Abstract
Thrombosed aneurysms of the middle cerebral artery (MCA) usually show large dimension and complex morphology with neck sclerosis and perforating vessels originating from the sac. Only limited experiences from case reports or small mixed series including thrombosed aneurysms in different locations are available in literature. To systematically review all the pertinent literature, a comprehensive literature review with the search terms “MCA, aneurysm, and thrombosis” and a pooled analysis including our institutional series were performed. We evaluated demographics, ruptured status, aneurysm morphology, topography and size, thrombosis extension, treatment, complications, final occlusion rate, and clinical outcome at follow-up. Data were individually extracted for each patient and included in a pool for the statistical analysis. Forty-two articles published between 1992 and 2016 were selected, including a total of 115 patients. Most of thrombosed aneurysms were saccular (67.6%), large or giant (86.7%), and located at the MCA bifurcation (67.3%). The treatment of choice was surgery in more than 80% of cases compared with the endovascular techniques, though the overall percentage of complications reported in the two groups was similar and around 20% of cases. Clinical outcome was favorable in more than 85% of patients after treatment. This is the first systematic review focusing on treatment and outcome of thrombosed MCA aneurysms. Our data depict their main angioarchictectural and clinical characteristics, proving the feasibility of their treatment with good prognosis in a high percentage of patients. However, complication and mortality rates of about 20 and 3.5%, respectively, are not negligible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thrombosed aneurysms of the middle cerebral artery (MCA) account for 63% of all giant MCA aneurysms [47].
They usually show complex morphology, large dimensions, neck sclerosis, and efferent or perforating vessels originating from the sac. Clinical onset is often characterized by mass effect and brain edema. Due to these peculiar features, traditional clipping or simple endovascular coiling are generally not adequate to treat these aneurysms, but the thrombus debulking is necessary to reduce the mass effect and to exclude the sac.
Only a few neurosurgical centers have a sufficient expertise to manage these complex cases, and large institutional experiences are almost absent in literature. By an extensive search, we retrieved only a small series reporting heterogeneous information on thrombosed aneurysms in different locations of the Willis circle [13, 35, 47, 56] and mixed series including thrombosed and non-thrombosed MCA aneurysms [5, 43]. Moreover, most of these papers are case reports.
Accordingly, a detailed information about treatment, complications, and short- and long-term clinical outcomes of thrombosed MCA aneurysms are currently missing.
In this paper, we performed a comprehensive review of the literature and a pooled analysis of demographics, morphology, topography, ruptured status, types of treatment, and clinical and radiological outcomes of patients who underwent treatment of thrombosed MCA aneurysms. Our institutional series was also included in the pooled analysis.
Materials and methods
Literature review
A comprehensive review of the literature was performed with the following keywords “(middle cerebral artery and aneurysm and (thrombosed or thrombotic))” to be searched in PubMed and Scopus databases.
The search strategy followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement where applicable, and this checklist was used in designing and reporting our review.
The last search was launched in March 2017.
All studies reporting patients who underwent surgical or endovascular treatments of partially or totally thrombosed MCA aneurysms were first included in the database.
Two reviewers (A.S. and G.S.) independently collected data from the included articles. Any differences were resolved by consensus discussed with a third author (C.L.S.).
Then, English studies reporting detailed information regarding operative procedures (both surgical and endovascular), perioperative and postoperative complications, and aneurysm occlusion rates were included in the following analysis.
Two pools of patients including all case reports and clinical series were initially collected accordingly to the different treatment options (surgery or endovascular embolization), and the data extracted were analyzed (a) separately for each cohort, (b) comprehensively, and (c) comparatively with the largest series.
Clinical series where no possible individual data can be extracted for each patient were excluded by our analysis.
We evaluated the following variables: demographics, ruptured status, morphology, topography and size of the aneurysms, extension of the thrombosis, modality of the treatment, complications, occlusion rate, and clinical outcome at follow-up.
Periprocedural complications studied were the occurrence of transient or permanent intraprocedural thrombosis of the efferent branch hesitating in transient hemiparesis or stroke and intraoperative rupture.
Concerning the localization, we distinguished the aneurysms according to MCA segment involved: bifurcation, M1, M2, and M3. Aneurysms were also divided in small (< 1 cm), large (> 1 < 2.5 cm) and giant (> 2.5 cm) according to those reported by the investigators.
Radiological outcomes for surgery or endovascular procedures were stratified into three levels based on the degree of angiographic aneurysm obliteration. We distinguished: (1) complete/near-complete occlusion (> 95%), defined as a lack of angiographic filling of the sac and the neck or no filling of the sac but with small residual neck filling; (2) partial occlusion (< 95%), defined as persistent angiographic filling of a small portion of the sac; and (3) incomplete occlusion (< 90%), defined as a persistent angiographic filling of a significant portion of the sac.
Finally, clinical outcome was assessed through the Glasgow Outcome Scale (GOS) and the modified Rankin Scale (mRS).
Institutional series
All pertinent data of patients who underwent a surgical or endovascular treatment for partially or totally thrombosed MCA aneurysms between January 2006 and December 2016 at the “A. Gemelli” Hospital, Catholic University School of Medicine of Rome, were included in the database and pooled with those retrieved in literature.
Statistical analysis
Data were individually extracted for each patient and included in a pool. Percentage of incidence and 95% confidence intervals (CI) were calculated for all considered variables and outcomes.
Quantitative variables were expressed as mean ± standard deviation, and Student’s t test was used to compare their means. Fisher’s exact test (two-sided) was used to compare categorical variables. Statistical significance was assessed at α < 0.05, and the Bonferroni correction for multiple comparisons was applied for each outcome analysis.
Results
Institutional series
Five patients were retrieved from the database of our tertiary referral center for neurovascular surgery: “Agostino Gemelli Hospital”—Catholic University School of Medicine of Rome. Two of them were males and three females with a mean age of 51.8 ± 20.3 years.
Three patients harbored a left MCA aneurysm and two a right one (Figs. 1, 2, and 3). Three aneurysms were saccular (60%), one fusiform, and one dissecting. Two were located at the bifurcation, one on the M1 segment, and 2 on M2; five were large and one giant. All aneurysms were partially thrombosed.
