Abstract
To assess automated volumetric analysis as a potential presurgical diagnostic tool or as a method to potentially shed light on normal pressure hydrocephalus (NPH) pathophysiology. MRI imaging according to our protocol was performed in 29 NPH patients, 45 non-NPH (but suspected) patients and 15 controls. Twenty patients underwent a second MRI 3 months after ventriculoperitoneal (VP) shunt surgery. All structures relevant to NPH diagnosis were automatically segmented using commercial software. The results were subsequently tested using ANOVA analysis. Significant differences in the volumes of the corpus callosum, left hippocampus, internal globus pallidus, grey and white matter and ventricular volumes were observed between NPH group and healthy controls. However, the differences between NPH and non-NPH groups were non-significant. Three months after, VP shunt insertion decreased ventricular volume was the only clearly significant result (p value 0.0001). Even though a detailed volumetric study shows several significant differences, volumetric analysis as a standalone method does not provide a simple diagnostic biomarker, nor does it shed a light on an unknown NPH aetiology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Normal pressure hydrocephalus (NPH) was first described in 1964 by Hakim [1] as ventriculomegaly with normal cerebrospinal fluid (CSF) pressure during lumbar puncture. Typical presentation is characterized by the triad of gait disturbance, mental deterioration and urinary incontinence. Unlike other types of dementias with similar symptoms and findings, NPH is treatable with CSF diversion, by means of ventriculoperitoneal (VP) or ventriculoatrial shunt placement. It is estimated that there are approximately 2 million people in Europe and 700,000 in the USA that may have the diagnosis of NPH [19]. Only a few percentage of these patients receive a proper diagnosis and treatment. Shunt surgery significantly prolongs life and adds quality-adjusted life years [45]. Unfortunately, the procedure is also burdened with a significant complication rate [13]. Despite the considerable surgical treatment, very little is known about NPH aetiology itself. This is compounded by the ambiguity of available diagnostic tests.
In current practice, functional testing predominates: The spinal tap test, external lumbar drainage or lumbar infusion test [40]. These tests can achieve high predictive accuracy [25], but they are painful and associated with rare, but potentially serious complications [12]. Standard CT and MRI scans are equally good for assessing the basic radiological signs associated with NPH [24], such as Evan’s index, callosal angle, tight high convexity, focal sulcal dilation and dilated Sylvian fissures. The listed parameters are definitely predictive of shunt responsiveness [38] but are not sufficiently sensitive and specific. Some clinical symptoms can be explained by the compression of periventricular white matter and the corpus callosum. Diffusion tensor images (DTI) show changes in mean diffusivity and fractional anisotropy in the posterior limb of the internal capsule, the hippocampus and the fornix. Even though the reported specificity and sensitivity of DTI are high, the technical difficulty along with the absence of a standardized protocol limits its broad utilization [39]. Frequently observed CSF flow void phenomenon in the aqueductal region has directed research towards CSF dynamics. Aqueductal stroke volume measured on phase-contrast MRI shows promising results, but the examination is technically demanding and hardware dependent [8, 36].
It is likely that the key to understanding NPH lies in the ability to correlate novel neuroimaging tools with clinical biomarkers [22]. In our study, we focused on volumetric changes in the subcortical grey and white matter structures since their altered function may have origins in the respective morphometric changes. Recently introduced voxel-based morphometry enables precise volumetric measurements with the help of automatic segmentation software, such as FSL [20] and SPM [4]. In this study, we used Anatomical Mapping Ver. 1.1, Brainlab AG. Our goal was to evaluate volumetry as a potential presurgical diagnostic tool or as a method potentially shedding light on the NPH pathophysiology.
