J (1983) The biological significance of platelet volume: its relationship to bleeding time, platelet thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb Res 32:443–460. https://doi.org/10.1016/0049-3848(83)90255-4
Thompson CB, Eaton KA, Princiotta SM, Rushin CA, Valeri CR (1982) Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br J Haematol 50:509–519. https://doi.org/10.1111/j.1365-2141.1982.tb01947.x
Gladwin AM, Martin JF (1990) The control of megakaryocyte ploidy and platelet production: biology and pathology. Int J Cell Cloning 8:291–298. https://doi.org/10.1002/stem.5530080414
Tuncel T, Ozgun A, Emirzeoglu L, Celik S, Bilgi O, Karagoz B (2014) Mean platelet volume as a prognostic marker in metastatic colorectal cancer patients treated with bevacizumab-combined chemotherapy. Asian Pac J Cancer Prev APJCP 15:6421–6423. https://doi.org/10.7314/apjcp.2014.15.15.6421
Brandt L, Ljunggren B, Andersson KE, Hindfelt B, Uski T (1983) Prostaglandin metabolism and prostacyclin in cerebral vasospasm. Gen Pharmacol 14:141–143
Paoletti P, Gaetani P, Grignani G, Pacchiarini L, Silvani V, Rodriguez y Baena R (1988) CSF leukotriene C4 following subarachnoid hemorrhage. J Neurosurg 69:488–493. doi: https://doi.org/10.3171/jns.1988.69.4.0488
Rodriguez y Baena R, Gaetani P, Folco G, Viganó T, Paoletti P (1986) Arachidonate metabolites and vasospasm after subarachnoid haemorrhage. Neurol Res 8:25–32
Rodriguez y Baena R, Gaetani P, Paoletti P (1988) A study on cisternal CSF levels of arachidonic acid metabolites after aneurysmal subarachnoid hemorrhage. J Neurol Sci 84:329–335
Buller KM (2001) Role of circumventricular organs in pro-inflammatory cytokine-induced activation of the hypothalamic-pituitary-adrenal axis. Clin Exp Pharmacol Physiol 28:581–589
Sisó S, Jeffrey M, González L (2010) Sensory circumventricular organs in health and disease. Acta Neuropathol (Berl) 120:689–705. https://doi.org/10.1007/s00401-010-0743-5
Hinson H, Sheth K (2012) Manifestations of the hyperadrenergic state after acute brain injury. Curr Opin Crit Care 18:139–145. https://doi.org/10.1097/MCC.0b013e3283513290
Naredi S, Lambert G, Edén E, Zäll S, Runnerstam M, Rydenhag B, Friberg P (2000) Increased sympathetic nervous activity in patients with nontraumatic subarachnoid hemorrhage. Stroke 31:901–906. https://doi.org/10.1161/01.STR.31.4.901
Naredi S, Lambert G, Friberg P, Zäll S, Edén E, Rydenhag B, Tylman M, Bengtsson A (2006) Sympathetic activation and inflammatory response in patients with subarachnoid haemorrhage. Intensive Care Med 32:1955–1961. https://doi.org/10.1007/s00134-006-0408-y
Osteraas ND, Lee VH (2017) Neurocardiology. Handb Clin Neurol 140:49–65. https://doi.org/10.1016/B978-0-444-63600-3.00004-0
Lordkipanidzé M, Lowe GC, Kirkby NS, Chan MV, Lundberg MH, Morgan NV, Bem D, Nisar SP, Leo VC, Jones ML, Mundell SJ, Daly ME, Mumford AD, Warner TD, Watson SP (2014) Characterization of multiple platelet activation pathways in patients with bleeding as a high-throughput screening option: use of 96-well Optimul assay. Blood 123:e11–e22. https://doi.org/10.1182/blood-2013-08-520387
Tschuor C, Asmis LM, Lenzlinger PM, Tanner M, Härter L, Keel M, Stocker R, Stover JF (2008) In vitro norepinephrine significantly activates isolated platelets from healthy volunteers and critically ill patients following severe traumatic brain injury. Crit Care 12:R80. https://doi.org/10.1186/cc6931
Chen S, Du C, Shen M, Zhao G, Xu Y, Yang K, Wang X, Li F, Zeng D, Chen F, Wang S, Chen M, Wang C, He T, Wang F, Wang A, Cheng T, Su Y, Zhao J, Wang J (2016) Sympathetic stimulation facilitates thrombopoiesis by promoting megakaryocyte adhesion, migration, and proplatelet formation. Blood 127:1024–1035. https://doi.org/10.1182/blood-2015-07-660746
Chen S, Hu M, Shen M, Xu Y, Wang C, Wang X, Li F, Zeng D, Chen F, Zhao G, Chen M, Wang F, Cheng T, Su Y, Zhao J, Wang S, Wang J (2018) Dopamine induces platelet production from megakaryocytes via oxidative stress-mediated signaling pathways. Platelets 29:702–708. https://doi.org/10.1080/09537104.2017.1356451
Ozdemir O, Soylu M, Alyan O, Geyik B, Demir AD, Aras D, Cihan G, Cagirci G, Kacmaz F, Balbay Y, Sasmaz H, Korkmaz S (2004) Association between mean platelet volume and autonomic nervous system functions: increased mean platelet volume reflects sympathetic overactivity. Exp Clin Cardiol 9:243–247
