Abstract
Homocysteine (tHcy) has been hardly studied among patients with head injury. This study was to evaluate whether there is any independent impact of tHcy levels on neurological outcome following head injury in a multivariate model. Patients admitted within 24 h of injury were included in the study, along with 20 age- and gender-matched controls. Plasma levels of tHcy were measured at admission using direct immunoassay. All the variables were analyzed with respect to tHcy levels and outcome according to Glasgow Outcome Score (GOS) at 3 months. Univariate and multivariate analyses were performed using SPSS 21. There were a total of 72 patients in the study. tHcy levels were significantly higher after head injury (mean 24.03[SD ± 16.0] μmol/L), compared to matched controls (mean 16.62 [SD ± 10.4] μmol/L) (p = 0.05). Patients with severe head injury, acute SDH, or diffuse higher radiological grades had greater levels of tHcy compared to others. There was a significant relationship between tHcy level and neurological outcome. tHcy levels were significantly higher in patients who had unfavorable GOS (mean 36.22[±25.3] μmol/L), compared to those with favorable GOS (mean 22.71[±14.3] μmol/L) (P = 0.03). In multivariate analysis, tHcy level (adj. odds ratio [OR] 1.17, P = 0.05) and Glasgow Coma Scale (adj. OR 5.17, P = 0.01) had significant association with neurological outcome at 3 months independent of age, dietary habit, radiological grading and of each other. tHcy level has significant independent impact on neurological outcome and may be useful as a prognostic marker following head injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head injury is one of the most common causes of mortality and morbidity in young people, causing immense socio-economic burden [1]. The imaging studies of brain are useful in therapeutic management, but have limited role in prognostication, as important pathophysiological events of secondary neuronal injury, such as cellular ischemia, oxidative stress and inflammatory response are not associated with visible radiological abnormalities [2,3,4]. The biochemical prognostic markers of these have also not been clearly established.
Homocysteine (tHcy) is a sulfhydryl non-protein α-amino acid and branch-point intermediate of methionine metabolism [5, 6]. It has been used as a marker of oxidative stress leading to lipid peroxidation and reactive oxygen species. Higher levels of tHcy are associated with an increased risk of various neurovascular diseases such as stroke, Alzheimer disease, and subarachnoid hemorrhage. However, there are hardly two clinical studies of tHcy in head injury. While they showed some association with mortality, there was no clear independent association with neurological outcome after adjusting for all relevant prognostic factors [7, 8]. This study was conducted to evaluate whether there is any independent impact of plasma levels of tHcy on neurological outcome at 3 months following head injury in a multivariate model.
Material and methods
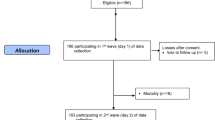
Patients admitted within 24 h of head injury at the Neurosurgery Department of the Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India, were prospectively enrolled for the study following ethics committee approval. Patients with hypotension, renal or liver dysfunction, un-consenting guardians, and those who did not continue the entire treatment were excluded from the study.
The admission EDTA blood samples of patients were analyzed post-centrifugation for plasma total homocysteine (tHcy) levels by competitive immunoassay using direct, chemiluminescent technology with the ADVIA Centaur Immunoassay System (Siemens AG, Munich, Germany). The tests were also performed on 20 (age- and gender-matched) normal healthy volunteers from the same community to arrive at a local control range.
The clinico-demographic data collected included age, sex, dietary habit, mechanism and type of injury, systemic injury, and severity of head injury based on Glasgow Coma Scale (GCS) [9, 10]. After initial resuscitation, they underwent evaluation with non-contrast computed tomography (CT) of the head and graded according to Marshall et al. [11]. Standard care provided to patients consisted of intubation and ventilation (whenever GCS <9), fluid and electrolyte homeostasis, seizure prophylaxis with Phenytoin, and routine tetanus prophylaxis as a policy. Baseline demographic characteristics of patients, mechanism of injury, GCS, and surgical intervention details were noted in a prospective database and followed up.
