Abstract
The use of computed tomography angiography (CTA) for the evaluation of peripheral vascular trauma has become increasingly prevalent in the past decade with the development of multidetector CT (MDCT) and multiple studies subsequently demonstrating high sensitivity, specificity, and diagnostic accuracy when compared with conventional angiography. Additional benefits of MDCT include the ability to rapidly acquire the images, perform multiplanar and 3D reconstructions, and assess the adjacent soft tissues and bones. Rapid intravenous injection of iodinated contrast material is required for optimal arterial enhancement. CTA manifestations of an arterial injury may be direct, and include active contrast extravasation, pseudoaneurysm, arteriovenous fistula (AVF), intimal injury, dissection, or occlusion. There are also indirect signs which have a high association with vascular injury, and should raise suspicion, when present. Pitfalls related to image acquisition or patient factors can be mitigated with appropriate planning and post-processing techniques.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Injuries to the vascular structures of the extremities comprise approximately 34% of traumatic vascular injuries, and are relatively evenly distributed between the upper and lower extremities [1]. Vascular injury to the extremities can occur with both blunt and penetrating trauma as well as in civilian and combat settings. Penetrating trauma is more commonly associated with vascular injuries [1]. Traumatic vascular injuries are on rise, especially in combat, with the most commonly injured vessels during recent wars in Iraq and Afghanistan being those of the distal extremities [2]. Vascular injury to the extremities is associated with a high rate of morbidity, including amputation [3,4,5].
While catheter-based angiography is the gold standard for the diagnosis of peripheral vascular trauma, it has largely been replaced by CTA over the past decade owing to easy integration of extremity CTA with torso imaging in trauma patients and improved image quality with advances in CT technology. As a result, CTA is now widely accepted as the first-line imaging investigation when an upper or lower extremity vascular injury is in question. Compared with conventional angiography, CTA has the advantages of being less invasive, more readily available, and allowing for the evaluation of the adjacent soft tissues and bones [6, 7]. CTA avoids potential iatrogenic complications associated with catheter angiography, such as pseudoaneurysm, hematoma, thrombosis of the access vessel, and peripheral embolization [8]. CTA is also less expensive and can be obtained quickly, as opposed to the delay often required to assemble a specialized team to perform conventional angiography [9], a particular advantage of CTA when reducing ischemic time is known to be an important factor in limb salvage [4, 10, 11]. Additional advantages of CTA include the ability to be performed in combination with protocols for trauma CTs of the chest, abdomen, and pelvis, and the production of multiplanar reformats and 3D renderings after initial image acquisition. The major disadvantage of CTA is the inability to perform concurrent percutaneous intervention at the time of diagnosis, unlike conventional angiography; in cases where subsequent intervention is required, performing CTA first increases the overall radiation and contrast exposure.
Compared with magnetic resonance angiography (MRA), CTA has better spatial resolution, faster isotropic image acquisition, is more readily available, and often located in closer physical proximity to emergency departments. Additionally, CTA avoids potential hazards of placing an unstable patient into a magnet and safety concerns with MR unsafe shrapnel or medical equipment [12]. Multiple studies have demonstrated the high sensitivity, specificity, accuracy, and diagnostic quality of CTA [6, 12,13,14,15]. Analyses of trauma patients who underwent upper or lower extremity CTA showed sensitivities in the range of 95–100%, specificities of 87–100%, a low nondiagnostic imaging rate, and good inter-observer agreement between radiologists indicating that CTA can replace conventional diagnostic angiography in the acute trauma setting [12, 13, 15, 16]. Several of these studies are limited by verification and follow-up bias, and although some injuries may have been missed by CTA, none of the missed injuries was likely to have been clinically significant [6, 13].
Vascular injuries of the extremities are encountered by radiologists across multiple subspecialties including emergency, vascular, and musculoskeletal radiology. Knowledge of specific extravascular patterns of trauma commonly associated with vascular injuries, as well as prompt recognition of the direct and indirect imaging signs of vascular trauma, is crucial to allow for expedient treatment, and thus reduces the morbidity and mortality of these injuries.
Indications for performing CTA in the setting of suspected peripheral vascular trauma
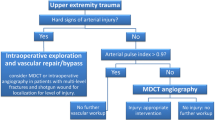
Traumatic vascular injuries to the extremities can occur in the setting of both blunt and penetrating trauma. The decision of whether to pursue CTA in the setting of a suspected peripheral vascular injury largely depends on patient presentation and physical examination findings. “Hard signs” of arterial injury in an extremity include external bleeding, a rapidly expanding hematoma, a palpable thrill or audible bruit, or signs of severe acute extremity ischemia (pulselessness, pallor, paresthesias, pain, and paralysis) [17]. Most patients with signs of hard injury will undergo operative management. “Soft signs” of arterial injury in an extremity include history of arterial bleeding in the pre-hospital setting, proximity of a penetrating wound or blunt trauma to an artery, a small nonpulsatile hematoma over an artery, and a neurologic deficit originating in a nerve adjacent to a named artery [17]. The incidence of arterial injury in patients with soft signs ranges from 3 to 25% [17]. When soft signs are present in the setting of an abnormal physical examination or arterial pressure index < 0.9, imaging (CTA or duplex ultrasound) is indicated. Imaging is also recommended when the arterial peripheral index or pulses are unclear and/or the extremity remains cool [17]. With increased utilization of CT in polytrauma, there is a trend towards increased use of extremity CTA as well. CTA findings often dictate whether patient disposition should be to surgery, interventional radiology, or conservative management.
