Abstract
The use of the internal maxillary artery (IMA) in intracranial artery bypass or subcranial-intracranial (SC-IC) bypass has recently been described as an alternative to traditional bypass. This study explores cerebral glucose metabolism characteristics of SC-IC bypass. Ten crescendo transient ischemic attack (TIA) patients with chronic occlusion of the middle cerebral artery (MCA) received bypass surgery of IMA with the radial artery graft (RAG) to the branch of MCA. The graft’s flow volume (FV) was measured by operative intraoperative duplex ultrasonography. Positron emission tomography (PET)/computed tomography (CT) was used to calculate the preoperational and postoperational average of the standard uptake value (SUVavg) of the 18-fluoro-2-deoxy-d-glucose (18F-FDG) in the region of interest (ROI). The asymmetric index (AI) is recommended to reflect the SUVavg changes, and subsequently, cerebral glucose metabolism changes are supposedly clarified. Patent IMA-RAG-MCA bypass in ten chronic ischemia patients was confirmed by angiography after surgery. The intraoperative FV measurement value was 65.64 ± 10.52 (58.11–73.17) ml/min. Before the operation, the SUVavg of the ROI in the ischemic hemisphere (4.76 ± 2.35 (3.08–6.04)) clearly decreased compared to the one (5.99 ± 2.63 (4.11–7.87)) in the contralateral mirror region (P = 0.003). The result of AI of preoperation minus AI of postoperation was more than 10% (P = 0.031), which indicated suspicious significant changes in cerebral metabolism. All symptoms of study patients having crescendo ischemia were resolved in 1 month after the operation. In the cerebral hypoperfusion territory, uptake of 18F-FDG deceased. Improving the flow volume via SC-IC bypass makes available an elevated uptake of 18F-FDG.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since conventional extracranial-intracranial (EC-IC) bypass was first reported by Yasagil in 1967 [19], it has gained popularity for the treatment of aneurysms, moyamoya disease, and ischemic cerebrovascular diseases. The report of EC-IC bypass in the treatment of ischemic cerebrovascular diseases in 1985 [14, 15] and the Carotid Occlusion Surgery Study (COSS) in 2011 [11] stated that the surgery did not confer additional benefit compared to conservative drug therapy. However, this procedure ameliorated the oxygen metabolism evaluated by positron emission tomography (PET) with 15O-labeled tracers [11]. Collectively, while PET with 15O-labeled tracers provides essential physiological information that could predict the final fate of tissue during cerebral ischemia, the requirement of an in-house cyclotron to produce ultra-short half-life 15O-labeled tracers (~ 2 min) significantly hampers its clinical utility. Alternatively, fluorine-18 (or 18F) has a half-life of 110 min, and [18F]-fluoro-2-deoxy-d-glucose (18F-FDG) has been used extensively to reveal in vivo glucose utilization in different clinical settings, most notably in oncology and cardiology. Therefore, it is highly plausible that 18F-FDG may also offer valuable insights into cerebral glucose metabolism during cerebral ischemia, which consequently discerns tissue viability. At present, the uptake of 18F-FDG in the chronic ischemia territory remains unknown, as do the changes of the uptake of 18F-FDG after the augmentation flow bypass. Here, we have chosen internal maxillary artery (IMA) instead of superficial temporal artery (STA) as the donor artery due to the former is expected to supply more blood flow to the ischemic territory than the latter. Since 2018, ten patients completed 18F-FDG PET/computed tomography (CT) before and after the augmentation flow bypass. This paper will review our results in order to evaluate the glucose metabolism changes in the chronic ischemia patients after the IMA bypass.
Materials and methods
Patients
We have analyzed ten patients between August 2018 and January 2019. Patients who fulfilled the initial clinical eligibility criteria provided written informed consent according to local institutional review board regulations. Before surgery, an independent neurologist and a vascular neurosurgeon assessed each patient according to the National Institutes of Health Stroke Scale (NIHSS). All patients received digital subtraction angiography (DSA) to evaluate the relevant extracranial and intracranial vessels. The extent and shape of the cerebral ischemic lesion in five patients were evaluated by magnetic resonance imaging (MRI). The regional cerebral blood flow (rCBF), regional cerebral blood volume (rCBV), mean transit time (MTT), and time to peak (TTP) were investigated by perfusion-weighted imaging (PWI)/MRI in order to assess the associated hemodynamic changes consistent with major cerebral artery occlusion. 18F-FDG PET/CT was utilized to evaluate the average standard uptake value (SUVavg) of the region of interest (ROI). In this series, the indication of internal maxillary artery (IMA) augmentation flow bypass surgery was for patients who (1) had atherosclerotic occlusion of the internal carotid artery (ICA) or MCA trunk with poor collateral circulation demonstrated by DSA, (2) had a hypoperfused area of the brain on PWI/MRI consistent with occlusion angiography, (3) had more than a 4-week history of hemispheric ischemic attacks, and (4) were younger than 65 years of age.