Patients and methods
Participants
Between September 2016 and October 2019, 92 patients were referred to the Department of Neurosurgery and Neurooncology of the Central Military Hospital with suspicion of NPH. All patients suffered gait disturbance and at least one of the other two typical symptoms: mental deterioration or urinary incontinence. The gait disturbance was evaluated using the Dutch Gait Scale [6, 33]. All patients underwent profound examination by a neuropsychologist: Wechsler Memory Scale III, Mini Mental State Examination, Verbal Fluency test, Trial Making Test, Rey-Osterrieth Complex Figure Drawing Test, Beck Depression Inventory and others [10]. During these tests, six patients were removed from the group because they were diagnosed with another type of dementia (5 cases of Alzheimer’s and 1 case of Parkinson’s disease). Prior to being referred to our department, all patients had undergone standard MRI imaging and showed ventriculomegaly (Evans’ index greater than 0.3). All patients underwent lumbar infusion (modified Katzmann’s test, [21]. After finishing the test, lumbar drainage was performed with the same needle and cerebrospinal fluid was drained for 120 h. Shunt insertion was indicated to all patients with positive lumbar infusion test (resistance to outflow above 12 mmHg/ml/min) and showed at least 15% improvement in Dutch Gait Scale after the lumbar drainage. Part of the examination process was a detailed MRI imaging, including a study protocol. Images were reviewed by a radiologist and 9 patients had to be excluded because of coincidental other major pathology: ischemic, tumorous or post-traumatic lesions. Three additional patients were removed because of major movement artefacts preventing automatic segmentation.
The final study group comprises 74 patients—29 patients from this cohort were diagnosed as NPH after completing all the tests, 45 patients did not meet the criteria (in this article, this group is called non-NPH). Furthermore, 15 healthy controls underwent the MRI imaging protocol to compare with both groups. The age and sex characteristics of all groups are presented in Table 1.
Ventriculoperitoneal shunt was inserted to NPH patients, using OSV II Smart valve (Integra Neurosciences®). Three months after the procedure, the NPH patients underwent same neuropsychological and gait disturbance testing as prior to the surgery and MRI imaging according to the study protocol. We were able to completely examine 20 patients (4 people refused further controls, 1 MRI was not readable due to motion artefactss and 4 patients have not reached 3 months after the surgery). We observed at least 15% improvement in the Dutch Gait Scale in all patients who completed the 3 months control.
MRI imaging protocol
All the MRI images were performed on a 3T GE Signa HDx or GE Discovery 750w MR imaging system (GE Medical Systems, Milwaukee, WI) in the Central Military Hospital, Prague. Standard 8-channel (Signa HDx) or 32-channel (Discovery 750w) head coil was used. Beyond the standard brain MRI protocol, the examination included high-resolution 3D T1 BRAVO and 3D T2 Cube PROMO sequences. Parameters of the 3D T1 BRAVO were the following: TR 10 ms, TE:4 ms, matrix 256 × 256, FOV 25.6 × 25.6 cm, flip angle 13°, in-plane resolution 1 × 1 mm2 and slice thickness 1 mm, and of the 3D T2 Cube PROMO sequence: TR 3000 ms, TE 125 ms, ETL 130, matrix 288 × 288, FOV 25.6 × 25.6 cm, in-plane resolution 0.8 × 0.8 mm2 and slice thickness1.2 mm.
Segmentation
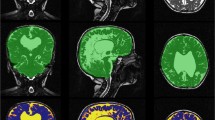
Anatomical Mapping Ver. 1.1 as part of Brainlab Elements software was used for automatic segmentation of high-resolution MRI images. All structures were manually checked for accuracy with no major adjustments needed. We have successively measured volumes of both white and grey matter, internal and external capsule, corpus callosum, hippocampus, amygdala, caudate nucleus, putamen, internal and external globus pallidus, thalamus, periaqueductal grey and three ventricles. The fourth ventricle was later manually removed from the ventricular system measurement in the level of the cerebral aqueduct because its volume is not related to NPH diagnosis [17]. Pictures from the segmentation process are presented in Fig. 1.
Statistical analysis
Comparisons of continuous variables were made using one-way ANOVA or t tests for repeated measures. Subsequent testing after ANOVA was performed using Fisher LSD test. Comparisons of categorical variables were done using chi-square test. In all cases, a p value of less than 0.05 was considered significant. All computations were performed using STATISTICA 13.5 software. Radar graphs were produced in OriginPro 2015 software.