Preoperative CT-scan and 3D-DSA (a, b) of a 68-year-old woman with a large thrombosed left MCA aneurysm, originating from the temporal branch, oriented frontally and inferiorly. Patient underwent a left pterional craniotomy, followed by a proximal-to-distal Sylvian fissure opening to secure proximal control; an intra-aneurysmal debulking was performed with ultrasonic aspirator, and the reconstruction of the vessel wall was obtained with a tandem of titanium clips. Postoperative angio-CT scan and 3D-DSA (c, d) showing a complete aneurysmal exclusion and the patency of the distal branches
Preoperative CT scan (a) and DSA (b) of a 41-year-old man with a giant left thrombosed MCA aneurysm originating from a frontal branch, surrounded by perilesional oedema. The aneurysm was dissected through a pterional approach, and proximal control was obtained at the carotid bifurcation, then, the aneurysm was opened and the thrombus was completely debulked with ultrasonic aspirator, paying attention to not extend beyond the endothelium of the neck. Once the aneurysmal neck was identified, the redundant aneurysmal wall was resected and the vessel wall reconstructed with two titanium clips. Postoperative CT scan (c) and DSA (d) showed the complete aneurysm exclusion and the preservation of the distal MCA flow
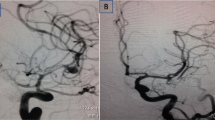
Preoperative DSA (a) showing a giant right MCA bifurcation aneurysm, previously treated with GDCs and partially thrombosed. The aneurysm appears re-perfused due to coil compaction in the super-lateral portion of the sac (type 6 according to Lawton’s classification). Two bifurcation branches originated from aneurysmal neck. The patient underwent aneurysm debulking with ultrasonic aspirator saving the neck endothelium; the redundant aneurysmal wall was resected, and neck reconstruction was performed with a tandem of titanium clips. No thrombization of the parent vessel or the distal branches occurred. Postoperative DSA (b) showed the complete aneurysmal exclusion and the preservation of the distal MCA flow
Three aneurysms were treated with clipping reconstruction and thrombus debulking; one underwent trapping, thrombus debulking, and STA-MCA bypass; and one showed spontaneous thrombosis after STA-MCA bypass.
Only one case of stroke was observed after clipping reconstruction, but the outcome was closely influenced from the Hunt-Hess grade at clinical onset. A complete occlusion was maintained for all the aneurysms at a mean radiological follow-up of 6.1 ± 6.1 years.
Data from our institutional series were pooled with those retrieved from literature in order to provide a meta-analysis.
Systematic review: study selection and characteristics
According to our search strategy, 753 articles in English language were retrieved through the electronic literature search.
Six hundred and twenty-nine were primarily excluded by abstract reading, while 124 were assessed for eligibility and analyzed in detail, since they met our inclusion criteria.
After full-text reading and a forward search from the bibliography of the selected papers, 82 articles were excluded because 56 of them did not report the thrombosed status of the aneurysm, 17 did not discuss about treatment, and 9 reported aneurysms not originating from MCA (Fig. 4).
Forty-two articles published between 1992 and 2016, which reported patients treated for thrombosed MCA aneurysms were included in this review. Two papers reported a series larger than 40 patients [13, 47], five case series between two and nine patients [24, 28, 42, 54, 56], and thirty-five were case reports [1,2,3, 6, 7, 9, 11, 14, 18, 20,21,22, 25,26,27, 29,30,31,32, 34, 37, 38, 41, 44,45,46, 48,49,50,51, 57, 60,61,62,63]. However, one of the two larger series reported pooled data about thrombosed and non-thrombosed MCA aneurysms, which could not be separately analyzed [47]. Therefore, it was considered only for a comparison with the results of our meta-analysis. Instead, the data extracted from the second larger series were mostly analyzable separately and included in our meta-analysis database [13].
Overall, 110 patients were collected from literature analysis and 5 from our institutional series, for a total of 115 cases. Among them, 20 patients underwent endovascular treatment, whereas 95 underwent open surgery (Table 1).
Demographics
Age was reported in all patients. Mean age of patients was 50.6 years (range, 1–78 years). Sex was reported in all patients, and the male/female ratio was 1.26 (64/51).
Morphology, topography, and thrombosed status of the aneurysms
Endovascular-treated group (20 patients)
Localization of the aneurysms on the MCA segment was reported in 19 out of 20 patients (95%; 95% CI, 76.39–99.11). The most common location was the MCA bifurcation in 47.37% out of cases (9/19; 95% CI, 27.33–68.29), followed by the M1 segment in 36.84% (7/19; 95% CI, 19.15–58.96), the M2 in 10.53% (2/19; 95% CI, 2.94–31.39), and the M3 in 5.26% (1/19; 95% CI, 0.94–24.64; Table 1).
Aneurysm size was reported in all patients. Seventy percent were giant (14/20; 95% CI, 48.1–85.45); 20% (4/20; 95% CI, 8.07–41.6) were large, and 10% (10% 2/20; 95% CI, 2.79–30.1) were small.
Approximately, the 52.63% of the aneurysms were fusiform (10/19; 95% CI, 31.71–72.67) and 47.37% were saccular (9/19; 95% CI, 27.33–68.29).
Partial thrombosis was reported in 95% of the aneurysms (19/20; 95% CI, 76.39–99.11) and total thrombosis in 5% (1/20; 95% CI, 0.89–23.61).
Surgical-treated group (95 patients)
Localization of the aneurysms on the MCA segment was reported in 86/95 out of patients (90.53%; 95% CI, 82.97–94.94). The most common location was the MCA bifurcation in 73.26% (63/86; 95% CI, 63.05–81.47), followed by M1 in 8.14% (7/86; 95% CI, 4.0–15.86), M2 in 13.95% (12/86; 95% CI, 8.17–22.82), and M3 in 4.65% (4/86; 95% CI, 1.82–11.36; Table 1).