Outcomes [12]
Outcome was evaluated according to the Glasgow Outcome Scale score (GOS) at 3 months after head injury, either directly or over the telephone. Good recovery (GOS 5) or moderate disability (GOS 4) was considered as favorable outcome, and severe disability (GOS 3), persistent vegetative state (GOS 2) or death (GOS 1) was considered as unfavorable outcome.
Statistical analyses [13]
Continuous variables were reported as a mean with standard deviation (SD). Categorical data were reported as counts and proportions in each group. SPSS 21 software (IBM Corp., New York, USA) was used for the statistical analyses. Univariate analyses of continuous variables across binary categories were compared using the independent samples T test and across multiple categories using ANOVA. The bivariate relationship between two continuous variables was assessed using the Pearson correlation coefficient. Proportions were compared using chi-square or Fisher’s exact test wherever appropriate. Two-sided significance tests were used throughout, and the significance level was kept at p < 0.05. Multivariate analyses were conducted using binary logistic regression with mandatory significance of the model coefficient being less than 0.05 for validity of outcome prediction independent of age, GCS and CT grading and definitive treatment in relation to tHcy levels.
Results
There was a total of 72 patients with mean age of 35 years comprising of 62 males and 10 females. The most frequent mechanism of trauma was vehicular accidents followed by fall and assault. Admission tHcy levels were significantly higher among patients after head injury (mean 24.03 [SD ± 16.0] μmol/L), compared to matched controls (mean 16.62 [SD ± 10.4] μmol/L) (p = 0.05) as shown in Fig. 1.
Table 1 shows the levels of homocysteine in relation to baseline demographic and clinical characteristics of the study patients. Patients who used to take vegetarian diet had non-significant trend towards higher homocysteine levels after head injury (27.01 ± 15.9 vs 20.50 ± 15.7 μmol/L) (P = 0.09). Patients with severe head injury had higher tHcy levels (29.24 ± 23.1 μmol/L) compared to those with mild to moderate injury. None of the other baseline demographic or clinical factor including age had any significant relationship with homocysteine levels after head injury.
Table 2 shows the levels of homocysteine in relation to radiological characteristics and the necessity for surgical intervention among study patients. Patients with acute subdural hematoma (SDH) had near-significant trend towards higher homocysteine levels compared to those with other CT findings (30.34 ± 22.1 vs 20.86 ± 10.8 μmol/L) (P = 0.06). Among Marshall grades, diffuse grade IV had the highest of tHcy levels (30.83 ± 19.7 μmol/L) compared to the rest. None of the other radiological parameters and the need for surgical intervention had any relationship with homocysteine levels.
There was a significant relationship between tHcy levels and neurological outcome. Admission tHcy levels were significantly higher among patients who developed unfavorable GOS (mean 36.22[±25.3] μmol/L), compared to those with favorable GOS (mean 22.71[±14.3] μmol/L) (P = 0.03) (Fig. 2).
In multivariate analysis using binary logistic regression, higher admission tHcy levels (adj. odds ratio [OR] 1.17, P = 0.05) and poorer GCS (adj. OR 5.17, P = 0.01) had significant association with unfavorable neurological outcome independent of age, dietary preference, Marshall grading and of each other (Table 3).
Discussion
The potential role of biomarkers in the pathophysiology and clinical prognostication after head injury is has still not been translated to clinical utility [14, 15]. Many markers are associated with either the clinical or the radiological severity of head injury, thereby impacting outcome. But unless the studied biomarker has significant association with neurological outcome independent of all prognostic factors, it may not have good utility.
Homocysteine has been implicated in a wide variety of neurological and vascular diseases [6, 16,17,18]. Despite the known impact of injury on neurovascular structures and humongous role of perfusion related pathophysiology in outcome after head injury, there have been not more than two clinical studies of tHcy in head injury [4, 7, 8, 19].
Higher levels of tHcy among patients with head injury than controls, in our study, are in line with the only other study by Rahmani et al. in head injury, as well as by Dhandapani et al. in subarachnoid hemorrhage [6, 8]. The increased levels of tHcy after head injury are probably due to the “metabolic stress response” similar to the result of subarachnoid hemorrhage, caused by downregulation of cystathionine-β-synthase [6, 20].