Results
Our study cohort consisted of 74 patients with suspicion of NPH and 15 age-matched healthy controls. After completing all the tests, 29 patients were diagnosed with NPH and underwent ventriculoperitoneal shunt insertion. Average age in the NPH group was 73.6 ± 1.6, in the non-NPH group 74.5 ± 2.2 and in the control group it was 71.4 ± 6.6 years. There were 19 men and 10 women in the NPH group, 33 men and 12 women in the non-NPH group and 9 men and 6 women in the control group (Table 1). Measured volumes of the selected structures in these three groups are presented separately for men and women in Tables 2 and 3, respectively.
Diagnostic volumetry
In this part of the study, we compared normalized volumes (counted to the cerebral volume) of selected structures in NPH patients before surgery to non-NPH group of suspected patients without other known neurodegenerative disease and to healthy age-matched controls. The results are presented in Table 4 with highlighted significant differences.
There were statistically significant differences in the volumes of the left hippocampus, the corpus callosum, the left internal globus pallidus, the white and grey matter (Figs. 2 and 3) and in the ventricular volume (Fig. 4) when both groups were compared to healthy volunteers. Relative volumes of subcortical structures are depicted in Fig. 5. The differences between NPH and non-NPH groups were minimal and not significant.
Volumetric changes after shunt insertion
In 20 patients, we managed to get clinical and graphical follow-up 3 months after the shunt surgery. In this group, there were 12 men and 8 women. Again both measurements were normalized to cerebral volume. The results are shown in Table 5.
Changes in both ventricular (Fig. 6) and cerebral volume and right putamen were statistically significant. The change in the volume of the corpus callosum was on the verge of significance. Relative volumes of subcortical structures are depicted in Fig. 7.
Discussion
In this study, we present comprehensive volumetric assessment of parenchymal structures in NPH patients both in an attempt to find a new reliable diagnostic biomarker and to enlighten the aetiology of NPH. Recent development of voxel-based morphometry and automatic segmentation software enabled simultaneous measurement of a larger number of key structures. We observed basic intracranial compartments such as ventricular volume and grey and white matter. However, previous studies proved no or minimal predictive value of volumetric assessment of the listed structures [27, 30]. From the NPH perspective, the most important white matter structures are the internal and external capsules and the corpus callosum. The internal capsule has combined roles both in psychiatric and movement disorders. Bilateral volume reductions of anterior limb were observed in first-episode psychosis subjects [41]. Posterior limb compression affects gait due to corticospinal tract compression. Fractional anisotropy in the posterior limb of the internal capsule compared with healthy controls and other types of dementia is significantly higher [23]. External capsule microstructure correlates with socially desirable behaviour due to connectivity between the prefrontal cortex and the striatum [3]. Another structure that plays an important role in neurodegenerative diseases is the corpus callosum. Previous studies have shown significant corpus callosum atrophy in both Alzheimer’s disease and mild cognitive impairment [48]. Previously, a minor but significant left-sided hippocampal volume decrease associated with cognitive decline was observed [37] and amygdala volume is supposed to be associated with anxiety and irritability in Alzheimer’s disease [32]. The basal ganglia are associated with both voluntary motor movements and cognition. The putamen plays a known role in Alzheimer’s disease due to amyloid deposits in the early stage of the disease process [7]. Its volume reduction correlates with impaired cognitive function [9]. The caudate nucleus is responsible for adaptable goal-directed behaviour [16] and the right caudate volume is associated with cognitive performance in older adults [5]. The pallidum plays an essential role in walking performance in elderly patients, probably through its involvement in the cortico-striato-pallido-thalamo-cortical circuit [11]. Pallidal volume was identified as the strongest correlate of walking performance in cases of multiple sclerosis [28]. Thalamic lesions were observed in cases of cognitive impairment. Left-sided thalamic lesions were observed in the vascular dementia patients and right-sided lesions in patients who did not meet the criteria for dementia. Left thalamic lesions are supposed to affect both verbal memory and language [42].