Aneurysm size was reported in 93/95 out of cases (97.89%; 95% CI, 92.65–99.42). About 56.99% of patients harbored giant thrombosed aneurysms (53/93; 95% CI, 46.85–66.58), the 40.86% showed large aneurysms (38/93; 95% CI, 31.43–51.02), and the 2.15% small aneurysms (2/93; 95% CI, 0.59–7.51).
Details about morphology were reported in 86/95 out of aneurysms (90.53%; 95% CI, 82.97–94.94): 72.09% were saccular (62/86; 95% CI, 61.83–80.47), 22.09% were fusiform (19/86; 95% CI, 14.62–31.95), 4.65% dissecting (4/86; 95% CI, 1.82–11.36), and 1.16% serpentine (1/86; 95% CI, 0.21–6.3).
Partial thrombosis was reported in about 93.62% out of aneurysms (88/94; 95% CI, 86.77–97.04), while a subtotal and a total thrombosis were both reported in 3.19% (3/94; 95% CI, 1.09–8.97).
Ruptured status
Endovascular-treated group (20 patients)
Unruptured aneurysms (HH = 0) were reported in 80% out of patients (16/20; 95% CI, 58.4–91.93), whereas ruptured aneurysms (with HH = 1–2) was described in 20% (4/20; 95% CI, 8.07–41.60; Table 1).
Surgical-treated group (95 patients)
Unruptured aneurysms (HH = 0) were found in about 27.37% out of patients (26/95; 95% CI, 19.41–37.08), while ruptured aneurysms in 72.63% (with HH = 1–4) (69/95; 95% CI, 62.92–80.59; Table 1).
Type of treatment and occlusion rate
Endovascular-treated group (20 patients)
Twenty patients out of 115 (17.39%; 95%CI 11.55–25.34) underwent endovascular treatment.
Coiling stand-alone was the procedure of choice in 65% out of patients (13/20; 95% CI, 43.29–81.88), followed by stent or stent assisted coiling in 30% (6/20; 95% CI, 14.55–51.9), and trapping in 5% (1/20; 95% CI, 0.89–23.61).
The percentage of occlusion was reported in all patients. A total occlusion was described in 65% out of cases (13/20; 95% CI, 43.29–81.88), a subtotal occlusion in 25% (5/20; 95% CI, 11.19–46.87), and a partial occlusion in 10% (2/20; 95% CI, 2.79–30.1).
Surgical-treated group (95 patients)
Ninety-five patients out of 115 (82.61%; 95% CI, 74.66–88.45) underwent surgery. About 63.16% out of them (60/95; 95% CI, 53.12–72.17) underwent clipping reconstruction, about 2.11% (2/95; 95% CI, 0.58–7.35) were treated with a bypass, 10.53% (10/95; 95% CI, 5.82–18.3) with trapping with no by-pass, 18.95% (18.95%; 95% CI, 12.33–27.97) with trapping with bypass, and about 5.26% (5/95; 95% CI, 2.27–11.73) with wrapping.
The percentage of exclusion was reported only in 36 out of 95 patients (37.89%; 95% CI, 28.79–47.94). Among them, a total exclusion was described in about 94.44% out of cases (34/36; 95% CI, 81.86–98.46), and a subtotal exclusion in 5.56% (2/36; 95% CI, 1.54–18.14).
Complications and clinical outcomes
Endovascular-treated group (20 patients)
The occurrence of complications after endovascular procedures was reported overall in 20% out of patients: in particular, a permanent hemiparesis was reported in 15% (3/20; 95% CI, 5.24–36.04) and a transient hemiparesis in 5% (1/20; 95% CI, 0.89–23.61), whereas no complications were reported in 80% out of cases (16/20; 95% CI, 58.4–91.93).
Clinical and radiological outcomes were reported in 19/20 out of patients (95%; 95% CI, 76.39–99.11), with a mean follow-up of 22.6, and 15.5 months, respectively. According to GOS, a good outcome (GR or MD) was described in 89.47% out of cases (17/19; 95% CI, 68.61–97.06) out of cases, and a SD in 10.53% (2/19; 95% CI, 2.94–31.39). In agreement, the same 89.47% out of patients showed a mRS score between 0 and 2 and 10.53% between 3 and 4. Fatal complications were not reported in any case.
Surgical-treated group (95 patients)
The occurrence of complications after surgery was overall reported in 20.22% out of patients (18/89; 95% CI, 13.19–29.87). A transient hemiparesis was reported in 6.74% (6/18; 95% CI, 3.13–13.94), and a permanent hemiparesis in 5.62% (5/18; 95% CI, 2.42–12.49), while an intraoperative aneurysm rupture was reported in 7.87% out of cases (7/18; 95% CI, 3.86–15.36). No complications were described in 79.78% out of patients (71/95; 95% CI, 70.28–86.81).
Clinical and radiological outcomes were reported in about 98.95% out of cases (94/95; 95% CI, 94.28–99.81), with a mean follow-up of 17.8 and 11.5 months, respectively.
According to GOS, a good outcome (GR and MD) was reported in 86.17% out of patients (81/94; 95% CI, 77.76–91.74) and a poor outcome (SD, VS, and D) in 13.83% (13/94; 95% CI, 8.26–22.24; Table 1). In agreement, the same 86.17% out of patients showed a mRS score between 0 and 2, 9.57% a mRS between 3 and 4, and 4.26% between 5 and 6. The mortality rate was 4.26% (4/94 out of patients; 95% CI, 1.67–10.44).
Outcomes comparison between patients with saccular and fusiform aneurysms
Patients were divided according to the aneurysm morphology in saccular and fusiform (also including the four dissected aneurysms, which were morphologically fusiform); the only serpentine aneurysm was excluded from this dichotomic analysis (Table 2).
A significantly higher percentage of saccular aneurysms involved the MCA bifurcation (87.32 vs. 23.07%; p = 0.0001); on the other hand, fusiform aneurysms were significantly more frequent on M1 and M2 segments.
Ruptured aneurysms were significantly more frequent among saccular (78.87 vs. 21.21%), whereas the fusiform ones were more frequently unruptured (78.78 vs. 21.12%; p = 0.0001).