Surgical procedures
Frontotemporal craniotomy was used, followed by zygomatic osteotomy with subsequent temporal fossa craniotomy reaching its medial border, as designated by the same virtual line connecting the foramen rotundum and the foramen ovale. The IMA is divided into three segments. We used the second segment, called the pterygoid muscle segment, which lies on the superficial part of the lateral pterygoid muscle [10]. Usually, the left radial artery was utilized as a graft vessel after a preoperative negative Allen’s test, and the required length ranged from 7 to 8 cm. An end-to-end anastomosis was created between the proximal end of the radial artery graft and the second segment of the IMA, using 9–0 nylon suture for 18.9 ± 1.2 (range 17–21) minutes, and the distal end of the graft was later anastomosed to the side of the recipient artery-M2 or M3 segment of MCA. During the end-to-side anastomosis, the recipient artery would be clipped for 22.4 ± 1.5(range 20–25) minutes; meanwhile, the blood pressure was raised, and the somatosensory evoked potentials (SEP) were monitored during the entire anastomosis procedure. After the temporary clips were removed, the Pro Focus 2202 ultra-view 8863 probe (BK Medical, Herlev, Denmark) would measure flow volume (FV) on the graft. The systolic blood pressures of patients were sustained at 123.7 ± 2.3 (range 120–127) mmHg during FV measurements.
Intravenous heparin (2000 units) was used intraoperatively at the time of vascular clipping for anastomosis. During the first three postoperative days, the patient received low molecular weight heparin calcium subcutaneous injection, 2050 IU, every 12 h, daily. Aspirin (300 mg/day) was also administered perioperatively and continued for 1 month postoperatively. Subsequently, aspirin was reduced to 100 mg daily and continued unless severe complications appeared. On the first three postoperative days, all patients received a computed tomography angiogram (CTA) to confirm if the graft was patent.
PET/CT examination
All patients underwent 18F-FDG PET/CT imaging of the brain tissue with a hybrid scanner (64-multidetector Biograph mCT, Discovery VCT, General Electric Company, State of Connecticut, America) before the operation and at 1 month after surgery. Before examination, the patients fasted for at least 8 h, and blood glucose measurements were obtained in these patients before the administration of 18F-FDG. Blood glucose levels in all patients were less than 126 mg/dl (7 mmol/l). Study subjects were administered a target dose of 3.7 to 5.55 MBq (0.10–0.15 mCi)/kg 18F-FDG intravenously and subsequently rested in a quiet environment for 60 min. PET scan parameters: 3D mode acquisition, transmission, and acquisition of 10 min, 4.25ram, 5 mm. An attenuation correction CT scan (non-enhanced 140 kV and 110 mA) was then performed, followed by PET imaging of the head in list mode for 20 min. The rotating speed of the ball tube speed was 0.8 s/c; the bed speed was 15 mm. A nuclear medicine physician and a radiologist made visual judgments and undertook semi-quantitative analysis to obtain the average SUV of the ROI (SUV = radioactivity concentration of ROI (kBq/ml)/injection dose(MBq)/body weight(kg)). SUVavg stands for the average value of radioactivity concentration of ROI an indirectly calculated by PET/CT. The ROI consisted of a 3-cm3 spherical volume along the cortical rim, avoiding the infarcted area, and was manually placed over the frontal or temporal cortex (targeted to the central area where anastomosis was planned).
Data analysis
The continuous variables are expressed as the mean ± standard deviation at 95% confidence intervals (95% CI), and the categorical variables are expressed as percentages. Differences between preoperative and postoperative hemodynamic parameters (rCBF, rCBV, MTT, TTP) were assessed by Fisher’s exact test. Differences between ipsilateral SUVavg and contralateral SUVavg were assessed by a paired t test. Decline of preoperative and postoperative asymmetric index (AI) ([SUVavg of normal region-SUVavg of abnormal region] / [SUVavg of normal region + SUVavg of abnormal region] × 200%) compared to 0 or 10% was assessed by one-sample t test. To examine whether the degree of cerebral metabolism change is associated with an increase in cerebral perfusion after bypass, Pearson correlation analysis was performed to evaluate the relationship between the FV and the decline of AI in ROI (preoperational AI-postoperational AI). The data was analyzed using SPSS 19.0 software (IBM, New York, USA). The level of significance was set at P < 0.05.