Volume of various structures and NPH triad
Gait
NPH gait is characterized by short steps with reduced step height, impaired dynamic equilibrium accented during turning and reduced speed of walking [26]. The gait pattern is sometimes characterized as “glued to the floor” or “magnetic” [14]. Reasons may be due to the impairment of cortico-basal ganglia-thalamo-cortical loops and hippocampi [2]. Our study has shown decreased volume of left internal globus pallidus, left hippocampus and white-matter compared with controls which might contribute to the NPH gait presentation.
Neuropsychology
NPH patients present with alteration of working memory, attention, learning, processing and psychomotor speed, executive, visuospatial and visuoconstructional functions [18]. Memory is presented by delayed recall and recognition [29] and is less severely impaired compared with AD [47]. NPH is a subcortical dementia [46] and decrease in volumes of striatum, hippocampus, thalamus and nucleus accumbens has been previously described [31] compared to healthy controls. We have observed decreased volume of left hippocampus and left internal globus pallidus compared to healthy controls and nucleus accumbens was not segmentated in our study. Striatum is linked to the frontal cortex and lesions to the connections can cause executive dysfunction, impaired motivation, apathy and personality changes [43]. Interesting study found decrease in the cerebral blood flow in normal appearing and periventricular white matter, the lentiform nucleus and global parenchyma of NPH shunt responders or non-responders preoperatively compared to healthy controls with no significant changes between responders and non-responders [51]. These changes may precede atrophy [34], but it is not clear how or whether it is related to volumetric alterations in NPH patients. Lastly, prevalence of another comorbid neurodegenerative disease is high, profound neuropsychologic assessment contributed on the selection of shunt candidates. Prospective studies with autopsy-proven NPH with absence of other neurodegenerative disease may bring other results [40]; however, this study was dedicated on prediction shunt-responsiveness mainly and we did not found differences between NPH and non-NPH groups.
Urinary incontinence
Lower urinary symptoms are frequent in people older than 60 years. Typically, these symptoms in NPH patients result from the impairment of micturition centers above the pontine level and manifest as detrusor overactivity and later as urge incontinence [35]. An fMRI study on the role of suprapontine brain structures involved in voiding control has shown that medial prefrontal cortex, basal ganglia and cerebellum are involved in the control of micturition, parietal cortex and limbic system are involved in inhibitory voiding control mechanism and right hemisphere seems to be more dominant in these mechanisms [50]. Periaqueductal grey has a paramount role in the control of micturition and its role in NPH symptoms has been suggested for MRI evaluation [49]. Proximity of some of the centers or the connecting pathways to the ventricular system may be the reason of urinary incontinence in NPH [44].
There are few volumetric studies of NPH. In the past, manual segmentation by drawing regions of interest on imaging and subsequent volume calculation was needed. This process was very time-consuming with questionable accuracy. During the past two decades, voxel-based morphometry has changed the accuracy and difficulty of this process. Steadily evolving technology has enabled automated, easy-to-use and time-efficient segmentation of several structures simultaneously. Nevertheless, the vast majority of published studies are dealing with one or just few of the crucial structures. However, in our study, we are evaluating volumes of all relevant structures in every patient at once, which significantly extends the possibilities of statistical comparison.
Firstly, we have assessed volumetry as a diagnostic tool for NPH suspected patients. All structures mentioned above were measured both in NPH and non-NPH group and compared to healthy control. Statistically significant difference in ventricular volume was confirmed (p value < 0.000001). The difference in white and grey matter was also significant, with p value 0.007 for white matter and 0.005 for grey matter. However, the difference between the NPH and non-NPH group was minimal and the groups were overlapping. Furthermore, we have observed significant differences in the corpus callosum (p value 0.002), left hippocampus (p value 0.02) and left internal globus pallidus (p value 0.04). Again the difference was mainly compared to control group, whereas compared to non-NPH group it was minimal. Moreover, we have observed larger corpus callosum and hippocampal volume in the NPH group than in the healthy controls, which is in contrast to published data [48]. All observed significant differences were between the NPH suspect group (NPH and non-NPH) and healthy controls. Within the NPH suspect group, where the only difference between NPH and non-NPH was the positivity in functional testing and response to lumbar drainage, the volumetric differences were rather negligible. Our data contradict the routine use of volumetry in NPH diagnostics.