Surgery was the treatment of choice in 87.32% of saccular aneurysms compared with 69.69% of the fusiform ones, while endovascular treatment was preferred in 30.30% of fusiform aneurysms compared with 12.67% of the saccular ones; however, this difference only showed a trend of significance. Rate of complications and clinical outcome did not significantly differ between the two groups.
Outcomes comparison between patients with ruptured and unruptured aneurysms
Patients were also divided according to the ruptured status of the aneurysms (Table 3). The percentage of saccular aneurysms was significantly higher among ruptured ones (77.77 vs. 45.45%; p = 0.0016), whereas fusiform aneurysms were significantly more frequent among non-ruptured (48.48 vs. 18.05%; p = 0.002). The percentage of dissecting and serpentine aneurysms were similar between the two groups.
In addition, ruptured aneurysms were significantly more frequent at the MCA bifurcation compared with the other MCA segments (87.67 vs. 46.87%; p = 0.0001).
Concerning the size, the percentage of large ruptured aneurysms was higher than that of large unruptured (44.4 and 24.3%); instead, there were no differences by comparing small and giant aneurysms.
Surgical treatment was the treatment of choice in ruptured aneurysms (94.72 vs. 41.93%), whereas the endovascular approach was mostly preferred in unruptured ones (p = 0.0001). Nevertheless, the angiographic outcomes were comparable between the two groups.
The total rate of treatment related complications was slightly higher for ruptured aneurysms (26 vs. 20%), but a significantly higher incidence of a new postoperative hemiparesis was reported in patients treated for unruptured aneurysms (p = 0.0098). However, the outcome was not significantly different between the two groups.
Outcomes comparison between patients who underwent surgery or endovascular treatment
Patients were finally divided according to the treatment in those who underwent surgery (including clipping, bypass stand-alone, trapping + bypass, trapping stand-alone, wrapping) and endovascular treatment (including stenting, stent-assisted coiling, trapping, Table 1).
Surgery was the treatment of choice for ruptured aneurysms, while the endovascular procedures were preferably adopted for the unruptured ones, particularly when fusiform, and regardless of their size. There were no different rates of complications and clinical outcome between the two treatment modalities.
Discussion
Thrombosed intracranial aneurysms are heterogeneous lesions with complex vascular anatomy, and the MCA is considered a very common site for their formation, with a frequency of up to 41% [23].
Spontaneous thrombosis of saccular aneurysms may occur up to 40% of giant aneurysms, which eventually become symptomatic due to mass effect or stroke. On the other hand, spontaneous thrombosis of non-giant (< 25 mm) saccular aneurysm is rarer [9].
Black and German demonstrated the relationship among aneurysmal volume, aneurysmal orifice (neck size), and intra-aneurysmal thrombus formation by biophysical and hemodynamic studies. A larger volume/orifice ratio causes slower flow and longer blood retention time, resulting in intra-aneurysmal thrombosis [4]. Hence, this may be a reason why giant intracranial aneurysms have a higher incidence of thrombus formation compared with the smaller ones.
This kind of aneurysms often possess complex characteristics like morphology (fusiform or serpentine), origin of MCA branches, and mural calcifications. They can clinically present with rupture or, if unruptured, they can cause mass effect due to their dimension or perilesional edema. They can also cause ischemic stroke secondary to distal embolization. When dealing with a thrombotic aneurysm, the standard surgical clipping techniques collide with the solid mass caused by the thrombus, requiring the surgeon to deploy more risky and complex strategies such as trapping the aneurysm with or without revascularization of downstream regions or removing the thrombus and reconstructing the neck with one or more clips.
In recent years, the interest of the literature on this topic has been progressively increasing, but the majority of the published papers are case reports, small series reporting on thrombosed aneurysms in different locations, and mixed series including thrombosed and non-thrombosed MCA aneurysms.
Our paper is the first systematic review focusing on thrombosed aneurysms involving the MCA. We collected the data from literature and from our own experience, including a total of 115 thrombosed MCA aneurysms. Then we performed a pooled analysis of demographics, angioarchitectural features, treatments, and outcomes.
We found that most of the thrombosed MCA aneurysms had a saccular morphology (67.6%) and large or giant dimensions (86.7%). The thrombosis was partial in almost all cases (96.1%). From the topographic point of view, more than 2/3 of these aneurysms were located at the MCA bifurcation (67.3%), suggesting underlying flow-related factors involved in the pathophysiology similar to those reported for non-thrombosed aneurysms [58, 59].
The most common angioarchitectural features of thrombosed MCA aneurysms found in this pooled analysis appeared in agreement to those already reported in the two largest published series by Lawton et al. [35] and Nurminem et al. [47], who respectively described 11 and 26 cases, with a prevalence of saccular aneurysms, partially thrombosed, and mainly located at the bifurcation.
In particular, we found that the saccular aneurysms were those more frequent at the MCA bifurcation, whereas the fusiform ones primarily involved the distal MCA segments (p > 0.02; Table 2). The percentage of ruptured aneurysms was significantly higher among the saccular compared with the fusiform (p = 0.0001).
Pathophysiology of aneurysms’ thrombosis
During aneurysms’ formation, the occurrence of thrombosis represents a healing mechanism by which the aneurysm cavity may be occluded and the parent artery remodeled [39]. Its role is therefore to form a plug on the injured vessel to prevent rupture and allow a tissue remodeling. In fact, the absence of wall calcifications and intraluminal thrombosis were associated with increased risk of rupture of giant aneurysms in a previous study [12].
The mechanisms leading to thrombosis depend on biochemical factors, morphometric characteristics of the aneurysm, and the related blood flow pattern. Several major points involve the triggering of specific red blood cells—platelet dynamics along with a complex network of wall proteins interactions [15], and the production of thrombin by the endothelial cells exposed to a low wall shear stress [39].
Computational fluid dynamics (CFD) models provided detailed analysis of flow (pressure, velocity, wall shear stress) in aneurysms [55]. Slow recirculating flows may cause extended residence time and lead to compromised mass transfer, potentially causing blood coagulation [58, 59]. The pulsatility influences the flow patterns changing the recirculating flow regions over the cardiac cycle. Moreover, the formation of thrombus may occur over a short time rather than as a slow layering process [52].