Results
Patient characteristics
Of the ten patients, there were eight males (80%) and two females (20%) and the mean age was 49 ± 9.3 years (range 35–65). Although each of the ten patients’ NIHSS scores was zero, they had focal limb weakness (20%), aphasia (40%), dizziness (20%), or quadrantanopia (20%) that resolved within 24 h but occurred frequently and affected their daily life. The symptom duration was 2 ± 0.3 months (range 1.5–2.5). Five patients had a history of focal cerebral ischemic infarction for more than 3 months. Three patients had a history of hypertension. All the patients had hyperlipidemia, and triglycerides were 2.59 ± 0.24 mmol/l. Five patients had smoked for more than 5 years. Out of ten patients, complete occlusion of the single MCA and the ICA was shown in five patients and one patient, respectively. Two patients had both MCAs occluded and one patient had both ICAs occluded (Table 1). During the operation, we can see one or more atherosclerotic intracranial arteries. The patients’ crescendo ischemia symptoms resolved after the operation. The NIHSS score after surgery was also zero. There were no procedure-related stroke, postoperative hemorrhagic complication that required reoperation, and hyperperfusion syndrome after IMA-MCA bypass.
Results of flow volume and glucose metabolism after surgery
The intraoperative FV measurement value was 65.64 ± 10.52(58.11–73.17) ml/min (Table 2) when the systolic pressure was sustained at 123.7 ± 2.3(range 120–127) mmHg. During the first 3 days after the operation, postoperative CTA demonstrated graft vessel patency in all ten patients (Fig. 1). PWI indicated that significant changes occurred in MTT (P = 0.031) and in TTP (P = 0.016) but not in rCBF (P = 0.125) or in rCBV (P = 1) (Fig. 2). Before surgery, the SUVavg of ROI in the ischemic hemisphere was 4.76 ± 2.35 (3.08–6.04), which was obviously decreased compared to the SUVavg of 5.99 ± 2.63 (4.11–7.87) in the contralateral mirror region (P = 0.03). After augmentation flow bypass, the SUVavg of ROI in the ischemic hemisphere was 5.26 ± 2.23 (3.67–6.86), which was not significantly different (P = 0.45) compared to the SUVavg in the contralateral mirror region of 5.05 ± 1.58 (3.91–6.18). The bypass ameliorated the SUVavg of the ROI in the ischemic hemisphere (Fig. 1). To further elaborate the results, the AI is recommended to reflect the SUVavg difference between the ischemic hemisphere and contralateral mirror region. Before operation, the AI was 23.91% ± 19.32% (10.09–37.73%). The result of preoperative AI minus postoperative AI (decline of AI) was 24.82% ± 18.32% (11.72–31.93%) which was greater than not only zero (P = 0.002) but also 10% (P = 0.031). FV correlated significantly with the decline of AI on linear regression analysis (P = 0.002) (Fig. 3).
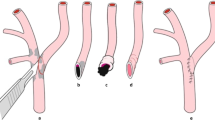
Representative case. A 45-year-old male who was admitted for crescendo left-sided limb weakness for 1 month. The preoperative CTA indicated that the right MCA was occluded (a, arrow).18F-FDG PET/CT showed the right hemisphere had low metabolism (b, arrow). Repeated CTA confirmed that the graft was patent (c, arrowhead) on the third day after the IMA-RAG-MCA flow augmentation bypass and ipsilateral territory metabolism increased in the postoperative metabolism map (d, arrowhead)
Discussion
Recently, cerebral hypotension has received considerable attention, as it is the possible cause of ischemic stroke, rather than embolism or local vasculopathy [4]. Patients with untreated athero-occlusive disease of stage II hemodynamic failure (increased oxygen extraction fraction) have a 13.2% first year and a 29.2% second year of risk of stroke [3]. Approximately 10–12% of the patients with carotid artery steno-occlusive disease suffer a stroke on the basis of chronic hemodynamic impairments as a consequence of an occlusion of the major cerebral blood vessels [18]. Although most trials confirmed that conventional EC-IC bypass does not benefit selected patients, cerebrovascular neurosurgeons persist in trying a novel EC-IC bypass [6, 13, 16, 17]. Recently, a new EC-IC bypass of the IMA, which supplies the blood flow to the penumbra, was applied in clinical trials [1, 8, 12]. As is well-known, maintaining normal brain homeostasis is an energy-consuming process that depends on a continuous supply of oxygen and glucose because the brain cannot store energy substrates. Theoretically, this new bypass ameliorates oxygen metabolism on the basis of the former trial [11]. The cerebral glucose metabolism changes in ischemic patients before and after augmentation flow bypass surgery were assessed using 18F-FDG PET/CT.