The statistical analysis of changes in structural volumes 3 months after the VP shunt surgery showed expected significant changes in ventricular (p value 0.0001) and cerebral volumes (p value 0.05). The volume of right putamen significantly increased (p value 0.04). This finding could be explained with better drainage of right hemisphere due to the routine placement of ventricular catheter into the right lateral ventricle. This hypothesis is supported by other findings of right-sided structural enlargement, even though these were not statistically significant. All in all, this part of the study does not reveal possible NPH aetiology.
The main limitation of the present study is the limited number of patient controls in 3 months (20/29). It was caused both by a low compliance of some patients and motion artefacts on either preoperative or postoperative images. Only patients with a good quality of both scans were enrolled to the study. Another limitation is the certain inaccuracy of the segmentation process in the case of large ventricles observed by some authors [15].
Conclusion
A detailed volumetric study of NPH patients in both presurgical diagnostics and after VP shunt insertion shows several significant differences. According to our findings, volumetry as a standalone method fails to provide a simple diagnostic biomarker, nor does it shed a light on an unknown NPH aetiology.
References
Adams RDFC, Hakim S (1965) Symptomatic occult hydrocephalus with “normal” cerebrospinal fluid pressure. A treatable syndrome. N Engl J Med 273:117–226
Allali G, Laidet M, Armand S, Saj A, Krack P, Assal F (2018) Apathy in idiopathic normal pressure hydrocephalus: a marker of reversible gait disorders. Int J Geriatr Psychiatry 33:735–742. https://doi.org/10.1002/gps.4847
Andrejevic M, Meshi D, van den Bos W, Heekeren HR (2017) Individual differences in social desirability are associated with white-matter microstructure of the external capsule. Cogn Affect Behav Neurosci 17:1255–1264. https://doi.org/10.3758/s13415-017-0548-2
Ashburner J (2012) SPM: a history. Neuroimage 62:791–800. https://doi.org/10.1016/j.neuroimage.2011.10.025
Bauer E, Toepper M, Gebhardt H, Gallhofer B, Sammer G (2015) The significance of caudate volume for age-related associative memory decline. Brain Res 1622:137–148. https://doi.org/10.1016/j.brainres.2015.06.026
Boon AJ, Tans JT, Delwel EJ, Egeler-Peerdeman SM, Hanlo PW, Wurzer JA, Hermans J (1997) Dutch normal pressure hydrocephalus study: baseline characteristics with emphasis on clinical findings. Eur J Neurol 4:39–47. https://doi.org/10.1111/j.1468-1331.1997.tb00297.x
Braak H, Braak E (1990) Alzheimer’s disease: striatal amyloid deposits and neurofibrillary changes. J Neuropathol Exp Neurol 49:215–224
Bradley WG Jr (2016) Magnetic resonance imaging of normal pressure hydrocephalus. Semin Ultrasound CT MR 37:120–128. https://doi.org/10.1053/j.sult.2016.01.005
de Jong LW, van der Hiele K, Veer IM, Houwing JJ, Westendorp RG, Bollen EL, de Bruin PW, Middelkoop HA, van Buchem MA, van der Grond J (2008) Strongly reduced volumes of putamen and thalamus in Alzheimer’s disease: an MRI study. Brain 131:3277–3285. https://doi.org/10.1093/brain/awn278
Devito EE, Pickard JD, Salmond CH, Iddon JL, Loveday C, Sahakian BJ (2005) The neuropsychology of normal pressure hydrocephalus (NPH). Br J Neurosurg 19:217–224. https://doi.org/10.1080/02688690500201838
Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, Deichmann R, Ashburner J, Frackowiak RS (2008) Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci 28:7143–7152. https://doi.org/10.1523/JNEUROSCI.1486-08.2008
El Ahmadieh TY, Wu EM, Kafka B, Caruso JP, Neeley OJ, Plitt A, Aoun SG, Olson DM, Ruchinskas RA, Cullum CM, Barnett S, Welch BG, Batjer HH, White JA (2019) Lumbar drain trial outcomes of normal pressure hydrocephalus: a single-center experience of 254 patients. J Neurosurg:1–7. https://doi.org/10.3171/2018.8.JNS181059
Feletti A, d'Avella D, Wikkelso C, Klinge P, Hellstrom P, Tans J, Kiefer M, Meier U, Lemcke J, Paterno V, Stieglitz L, Sames M, Saur K, Kordas M, Vitanovic D, Gabarros A, Llarga F, Triffaux M, Tyberghien A, Juhler M, Hasselbalch S, Cesarini K, Laurell K (2019) Ventriculoperitoneal shunt complications in the European idiopathic normal pressure hydrocephalus multicenter study. Oper Neurosurg (Hagerstown) 17:97–102. https://doi.org/10.1093/ons/opy232
Ghosh S, Lippa C (2014) Diagnosis and prognosis in idiopathic normal pressure hydrocephalus. Am J Alzheimer’s Dis Other Dement 29:583–589. https://doi.org/10.1177/1533317514523485
Goto M, Abe O, Aoki S, Kamagata K, Hori M, Miyati T, Gomi T, Takeda T (2018) Combining segmented grey and white matter images improves voxel-based morphometry for the case of dilated lateral ventricles. Magn Reson Med Sci 17:293–300. https://doi.org/10.2463/mrms.mp.2017-0127
Grahn JA, Parkinson JA, Owen AM (2008) The cognitive functions of the caudate nucleus. Prog Neurobiol 86:141–155. https://doi.org/10.1016/j.pneurobio.2008.09.004
Greitz D (2004) Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg Rev 27:145–165; discussion 166-147. https://doi.org/10.1007/s10143-004-0326-9
Iddon JL, Pickard JD, Cross JJL, Griffiths PD, Czosnyka M, Sahakian BJ (1999) Specific patterns of cognitive impairment in patients with idiopathic normal pressure hydrocephalus and Alzheimer’s disease: a pilot study. J Neurol Neurosurg Psychiatry 67:723–732. https://doi.org/10.1136/jnnp.67.6.723
Jaraj D, Rabiei K, Marlow T, Jensen C, Skoog I, Wikkelso C (2014) Prevalence of idiopathic normal-pressure hydrocephalus. Neurology 82:1449–1454. https://doi.org/10.1212/WNL.0000000000000342
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) Fsl. Neuroimage 62:782–790. https://doi.org/10.1016/j.neuroimage.2011.09.015
Katzman R, Hussey F (1970) A simple constant-infusion manometric test for measurement of CSF absorption. I Rationale and method. Neurology 20:534–544. https://doi.org/10.1212/wnl.20.6.534
Keong NC, Pena A, Price SJ, Czosnyka M, Czosnyka Z, Pickard JD (2016) Imaging normal pressure hydrocephalus: theories, techniques, and challenges. Neurosurg Focus 41:E11. https://doi.org/10.3171/2016.7.FOCUS16194
Kim MJ, Seo SW, Lee KM, Kim ST, Lee JI, Nam DH, Na DL (2011) Differential diagnosis of idiopathic normal pressure hydrocephalus from other dementias using diffusion tensor imaging. AJNR Am J Neuroradiol 32:1496–1503. https://doi.org/10.3174/ajnr.A2531
Kockum K, Virhammar J, Riklund K, Soderstrom L, Larsson EM, Laurell K (2019) Standardized image evaluation in patients with idiopathic normal pressure hydrocephalus: consistency and reproducibility. Neuroradiology. 61:1397–1406. https://doi.org/10.1007/s00234-019-02273-2
Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM (2005) The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 57:S17–S28; discussion ii-v. https://doi.org/10.1227/01.neu.0000168184.01002.60