From a clinical point of view, spontaneous thrombosis is found more frequently in giant aneurysms than in smaller ones [8, 33]. Clotting can either facilitate the rupture through further vascular wall degradation or stabilize the sac by occluding the aneurysm and preventing rupture [8, 36, 52]. In giant aneurysms, the thrombus more often predispose to distal thrombo-embolism or increased the mass effect due to the distension of the aneurysmal wall, rather than prevent its rupture [10, 52, 65].
Despite continuous improvement in understanding the thrombosis physiopathology, there is still a great difficulty to predict its occurrence and progression due to the interdependence between the biochemical, morphological, and flow-related factors.
Treatment considerations
Despite the advancement and refinement of microsurgical and endovascular techniques, the treatment of thrombosed aneurysms of the MCA is still a challenging task.
The morphology (saccular or fusiform) and the anatomic relationship among the aneurysmal wall, thrombus position, and residual lumen represent the main criteria adopted for the classification of thrombosed aneurysms.
Lawton et al. distinguished six types of thrombosed aneurysms: type 1, characterized by saccular morphology with concentric thrombus; type 2, characterized by saccular morphology with eccentric thrombus; type 3, with saccular morphology with multiple lobes and lobulated thrombus; type 4, characterized by saccular morphology with complete thrombosis; type 5, characterized by fusiform or dolichoectatic morphology and canalized thrombus with an internal lumen; and type 6, a previously coiled saccular aneurysms with an incomplete thrombosis associated with compacted coils [35].
Similarly, Eliava et al. defined three types of thrombosed aneurysms: type I, characterized by partially thrombosed saccular aneurysms with thrombus extension into the neck; type II, characterized by partially thrombosed saccular aneurysms without thrombus in the neck; type III, characterized by partially thrombosed fusiform aneurysms [13].
The aim of these classifications is to identify categories suitable for different treatment strategies, such as direct clipping, thrombectomy-clip reconstruction, bypass occlusion, trapping, wrapping, or only observation. In fact, these surgical series represent the main experiences existing in literature [13, 35], whereas large series of endovascular treatment are not yet available, except for studies proposing combined approaches [47, 56].
In the series by Lawton et al., the management strategy was influenced by the thrombotic aneurysm type, but patient outcome was not, and the best results were observed in patients treated with direct clipping and bypass occlusion.
In our own experience, we found that the neck morphology or its remodeling by the thrombus extension are important risk factors by the surgical point of view. In fact, in the case of saccular aneurysms, the thrombus debulking usually stops at the narrow neck without damaging the endothelium of the parent vessel; then, the redundant aneurysmal wall is resected and the vessel wall is reconstructed with one or multiple clips. In the case of fusiform aneurysms or giant saccular aneurysms with larger neck size, the thrombus debulking may extend beyond the limit of the neck causing a de-endothelialization of the lumen of the parent vessels, which often may result in thrombosis of the efferent branch.
Our pooled analysis separately enrolled patients treated with surgery and endovascular procedures, overall showing that most of the patients reported in literature underwent surgery (82.6%).
Surgery was preferred by the most of authors as treatment of patients with ruptured aneurysms without comorbidities, in presence of (a) aneurysms with unfavorable angioarchitectural features, such as incorporation of key MCA branches or perforators emanating from the sac; (b) need for revascularization of the distal MCA territory in case of sacrifice of one of its major branches or for greatly atherosclerotic and calcified parent vessels; (c) need for thrombus debulking in cases of perianeurysmal edema, in order to relieve the mass effect on the cerebral parenchyma; and (d) in that cases considered untreatable by the endovascular point of view. In particular, an aneurysm trapping with or without bypass was performed in more than half of the surgeries (59.5%), while a simple clipping exclusion or clipping reconstruction in 31% out of cases.
Endovascular procedures are mainly performed on unruptured aneurysms located distal to the bifurcation, which were mostly fusiform, and rarely amenable to conventional clipping [13, 35].
The needful to relieve the aneurysm-related mass effect and to preserve the lenticulostriate arteries represent the two preponderant factors playing a role in the choice of treatment. The perianeurysmal edema, in fact, is a phenomenon frequently observed in giant thrombosed aneurysms (up to 66.7%), and its entity is related to the volume of the aneurysm [10], because the mass effect on the brain compresses the venous system, especially in case of MCA aneurysms, which are located into the Sylvian fissure. The presence of perianeurysmal edema rapidly aggravates the patient’s condition and increase the treatment morbidity [10, 17, 19]. Our data highlighted that edema is reported in more than 60% in giant MCA aneurysms, and it plays a very important role in the choice of treatment, encouraging a surgical approach.
The lenticulostriate territory, instead, is highly at risk of ischemia during the surgery of thrombosed aneurysms of MCA, especially in case of involvement of the M1 segment, because it lacks a collateral supply.
Therefore, the surgical strategy is mainly dictated by the presence or absence of perforators along the aneurysmal segment: if the aneurysm only displaces the perforators, a trapping can be safely performed; when the aneurysm involves the origin of the lenticulostriate arteries, an EC-IC interpositional bypass can be used to preserve the blood supply.
In order to avoid ischemic complications, temporary occlusion should be minimized, and a particular care should be taken during thrombectomy, with preservation of the neck endothelium, to reduce the risk of proximal and distal thrombosis and lenticulostriate occlusion. Eliava et al. also reported the injection of a fibrinolytic agent into thrombosed MCA branches as an effective method for the treatment of intraoperative thrombosis [13].
Nowadays, on the other hand, a traditional endovascular stenting still carries a high risk of an intrastent thrombosis, with consequent lenticulostriates occlusion, and large series and long-term angiographic follow-up of new intravascular devices for giant thrombosed MCA aneurysms are still unavailable [54, 56].
Efficacy and safety
Most of thrombosed aneurysms (particularly if large or giant) possess unfavorable features and in the past, have been considered untreatable or treatable with a high risk of poor clinical outcomes [64]. However, opinions changed in recent years: many vascular neurosurgeons now consider relatively feasible and effective surgery, endovascular or combined treatments [16, 40, 53].