Hypoperfused territories were confirmed via PWI/MRI in all patients in our study. In the hypoperfusion region, including the ischemic core, SUVavg obviously decreased compared with the contralateral mirror region. Chronic 18F-FDG PET/CT studies (days and months after ischemic stroke) in stroke patients have been reported by several groups, and a reduction of injury in the infarction areas is consistently reported [5, 7]. Nevertheless, there was no report about the uptake of 18F-FDG in the hypoperfusion region surrounding the ischemic core. To the best of our knowledge, this uptake represents a chronic ischemia process, and the brain cells only maintain homeostasis and have no surplus energy to actively uptake 18F-FDG when hypoperfusion is present. In ten cases, we found that four patients’ TTP and MTT were delayed but rCBF and rCBV were normal; meanwhile, 18F-FDG PET/CT showed decreasing SUVavg in the hypoperfusion region. After bypass surgery, TTP and MTT improved. Moreover, uptake of 18F-FDG increased. It is inferred that SUVavg is a sensitive indicator for predicting ischemia.
It was thought that all the patients’ SUVavg would be ameliorated after the bypass surgery. In fact, not only the ipsilateral SUVavg but also the contralateral SUVavg showed changes. The simple comparison of preoperative and postoperative SUVavg was not significant because there are so many non-controllable factors, such as blood glucose level, insulin level, injection of radionuclide dose, radionuclide concentration, metabolic time, excretion, and scan duration. Fortunately, there is no significant difference between the SUVavg in the ipsilateral ROI and that in the contralateral ROI after the augmentation flow bypass. To further illustrate the changes of SUVavg, the AI was utilized [9]. All the preoperative AIs were more than zero, and seven patients’ AIs were over 15%. The other three patients’ AIs were under 5%. This was because both ICAs or both MCAs were occluded in three patients, and both hemispheres were hypoperfused. In the dual-lesion cases, the symptomatic side was chosen for surgery. After augmentation flow bypass, if no new symptoms occurred, the contralateral lesion was preserved.
All of the grafts remained unobstructed during the study. Along with the increase in FV, the change in cerebral metabolism increased, which was in accordance with the theory of augmentation flow bypass. However, the decline of AI was not uniform; eight patients had more than 15% of a decline, and two patients had less than 10% (9.97% and 8.97%). Although the augmentation bypass restores the blood flow, it is not certain that the ischemia zone acquires more blood flow than before because of the pressure gradient. The new blood flow direction was not completely in accordance with the status before the augmentation bypass. Therefore, the final state resulting from the bypass surgery would not necessarily achieve the desired results. With the above hypothesis, sufficient blood flow guarantees the augmentation result. However, increased blood flow is associated with a greater impact on the recipient artery and tunica intima, and the long-term patency ratio is decreased. Augmentation bypass has lacked popularity until recently, possibly because valid flow was not supplied to the ischemia zone. The time from operation to reexamination may be another influencing factor. 18F-FDG uptake patterns 30, 60, 90, 120, and 150 min after middle cerebral artery occlusion (MCAO) were revealed in the ischemic stroke rat model [2]. It was observed that reduced 18F-FDG uptake in the ischemic core regions immediately after MCAO remained low across all time points. The quantitative and qualitative temporal and spatial patterns of 18F-FDG uptake in stroke patients are also of interest. If revascularization was successful, how would the 18F-FDG uptake change over time? In the COSS trial, surgical participants received a repeat 15O PET/CT scan, 30 to 60 days postoperatively [11]. The interval in our study is about a fortnight and this further follow-up scan may be a necessity.
While the previous discussion offers some of the potential factors related to the observed reduced uptake of 18F-FDG in the hypoperfusion areas and increased uptake of 18F-FDG after revascularization, one remaining major question is whether the changes of 18F-FDG uptake truly reflect changes of glucose metabolism. There are significant differences between the phosphorylation of 18F-FDG and glucose. Therefore, one must be cautious in the interpretation of 18F-FDG uptake as a marker of glucose metabolism.
Conclusions
In the hypoperfused region of the cerebral zone, uptake of 18F-FDG decreased. Improving the blood flow of the penumbra via a subcranial-intracranial bypass elevates uptake of 18F-FDG.