Marmarou A, Sawauchi S, Dunbar J (2005) Diagnosis and management of idiopathic normal-pressure hydrocephalus: a prospective study in 151 patients. J Neurosurg 102:11
Moore DW, Kovanlikaya I, Heier LA, Raj A, Huang C, Chu KW, Relkin NR (2012) A pilot study of quantitative MRI measurements of ventricular volume and cortical atrophy for the differential diagnosis of normal pressure hydrocephalus. Neurol Res Int 2012:718150. https://doi.org/10.1155/2012/718150
Motl RW, Hubbard EA, Sreekumar N, Wetter NC, Sutton BP, Pilutti LA, Sosnoff JJ, Benedict RH (2015) Pallidal and caudate volumes correlate with walking function in multiple sclerosis. J Neurol Sci 354:33–36. https://doi.org/10.1016/j.jns.2015.04.041
Ogino A, Kazui H, Miyoshi N, Hashimoto M, Ohkawa S, Tokunaga H, Ikejiri Y, Takeda M (2006) Cognitive impairment in patients with idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord 21:113–119. https://doi.org/10.1159/000090510
Palm WM, Walchenbach R, Bruinsma B, Admiraal-Behloul F, Middelkoop HA, Launer LJ, van der Grond J, van Buchem MA (2006) Intracranial compartment volumes in normal pressure hydrocephalus: volumetric assessment versus outcome. AJNR Am J Neuroradiol 27:76–79
Peterson KA, Mole TB, Keong NCH, DeVito EE, Savulich G, Pickard JD, Sahakian BJ (2018) Structural correlates of cognitive impairment in normal pressure hydrocephalus. Acta Neurol Scand. https://doi.org/10.1111/ane.13052
Poulin SP, Dautoff R, Morris JC, Barrett LF, Dickerson BC, Alzheimer’s Disease Neuroimaging I (2011) Amygdala atrophy is prominent in early Alzheimer’s disease and relates to symptom severity. Psychiatry Res 194:7–13. doi:https://doi.org/10.1016/j.pscychresns.2011.06.014
Ravdin LD, Katzen HL, Jackson AE, Tsakanikas D, Assuras S, Relkin NR (2008) Features of gait most responsive to tap test in normal pressure hydrocephalus. Clin Neurol Neurosurg 110:455–461. https://doi.org/10.1016/j.clineuro.2008.02.003
Ruitenberg A, den Heijer T, Bakker SLM, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MMB (2005) Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Ann Neurol 57:789–794. https://doi.org/10.1002/ana.20493
Sakakibara R, Kanda T, Sekido T, Uchiyama T, Awa Y, Ito T, Liu Z, Yamamoto T, Yamanishi T, Yuasa T, Shirai K, Hattori T (2008) Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourol Urodyn 27:507–510. https://doi.org/10.1002/nau.20547
Sakhare AR, Barisano G, Pa J (2019) Assessing test-retest reliability of phase contrast MRI for measuring cerebrospinal fluid and cerebral blood flow dynamics. Magn Reson Med 82:658–670. https://doi.org/10.1002/mrm.27752
Savolainen S, Laakso MP, Paljarvi L, Alafuzoff I, Hurskainen H, Partanen K, Soininen H, Vapalahti M (2000) MR imaging of the hippocampus in normal pressure hydrocephalus: correlations with cortical Alzheimer’s disease confirmed by pathologic analysis. AJNR Am J Neuroradiol 21:409–414
Shinoda N, Hirai O, Hori S, Mikami K, Bando T, Shimo D, Kuroyama T, Kuramoto Y, Matsumoto M, Ueno Y (2017) Utility of MRI-based disproportionately enlarged subarachnoid space hydrocephalus scoring for predicting prognosis after surgery for idiopathic normal pressure hydrocephalus: clinical research. J Neurosurg 127:1436–1442. https://doi.org/10.3171/2016.9.JNS161080
Siasios I, Kapsalaki EZ, Fountas KN, Fotiadou A, Dorsch A, Vakharia K, Pollina J, Dimopoulos V (2016) The role of diffusion tensor imaging and fractional anisotropy in the evaluation of patients with idiopathic normal pressure hydrocephalus: a literature review. Neurosurg Focus 41:E12. https://doi.org/10.3171/2016.6.FOCUS16192