In our pooled analysis, we found that more than 80% of the included patients underwent a surgical treatment, most of them (72.6%) after a SAH (p = 0.0001). An endovascular management was instead reported in about 80% of unruptured aneurysms. However, we did not find any significant difference in terms of complications and clinical outcome (GOS or mRS scores) between the two groups.
In the opinion of experienced authors, surgery is the primary therapy for most thrombotic aneurysms, whereas endovascular treatment remains a critical adjunct to surgery, particularly in occluding parent arteries and aneurysms after bypass, and it is preferable only in selected cases in which the surgical risks are deemed unacceptably high [35].
In fact, when primary surgical reconstruction of the parent vessel is not possible, for example in case of giant fusiform aneurysms or anatomic or functional limitations, such as aggressive swelling or critical branch proximity, combined approach with EC-IC bypass and endovascular coiling may provide better results [56].
Our review confirmed that nearly 50% of thrombosed MCA aneurysms reported in literature that underwent an endovascular management were part of a combined approach, and clinical outcomes between surgery and endovascular treatment were not significantly different.
Complications and clinical outcome
The overall percentages of complications reported in the two treatment groups were similar, and around 20% of cases.
A good outcome (GR/MD at GOS or mRS between 0 and 2) was instead reported in 85.2% of patients, supporting the idea that these aneurysms are treatable, although a rate of about 3.5% of mortality is not negligible.
These data are comparable with those reported in the series by Lawton et al. where neurological condition was unchanged or improved in more than 90% of patients after direct clipping or bypass occlusion and in more than 70% after thrombectomy plus clip reconstruction [35].
Strengths and limitations
This study presents several limitations. First, 35 of the included papers were case reports and 5 were case series including a small number of patients (from 2 to 9). In addition, the reported evidence is observational and non-comparative. Therefore, the proportions reported in this review only reflect the published cases and may be influenced by some forms of publication bias. Finally, before the 1990s, the endovascular procedures were not available.
However, among the major strengths is that the evaluation of long-term prognosis included in this study is rather reliable, since we were able to obtain the clinical outcome at follow-up in 113/115 of patients.
Conclusions
This is the first systematic review exclusively focusing on treatment and outcome of thrombosed MCA aneurysms. Data from our pooled analysis depict their main structural and clinical characteristics, proving that this kind of pathology is treatable with good prognosis in more than 85% of patients, mainly by surgery, and by endovascular techniques in selected cases.
References
Abiko M, Ikawa F, Ohbayashi N, Mitsuhara T, Nosaka R, Inagawa T (2009) Giant serpentine aneurysm arising from the middle cerebral artery successfully treated with trapping and anastomosis: case report. Neurol Med Chir (Tokyo) 49(2):77–80
Ahn JH, Phi JH, Kang HS, Wang KC, Cho BK, Lee JY, Kim GB, Kim SK (2010) A ruptured middle cerebral artery aneurysm in a 13-month-old boy with Kawasaki disease. J Neurosurg Pediatr 6(2):150–153
Biondi A, Jean B, Vivas E, le Jean L, Boch AL, Chiras J, van Effenterre R (2006) Giant and large peripheral cerebral aneurysms: etiopathologic considerations, endovascular treatment, and long-term follow-up. AJNR Am J Neuroradiol 27(8):1685–1692
Black SP, German WJ (1960) Observations on the relationship between the volume and the size of the orifice of experimental aneurysms. J Neurosurg 17:984–990
Cantore G, Santoro A, Guidetti G, Delfinis CP, Colonnese C, Passacantilli E (2008) Surgical treatment of giant intracranial aneurysms: current viewpoint. Neurosurgery 63(4 Suppl 2):279–289 discussion 289–90
Clarençon F, Nouet A, Redondo A, Di Maria F, Iosif C, Le Jean L, Chiras J, Sourour N (2014) Occlusion of M1 segment after superficial temporal artery-middle cerebral artery bypass in a giant M1 aneurysm with Onyx-34 injected via a double-lumen balloon under balloon inflation. J Neurointerv Surg 6(4):e27
Chen L, Yau I, deVeber G, Dirks P, Armstrong D, Krings T (2015) Evolution of a chronic dissecting aneurysm on magnetic resonance imaging in a pediatric patient. J Neurosurg Pediatr 15(2):192–196
Cohen JE, Itshayek E, Gomori JM et al (2007) Spontaneous thrombosis of cerebral aneurysms presenting with ischemic stroke. J Neurol Sci 254(1–2):95–98
Cohen JE, Rajz G, Umansky F, Spektor S (2003) Thrombosis and recanalization of symptomatic nongiant saccular aneurysm. Neurol Res 25(8):857–859
Dengler J, Maldaner N, Bijlenga P, Burkhardt JK, Graewe A, Guhl S, Hong B, Hohaus C, Kursumovic A, Mielke D, Schebesch KM, Wostrack M, Rufenacht D, Vajkoczy P, Schmidt NO, Giant Intracranial Aneurysm Study Group (2015) Perianeurysmal edema in giant intracranial aneurysms in relation to aneurysm location, size, and partial thrombosis. J Neurosurg 123(2):446–452
de Oliveira JG, Borba LA, Rassi-Neto A et al (2009) Intracranial aneurysms presenting with mass effect over the anterior optic pathways: neurosurgical management and outcomes. Neurosurg Focus 26(5):E3
dos Santos ML, Spotti AR, dos Santos RM et al (2013) Giant intracranial aneurysms: morphology and clinical presentation. Neurosurg Rev 36(1):117–122 discussion 122