References
Abdulrauf SI, Sweeney JM, Mohan YS, Palejwala SK (2011) Short segment internal maxillary artery to middle cerebral artery bypass: a novel technique for extracranial-to-intracranial bypass. Neurosurgery 68(3):804–809
Bunevicius A, Yuan H, Lin W (2013) The potential roles of 18F-FDG-PET in management of acute stroke patients. Biomed Res Int 2013:634598
Grubb RL Jr, Derdeyn CP, Frits SM, Carpenter DA, Yundt KD, Videen TO, Spitznagel EL, Powers WJ (1998) Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 280(12):1055–1060
Klijn CJ, Kappelle LJ (2010) Haemodynamic stroke: clinical features, prognosis and management. Lancet Neurol 9:1008–1017
Kuhl DE, Phelps ME, Kowell AP, Metter EJ, Selin C, Winter J (1980) Effects of stroke on local cerebral metabolism and perfusion: mapping by emission computed tomography of 18FDG and 13NH3. Ann Neurol 8(1):47–60
Morgan MK, Brennan J, Day MJ (1996) Interposition saphenous vein bypass graft between the common and intracranial internal carotid arteries. J Clin Neurosci 3(3):272–280
Nasu S, Hata T, Nakajima T, Suzuki Y (2002) Evaluation of 18F-FDG PET in acute ischemic stroke: assessment of hyper accumulation around the lesion. Kaku Igaku 39(2):103–110
Nossek E, Costantino PD, Eisenberg M, Dehdashti AR, Setton A, Chalif DJ, Ortiz RA, Langer DJ (2014) Internal maxillary artery to middle cerebral artery bypass: infratemporal approach for subcranial-intracranial (SC-IC) bypass. Neurosurgery 75(1):87–95
Ogasawara K, Ogawa A, Terasaki K, Shimizu H, Tominaga T, Yoshimoto T (2002) Use of cerebrovasular reactivity in patients with symptomatic major cerebral artery occlusion to predict 5-year outcome: comparison of xenon-133 and iodine-123-IMP single-photon emission computed tomography. J Cereb Blood Flow Metab 22(9):1142–1148
Otake I, Kageyama I, Mataga I (2011) Clinical anatomy of the maxillary artery. Okajimas Folia Anat Jpn 87(4):155–164
Powers WJ, Clarke WR, Grubb RL Jr, Videen TO, Adams HP Jr, Derdeyn CP (2011) Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA 306(18):1983–1992
Shi X, Qian H, K C KI, Zhang Y, Zhou Z, Sun Y (2011) Bypass of the maxillary to proximal middle cerebral artery or proximal posterior cerebral artery with radial artery graft. Acta Neurochir (Wien) 153(8):1649–1655
Story JL, Brown WE, Eidelberg E, Arom KV, Stewart JR (1978) Cerebral revascularization: proximal external carotid to distal middle cerebral artery bypass with a synthetic tube graft. Neurosurgery 3(1):61–65
The EC/IC Bypass Study Group (1985) Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med 313(19):1191–1200
The EC/IC Bypass Study group (1985) The International Cooperative Study of Extracranial/Intracranial Arterial Anastomosis (EC/IC Bypass Study): methodology and entry characteristics. Stroke 16(3):397–406
Ulku CH, Ustun ME, Buyukmumcu M, Cicekcibasi AE, Ziylan T (2004) Radial artery graft for bypass of the maxillary to proximal posterior cerebral artery: an anatomical and technical study. Acta Otolaryngol 124(7):858–862
Ustun ME, Buyukmumcu M, Ulku CH, Cicekcibasi AE, Arbag H (2004) Radial artery graft for bypass of the maxillary to proximal middle cerebral artery: an anatomic and technical study. Neurosurgery 54(3):667–670
Vajkoczy P (2009) Revival of extra-intracranial bypass surgery. Curr Opin Neurol 22:90–99
Yasargil MG, Yonekawa Y (1977) Results of microsurgical extra-intracranial arterial bypass in the treatment of cerebral ischemia. Neurosurgery 1:22–24
Funding
This study was funded by the China Postdoctoral Science Foundation (Grant number 2018M641390).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee-YuQuan Hospital Tsinghua University (reference number 20181664) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, Z., Shi, X., Zhou, Z. et al. Cerebral glucose metabolism changes in chronic ischemia patients following subcranial-intracranial bypass. Neurosurg Rev 43, 1383–1389 (2020). https://doi.org/10.1007/s10143-019-01177-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-019-01177-2