Skalický P, Mládek A, Vlasák A, De Lacy P, Beneš V, Bradáč O (2019) Normal pressure hydrocephalus—an overview of pathophysiological mechanisms and diagnostic procedures. Neurosurg Rev:1–14. https://doi.org/10.1007/s10143-019-01201-5
Spiegelhalder K, Regen W, Prem M, Baglioni C, Nissen C, Feige B, Schnell S, Kiselev VG, Hennig J, Riemann D (2014) Reduced anterior internal capsule white matter integrity in primary insomnia. Hum Brain Mapp 35:3431–3438
Swartz RH, Black SE (2006) Anterior-medial thalamic lesions in dementia: frequent, and volume dependently associated with sudden cognitive decline. J Neurol Neurosurg Psychiatry 77:1307–1312. https://doi.org/10.1136/jnnp.2006.091561
Tekin S, Cummings JL (2002) Frontal–subcortical neuronal circuits and clinical neuropsychiatry an update. J Psychosom Res 8
Tsakanikas D, Relkin N (2007) Normal pressure hydrocephalus. Semin Neurol 27:058–065. https://doi.org/10.1055/s-2006-956756
Tullberg M, Persson J, Petersen J, Hellstrom P, Wikkelso C, Lundgren-Nilsson A (2018) Shunt surgery in idiopathic normal pressure hydrocephalus is cost-effective-a cost utility analysis. Acta Neurochir 160:509–518. https://doi.org/10.1007/s00701-017-3394-7
van Harten B, Courant MNJ, Scheltens P, Weinstein HC (2004) Validation of the HIV dementia scale in an elderly cohort of patients with subcortical cognitive impairment caused by subcortical ischaemic vascular disease or a normal pressure hydrocephalus. Dement Geriatr Cogn Disord 18:109–114. doi:https://doi.org/10.1159/000077818
Walchenbach R, Geiger E, Thomeer RTWM, Vanneste JAL (2002) The value of temporary external lumbar CSF drainage in predicting the outcome of shunting on normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 72:503–506. https://doi.org/10.1136/jnnp.72.4.503
Wang XD, Ren M, Zhu MW, Gao WP, Zhang J, Shen H, Lin ZG, Feng HL, Zhao CJ, Gao K (2015) Corpus callosum atrophy associated with the degree of cognitive decline in patients with Alzheimer’s dementia or mild cognitive impairment: a meta-analysis of the region of interest structural imaging studies. J Psychiatr Res 63:10–19. https://doi.org/10.1016/j.jpsychires.2015.02.005
Zare A, Jahanshahi A, Rahnama'i MS, Schipper S, van Koeveringe GA (2019) The role of the periaqueductal gray matter in lower urinary tract function. Mol Neurobiol 56:920–934. https://doi.org/10.1007/s12035-018-1131-8
Zhang H, Reitz A, Kollias S, Summers P, Curt A, Schurch B (2005) An fMRI study of the role of suprapontine brain structures in the voluntary voiding control induced by pelvic floor contraction. NeuroImage 24:174–180. https://doi.org/10.1016/j.neuroimage.2004.08.027
Ziegelitz D, Arvidsson J, Hellström P, Tullberg M, Wikkelsø C, Starck G (2016) Pre-and postoperative cerebral blood flow changes in patients with idiopathic normal pressure hydrocephalus measured by computed tomography (CT)-perfusion. J Cereb Blood Flow Metab 36:1755–1766. https://doi.org/10.1177/0271678X15608521
Funding
Supported by institutional grant Czech Ministry of Defence MO1012.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Military University Hospital Ethical committee.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vlasák, A., Skalický, P., Mládek, A. et al. Structural volumetry in NPH diagnostics and treatment—future or dead end?. Neurosurg Rev 44, 503–514 (2021). https://doi.org/10.1007/s10143-020-01245-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01245-y