Eliava S, Pilipenko Y, Shekhtman O, Konovalov A (2016) Reversal of intraoperative arterial thrombosis with a fibrinolytic agent when treating large and giant partially thrombosed aneurysms of the middle cerebral artery. J Neurosurg 124(4):1114–1122
Esposito G, Albanese A, Sabatino G, Scerrati A, Sturiale C, Pedicelli A, Pilato F, Maira G, di Lazzaro V (2011) Large middle cerebral artery dissecting aneurysm mimicking hemorrhagic stroke. Clin Neurol Neurosurg 113(10):901–903
Fogelson AL, Neeves KB (2015) Fluid mechanics of blood clot formation. Annu Rev Fluid Mech 47:377–403
Gonzalez NR, Duckwiler G, Jahan R, Murayama Y, Vinuela F (2008) Challenges in the endovascular treatment of giant intracranial aneurysms. Neurosurgery 62(6 Suppl 3):1324–1335
Halbach VV, Higashida RT, Dowd CF, Barnwell SL, Fraser KW, Smith TP, Teitelbaum GP, Hieshima GB (1994) The efficacy of endosaccular aneurysm occlusion in alleviating neurological deficits produced by mass effect. J Neurosurg 80(4):659–666
Hayashi Y, Shima H, Kinoshita M, Nakada M, Miyashita K, Hamada J (2009) Ossified peripheral middle cerebral artery aneurysm in a 30-year-old man. J Clin Neurosci 16(8):1075–1077
Heros RC, Kolluri S (1984) Giant intracranial aneurysms presenting with massive cerebral edema. Neurosurgery 15(4):572–577
Hoit DA, Malek AM (2006) Fusion of three-dimensional calcium rendering with rotational angiography to guide the treatment of a giant intracranial aneurysm: technical case report. Neurosurgery 58(1 Suppl):ONS-E173 discussion ONS-E173
Horie N, Takahashi N, Furuichi S, Mori K, Onizuka M, Tsutsumi K, Shibata S (2003) Giant fusiform aneurysms in the middle cerebral artery presenting with hemorrhages of different origins. Report of three cases and review of the literature. J Neurosurg 99(2):391–396
Horowitz MB, Yonas H, Jungreis C, Hung TK (1994) Management of a giant middle cerebral artery fusiform serpentine aneurysm with distal clip application and retrograde thrombosis: case report and review of the literature. Surg Neurol 41(3):221–225
Huttunen T, von und zu Fraunberg M, Frosen J et al (2010) Saccular intracranial aneurysm disease: distribution of site, size, and age suggests different etiologies for aneurysm formation and rupture in 316 familial and 1454 sporadic eastern Finnish patients. Neurosurgery 66(4):631–638 discussion 638
Imai H, Watanabe K, Miyagishima T, Yoshimoto Y, Kin T, Nakatomi H, Saito N (2016) The outcome of a surgical protocol based on ischemia overprotection in large and giant aneurysms of the anterior cerebral circulation. Neurosurg Rev 39(3):505–517
Jeong SM, Kang SH, Lee NJ, Lim DJ (2010) Stent-assisted coil embolization for the proximal middle cerebral artery fusiform aneurysm. J Korean Neurosurg Soc 47(5):406–408
Jeong YH, Kim JY, Koo YM, Choi JW, Whang K, Hu C, Cho SM (2016) Endovascular treatment of Giant serpentine aneurysm of the middle cerebral artery. J Cerebrovasc Endovasc Neurosurg 18(3):264–270
Kato M, Kaku Y, Okumura A, Iwama T, Sakai N (2005) Thrombosed unruptured cerebral aneurysm causing brain infarction followed by subarachnoid hemorrhage—case report. Neurol Med Chir (Tokyo) 45(9):472–475
Kato N, Prinz V, Finger T, Schomacher M, Onken J, Dengler J, Jakob W, Vajkoczy P (2013) Multiple reimplantation technique for treatment of complex giant aneurysms of the middle cerebral artery: technical note. Acta Neurochir 155(2):261–269
Kim HJ, Lee SW, Lee TH, Kim YS (2015) Huge intramural hematoma in a thrombosed middle cerebral artery aneurysm: a case report. J Cerebrovasc Endovasc Neurosurg 17(3):234–238
Kim YJ, Jeun SS, Park JH (2015) Thrombosed large middle cerebral artery aneurysm mimicking an intra-axial brain tumor: case report and review of literature. Brain Tumor Res Treat 3(1):39–43
Koksal V, Kayaci S (2016) Unexpected rupture of a Giant lobulated thrombotic middle cerebral artery aneurysm and emergency surgical treatment with Thrombectomy: a case report and review of the literature. Iran Red Crescent Med J 18(8):e30608
Kuhn AL, Hou SY, Spilberg G, Wakhloo AK (2014) Visualization of a small hidden intracranial aneurysm during endovascular thrombectomy for acute MCA occlusion. J Vasc Interv Neurol 7(1):47–49
Kulcsar Z, Ugron A, Marosfoi M, Berentei Z, Paal G, Szikora I (2011) Hemodynamics of cerebral aneurysm initiation: the role of wall shear stress and spatial wall shear stress gradient. AJNR Am J Neuroradiol 32(3):587–594
Lasjaunias P, Wuppalapati S, Alvarez H, Rodesch G, Ozanne A (2005) Intracranial aneurysms in children aged under 15 years: review of 59 consecutive children with 75 aneurysms. Childs Nerv Syst 21(6):437–450
Lawton MT, Quinones-Hinojosa A, Chang EF, Yu T (2005) Thrombotic intracranial aneurysms: classification scheme and management strategies in 68 patients. Neurosurgery 56(3):441–454 discussion 441–54
Lee D, Yuki I, Murayama Y, Chiang A, Nishimura I, Vinters HV, Wang CJ, Nien YL, Ishii A, WU BM, Viñuela F (2007) Thrombus organization and healing in the swine experimental aneurysm model. Part I. A histological and molecular analysis. J Neurosurg 107(1):94–108
Lee YJ, Kim DJ, Suh SH, Lee SK, Kim J, Kim DI (2005) Stent-assisted coil embolization of intracranial wide-necked aneurysms. Neuroradiology 47(9):680–689
Lenthall R, Rodesch G (2001) Complete thrombosis of a giant distal middle cerebral artery aneurysm. Interv Neuroradiol 7(3):263–267
Malaspinas O, Turjman A, Ribeiro de Sousa D, Garcia-Cardena G, Raes M, Nguyen PTT, Zhang Y, Courbebaisse G, Lelubre C, Zouaoui Boudjeltia K, Chopard B (2016) A spatio-temporal model for spontaneous thrombus formation in cerebral aneurysms. J Theor Biol 394:68–76