While higher tHcy levels among more severe clinical and more diffuse radiological type of injuries are similar to the findings of Rahmani et al., the association between acute subdural hematoma and higher tHcy levels noted in our study has not been reported prior. Patients with acute SDH are likely to have more diffuse injury with mass effect and associated cerebral venous injury, accentuating the metabolic stress response and thereby the levels of tHcy.
While Rahmani et al. noted significantly higher tHcy levels among those who died than those who are alive in merely univariate analysis, we noted significant relationship between higher tHcy levels with overall neurological outcome (GOS 1-3), both in univariate as well as in multivariate analysis. This is probably the first study to show the independent impact of tHcy levels on neurological outcome after adjusting for other prognostic factors.
Hyper-homocysteinemia as a result of stress response has been known to be a double-edged sword with acute protective and protracted harmful effects in select groups of patients [21]. The independent harmful association of higher tHcy levels on neurological outcome noted in our study is likely be due to multiple mechanisms: microcirculation failure, glutamate-mediated excitotoxicity, oxidative stress, inflammatory responses, neuronal apoptosis, decreased activity of the Na+-K+-ATPase pump, calcium overload, metabolic derangements, epigenetic modifications, pathological aggregates deposition, endothelial damage, and atherothrombosis [5,6,7, 22]. Hatefi et al. had noted tHcy levels to be significantly associated with pulsatility index, cerebrovascular reactivity and markers of endothelial dysfunction [7]. Nevertheless, the dispute whether these associations are really causative or merely epiphenomena is still enigmatic [16].
The therapeutic relevance of these findings need not be overemphasized, especially when animal models have demonstrated enhanced functional recovery after head injury with folic acid supplementation potentially lowering levels of tHcy [23]. The limitations of our study are lack of serial assessment of tHcy and lack of CSF studies on homocysteine metabolites. More studies with larger number of patients with serial measurements of both tHcy and its metabolites in blood and CSF, in comparison with other markers such as CRP are required for definitive inference [24].
Conclusion
tHcy level has significant independent impact on neurological outcome after head injury and may be useful not only as a prognostic marker, but also probably in clinical decision making on folate supplementation.
References
Dhandapani SS, Manju D, Sharma BS, Mahapatra AK (2007) Clinical malnutrition in severe traumatic brain injury: factors associated and outcome at 6 months. Indian J Neurotrauma 4:35-39
Dhandapani S, Aggarwal A, Srinivasan A, Meena R, Gaudihalli S, Singh H, Dhandapani M, Mukherjee KK, Gupta SK (2015) Serum lipid profile spectrum and delayed cerebral ischemia following subarachnoid hemorrhage: Is there a relation? Surg Neurol Int 6(Suppl 21):S543-8
Dhandapani S, Kapoor A, Gaudihalli S, Dhandapani M, Mukherjee KK, Gupta SK (2015) Study of trends in anthropometric nutritional indices and the impact of adiposity among patients of subarachnoid hemorrhage. Neurol India 63:531–536
Dhandapani SS, Manju D, Vivekanandhan S, Agarwal M, Mahapatra AK (2010) Prospective longitudinal study of biochemical changes in critically ill patients with severe traumatic brain injury: factors associated and outcome at 6 months. Indian J Neurotrauma 7:23–27
Bonetti F, Brombo G, Zuliani G (2016) The relationship between hyperhomocysteinemia and neurodegeneration. Neurodegener Dis Manag 6:133–145
Dhandapani S, Goudihalli S, Mukherjee KK, Singh H, Srinivasan A, Danish M, Mahalingam S, Dhandapani M, Gupta SK, Khandelwal N, Mathuriya SN (2015) Prospective study of the correlation between admission plasma homocysteine levels and neurological outcome following subarachnoid hemorrhage: a case for the reverse epidemiology paradox? Acta Neurochir 157:399–407