Hirashima Y, Hamada H, Kurimoto M, Origasa H, Endo S (2005) Decrease in platelet count as an independent risk factor for symptomatic vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg 102:882–887. https://doi.org/10.3171/jns.2005.102.5.0882
Frontera JA, Provencio JJ, Sehba FA, McIntyre TM, Nowacki AS, Gordon E, Weimer JM, Aledort L (2017) The role of platelet activation and inflammation in early brain injury following subarachnoid hemorrhage. Neurocrit Care 26:48–57. https://doi.org/10.1007/s12028-016-0292-4
Ramchand P, Nyirjesy S, Frangos S, Doerfler S, Nawalinski K, Quattrone F, Ju C, Patel H, Driscoll N, Maloney-Wilensky E, Stein SC, Levine JM, Kasner SE, Kumar MA (2016) Thromboelastography parameter predicts outcome after subarachnoid hemorrhage: an exploratory analysis. World Neurosurg 96:215–221. https://doi.org/10.1016/j.wneu.2016.04.002
Darkwah Oppong M, Gembruch O, Pierscianek D, Köhrmann M, Kleinschnitz C, Deuschl C, Mönninghoff C, Kaier K, Forsting M, Sure U, Jabbarli R (2018) Post-treatment antiplatelet therapy reduces risk for delayed cerebral ischemia due to aneurysmal subarachnoid hemorrhage. Neurosurgery. 85:827–833. https://doi.org/10.1093/neuros/nyy550
Nagahama Y, Allan L, Nakagawa D, Zanaty M, Starke RM, Chalouhi N, Jabbour P, Brown RD, Derdeyn CP, Leira EC, Broderick J, Chimowitz M, Torner JC, Hasan D (2018) Dual antiplatelet therapy in aneurysmal subarachnoid hemorrhage: association with reduced risk of clinical vasospasm and delayed cerebral ischemia. J Neurosurg 129:702–710. https://doi.org/10.3171/2017.5.JNS17831
James RF, Khattar NK, Aljuboori ZS, Page PS, Shao EY, Carter LM, Meyer KS, Daniels MW, Craycroft J, Gaughen JR, Chaudry MI, Rai SN, Everhart DE, Simard JM (2018) Continuous infusion of low-dose unfractionated heparin after aneurysmal subarachnoid hemorrhage: a preliminary study of cognitive outcomes. J Neurosurg 1–8. doi: https://doi.org/10.3171/2017.11.JNS17894
Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, Cipolle MD, Cohn CS, Fung MK, Grossman BJ, Mintz PD, O’Malley BA, Sesok-Pizzini DA, Shander A, Stack GE, Webert KE, Weinstein R, Welch BG, Whitman GJ, Wong EC, Tobian AAR (2015) Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 162:205–213. https://doi.org/10.7326/M14-1589
Kumar A, Mhaskar R, Grossman BJ, Kaufman RM, Tobian AAR, Kleinman S, Gernsheimer T, Tinmouth AT, Djulbegovic B (2015) Platelet transfusion: a systematic review of the clinical evidence. Transfusion (Paris) 55:1116–1127. https://doi.org/10.1111/trf.12943
Harker LA, Slichter SJ (1972) The bleeding time as a screening test for evaluation of platelet function. N Engl J Med 287:155–159. https://doi.org/10.1056/NEJM197207272870401
Acknowledgments
The authors would like to thank Dr. Zbigniew Lewandowski of the Department of Epidemiology and Biostatistics of the Medical University of Warsaw for his helpful advice on the study’s conception.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology: Radosław Rzepliński; Formal analysis and investigation: Radosław Rzepliński, Mikołaj Sługocki, Tymon Skadorwa; Writing—original draft preparation: Radosław Rzepliński, Mikołaj Sługocki; Writing—review and editing: Tymon Skadorwa, Kacper Kostyra; Resources: Kacper Kostyra, Bogusław Kostkiewicz; Supervision: Bogusław Kostkiewicz.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was approved by The Ethics Committee of The Central Clinical Hospital of the Ministry of Interior and Administration, Warsaw, Poland (Number 109/2018).
Consent to participate
Due to a retrospective analysis of prospectively collected data, additional informed consent was deemed unnecessary. The Ethics Committee of The Central Clinical Hospital of the Ministry of Interior and Administration, Warsaw, Poland, has granted permission to conduct the study without obtaining written informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rzepliński, R., Kostyra, K., Skadorwa, T. et al. Acute platelet response to aneurysmal subarachnoid hemorrhage depends on severity and distribution of bleeding: an observational cohort study. Neurosurg Rev 44, 2647–2658 (2021). https://doi.org/10.1007/s10143-020-01444-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01444-7