Martin NA (1998) The combination of endovascular and surgical techniques for the treatment of intracranial aneurysms. Neurosurg Clin N Am 9(4):897
Maruya J, Nishimaki K, Minakawa T (2011) Hyperperfusion syndrome after neck clipping of a ruptured aneurysm on a dolichoectatic middle cerebral artery. J Stroke Cerebrovasc Dis 20(3):260–263
McLaughlin N, Bojanowski MW (2008) Unruptured cerebral aneurysms presenting with ischemic events. Can J Neurol Sci 35(5):588–592
Mortimer AM, Bradley MD, Mews P, Molyneux AJ, Renowden SA (2014) Endovascular treatment of 300 consecutive middle cerebral artery aneurysms: clinical and radiologic outcomes. AJNR Am J Neuroradiol 35(4):706–714
Mrak G, Duric KS, Nemir J (2016) Middle cerebral artery fusiform aneurysm presented with stroke and delayed subarachnoid hemorrhage trapping, thrombectomy, and bypass. Surg Neurol Int 7(Suppl 9):S209–S213
Nussbaum L, Defillo A, Zelensky A, Nussbaum ES (2011) A short segment intracranial-intracranial jump graft bypass followed by proximal arterial occlusion for a distal MCA aneurysm. Surg Neurol Int 2:98
Nguyen HS, Doan N, Eckardt G et al (2015) A completely thrombosed, nongiant middle cerebral artery aneurysm mimicking an intra-axial neoplasm. Surg Neurol Int 6:146
Nurminen V, Lehecka M, Chakrabarty A et al (2014) Anatomy and morphology of giant aneurysms—angiographic study of 125 consecutive cases. Acta Neurochir 156:1):1–1)10
Parenti G, Fiori L, Marconi F (1992) Intracranical aneurysm and cerebral embolism. Eur Neurol 32(4):212–215
Passacantilli E, Anichini G, Cannizzaro D et al (2013) Awake craniotomy for trapping a giant fusiform aneurysm of the middle cerebral artery. Surg Neurol Int 4:39
Pavesi G, Dimitriadis S, Baroni S, Vallone S, Valzania F, Costella GB, Feletti A (2015) Intraoperative functional and perfusion monitoring during surgery for Giant serpentine middle cerebral artery aneurysms. World Neurosurg 84(2):592.e15–592.e21
Pumar JM, Lete I, Pardo MI, Vazquez-Herrero F, Blanco M (2008) LEO stent monotherapy for the endovascular reconstruction of fusiform aneurysms of the middle cerebral artery. AJNR Am J Neuroradiol 29(9):1775–1776
Rayz VL, Boussel L, Lawton MT, Acevedo-Bolton G, Ge L, Young WL, Higashida RT, Saloner D (2008) Numerical modeling of the flow in intracranial aneurysms: prediction of regions prone to thrombus formation. Ann Biomed Eng 36(11):1793–1804
Ross IB, Weill A, Piotin M, Moret J (2008) Endovascular treatment of distally located giant aneurysms. Neurosurgery 62(6 Suppl 3):1354–1360
Sano H, Kato Y, Shankar K et al (1998) Treatment and results of partially thrombosed giant aneurysms. Neurol Med Chir (Tokyo) 38(Suppl):58–61
Sforza DM, Putman CM, Cebral JR (2009) Hemodynamics of cerebral aneurysms. Annu Rev Fluid Mech 41:91–107
Shi ZS, Ziegler J, Duckwiler GR et al (2009) Management of giant middle cerebral artery aneurysms with incorporated branches: partial endovascular coiling or combined extracranial-intracranial bypass—a team approach. Neurosurgery 65(6 Suppl):121–129 discussion 129–31
Smrcka M, Ogilvy C, Koroshetz W (2002) Small aneurysms as a cause of thromboembolic stroke. Bratisl Lek Listy 103(7–8):250–253
Steiger HJ, Poll A, Liepsch D, Reulen HJ (1987) Haemodynamic stress in lateral saccular aneurysms. An experimental study. Acta Neurochir 86(3–4):98–105
Steinman DA (2002) (2002) image-based computational fluid dynamics modeling in realistic arterial geometries. Ann Biomed Eng 30(4):483–497
Sugita M, Kinouchi H, Nishiyama Y, Kanemaru K, Yoshioka H, Horikoshi T (2009) Direct clipping of a thrombosed giant cerebral aneurysm after thrombectomy without bleeding to minimize the temporary occlusion time-technical case report. Neurol Med Chir (Tokyo) 49(12):600–603
Tecle NEE, Zammar SG, Hamade YJ, Ahmadieh TYE, Aoun RJN, Nanney AD, Batjer HH, Dumanian GA, Bendok BR (2016) Use of a harvested radial artery graft with preservation of the vena comitantes to reduce spasm risk and improve graft patency for extracranial to intracranial bypass: technical note. Clin Neurol Neurosurg 142:65–71
Wajnberg E, Silva TS, Johnson AK, Lopes DK (2014) Progressive deconstruction: a novel aneurysm treatment using the pipeline embolization device for competitive flow diversion: case report. Neurosurgery 10(Suppl 1):E161–E166 discussion E166
Wakui K, Kamijo Y, Seguchi K, Sakai T (1992) Thrombosed aneurysm of the middle cerebral artery with occlusion of the distal parent artery—case report. Neurol Med Chir (Tokyo) 32(11):842–845
Wiebers DO, Whisnant JP, Huston J 3rd et al (2003) Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362(9378):103–110
Whittle IR, Dorsch NW, Besser M (1982) Spontaneous thrombosis in giant intracranial aneurysms. J Neurol Neurosurg Psychiatry 45(11):1040–1047
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval is not mandatorily required in our Institution for literature review.
Informed consent
All patients treated at our Department of Neurosurgery give their informed consent to the treatment of their clinical and radiological data for scientific purposes at the same time of the informed consent for the surgical treatment.
Rights and permissions
About this article
Cite this article
Scerrati, A., Sabatino, G., Della Pepa, G.M. et al. Treatment and outcome of thrombosed aneurysms of the middle cerebral artery: institutional experience and a systematic review. Neurosurg Rev 42, 649–661 (2019). https://doi.org/10.1007/s10143-018-0984-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-018-0984-7