Hatefi M, Behzadi S, Dastjerdi MM, Ghahnavieh AA, Rahmani A, Mahdizadeh F, Hafezi Ahmadi MR, Asadollahi K (2017) Correlation of homocysteine with cerebral hemodynamic abnormality, endothelial dysfunction markers, and cognition impairment in patients with traumatic brain injury. World Neurosurg 97:70–79
Rahmani A, Hatefi M, Dastjerdi MM, Zare M, Imani A, Shirazi D (2016) Correlation between serum homocysteine levels and outcome of patients with severe traumatic brain injury. World Neurosurg 87:507–515
Dhandapani S, Sarda AC, Kapoor A, Salunke P, Mathuriya SN, Mukherjee KK (2015) Validation of a new clinico-radiological grading for compound head injury: implications on the prognosis and the need for surgical intervention. World Neurosurg 84:1244–1250
Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet (London, England) 2:81–84
Marshall LF, Marshall SB, Klauber MR, Van Berkum CM, Eisenberg H, Jane JA, Luerssen TG, Marmarou A, Foulkes MA (1992) The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma 9(Suppl 1):S287–S292
Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet (London, England) 1:480–484
Dhandapani S, Singh H, Negm HM, Cohen S, Anand VK, Schwartz TH (2016) Cavernous Sinus Invasion in Pituitary Adenomas: Systematic Review and Pooled Data Meta-Analysis of Radiologic Criteria and Comparison of Endoscopic and Microscopic Surgery. World Neurosurg 96:36-46
Adrian H, Marten K, Salla N, Lasse V (2016) Biomarkers of traumatic brain injury: temporal changes in body fluids. eNeuro 3
Bogoslovsky T, Gill J, Jeromin A, Davis C, Diaz-Arrastia R (2016) Fluid biomarkers of traumatic brain injury and intended context of use. Diagnostics (Basel, Switzerland) 6
Hannibal L, Blom HJ (2017) Homocysteine and disease: causal associations or epiphenomenons? Mol Asp Med 53:36–42
Schalinske KL, Smazal AL (2012) Homocysteine imbalance: a pathological metabolic marker. Adv Nutr (Bethesda, Md.) 3:755–762
Skovierova H, Vidomanova E, Mahmood S, Sopkova J, Drgova A, Cervenova T, Halasova E, Lehotsky J (2016) The molecular and cellular effect of Homocysteine metabolism imbalance on human health. International journal of molecular sciences 17
Dhandapani S, Gupta A, Singh J, Sharma BS, Mahapatra AK, Mehta VS (2013) Spinal dural arterio-venous fistula: clinico-radiological profile and outcome following surgical occlusion in an Indian neurosurgical center. Neurol India 61:406–410
Zhang M, Shan H, Wang Y, Wang T, Liu W, Wang L, Zhang L, Chang P, Dong W, Chen X, Tao L (2013) The expression changes of cystathionine-beta-synthase in brain cortex after traumatic brain injury. J Mol Neurosci MN 51:57–67
Dash PK, Hergenroeder GW, Jeter CB, Choi HA, Kobori N, Moore AN (2016) Traumatic brain injury alters methionine metabolism: implications for pathophysiology. Front Syst Neurosci 10:36
Wang JF, Li Y, Song JN, Pang HG (2014) Role of hydrogen sulfide in secondary neuronal injury. Neurochem Int 64:37–47
Naim MY, Friess S, Smith C, Ralston J, Ryall K, Helfaer MA, Margulies SS (2010) Folic acid enhances early functional recovery in a piglet model of pediatric head injury. Dev Neurosci 32:466–479
Srinivasan A, Aggarwal A, Gaudihalli S, Mohanty M, Dhandapani M, Singh H, Mukherjee KK, Dhandapani S (2016) Impact of Early Leukocytosis and Elevated High-Sensitivity C-Reactive Protein on Delayed Cerebral Ischemia and Neurologic Outcome After Subarachnoid Hemorrhage. World Neurosurg 90:91-5
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding information
No funding was received.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from either patients or relatives of patients included in the study.
Additional information
Sivashanmugam Dhandapani and Ankur Bajaj both to be considered first authors
Rights and permissions
About this article
Cite this article
Dhandapani, S., Bajaj, A., Gendle, C. et al. Independent impact of plasma homocysteine levels on neurological outcome following head injury. Neurosurg Rev 41, 513–517 (2018). https://doi.org/10.1007/s10143-017-0880-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-017-0880-6