CTA technique in peripheral vascular trauma
Ideally, CTA of the extremities should be performed on at least a 64-slice MDCT; however, in some cases, 16-slice MDCT may produce acceptable image quality [18]. Peripheral intravenous (IV) access with a large gauge cannula, typically 18-gauge or 20-gauge in average-sized adults, and use of a power injector to maintain a high flow rate are standard. Immobilization devices should be used if the patient is unable to lie still for the examination.
For evaluation of the lower extremity vasculature, the IV line can be placed in either antecubital fossa. The patient is positioned supine on the table with both legs together. Even if only one leg has sustained injury, bilateral evaluation should be performed. Typical coverage extends from the aorto-iliac bifurcation through the toes; however, the study can be combined with CTA of the chest and abdomen.
When unilateral upper extremity CTA is to be performed, an IV should be placed contralateral to the affected extremity to prevent dense venous contrast from obscuring the adjacent arteries. Optimal positioning is with the body supine with the arm over the head, palm facing upward with fingers extended [7]. If more comfortable for the patient, prone body positioning with the palm facing the table is also acceptable [7]. Positioning the arm above the head helps to reduce noise; however, this may not be possible in the setting of an upper extremity injury. In such cases, the arms can be positioned at the patient’s side with images acquired in the same field-of-view as the chest, abdomen, and pelvis CT. If bilateral upper extremity CTAs are required and no central line is present, bilateral arm IVs with two separate contrast injections and separate image acquisitions can be performed sequentially. Alternatively, a foot IV can be placed with imaging of both upper extremities simultaneously; however, patients often report discomfort with a foot IV, and insertion of an IV with large enough caliber to obtain optimal contrast injection rate may not be possible. Typical coverage for CTA of the upper extremities extends from the aortic arch through the fingertips.
At our institution, IV contrast with an iodine concentration of 350 mg/mL is most commonly used in patients without a contraindication to receiving iodinated IV contrast. The volume and rate of injection is based on patient for weight (Table 1). For example, for a lower extremity angiogram in a 100-kg patient, 25 mL of contrast is injected at 5 mL/s, followed by 120 mL at 4 mL/s, and then a 30 mL 0.9%NaCl flush at 4 ml/s. Patients with higher body mass index (BMI) require a higher volume or concentration of contrast and higher injection rates [7]. For both lower and upper extremity CTAs, an immediate delay of the distal half of the extremity is performed, as CT acquisition may outpace the contrast bolus transit time. Our institution uses bolus tracking with an enhancement threshold of 120 Houndsfield Units (HU) and minimum scan delay to ensure adequate arterial opacification. The monitoring region is placed on the abdominal aorta for lower extremity CTAs and the aortic arch for upper extremity CTAs. Alternatively, an empiric delay of 50 s and 25 s after the start of contrast injection, can be used for the lower extremity and upper extremity examinations, respectively [9]; however, this may not achieve adequate contrast enhancement due to individual variation in cardiac output [19]. Optimal enhancement of the artery in question is between 250 and 300 HU above baseline for arteries and 100 HU for veins [18]. Thin section images of 1 mm or less with reconstructions at 50% overlap are appropriate in most circumstances [18]. Multiplanar reconstructions in the coronal and sagittal planes are performed at the scanner. Curved planar reconstructions (CPR) and maximum intensity projection (MIP) images can be constructed at the workstation. 3D renderings produced at the workstation or in a dedicated 3D lab are offered upon ordering provider request. Vascular structures are usually best viewed with window width setting around 600 and window level setting around 80 [7].
Radiation dose depends upon the region of anatomy imaged, patient positioning, and patient BMI. Typically, the scanner automatically determines the mA and kV based on the initial topogram series; however, it should be noted that the kV should not be reduced below 100 in patients with stents in order to maintain image quality [7].
In patients with reduced renal function and a glomerular filtration rate (GFR) of < 30 mL/min, the contrast volume may be reduced, and diagnostic quality imaging maintained. This can be achieved using dual energy CT, with contrast volume as low as 0.75 mL/kg [20]. If dual energy CT is not available, reducing contrast volume and maintaining image quality may be possible by lowering the kV, and thus increasing contrast; however, this approach may be problematic in patients with stents and orthopedic hardware [20].
CTA manifestations and spectrum of arterial injuries
CTA findings of arterial injury can be categorized as either direct or indirect. Direct signs include active contrast extravasation, pseudoaneurysm, arteriovenous fistula (AVF), intimal defect, dissection, occlusion, and abrupt decrease in vessel caliber or wall irregularity. Indirect signs include a penetrating object trajectory near an artery, shrapnel less than 5 mm from a neurovascular bundle, and perivascular intramuscular hematoma [21] (Fig. 1).
Indirect signs of vascular injury. Gunshot wound to the right upper thigh resulting in a large intramuscular hematoma (*). Additional signs of indirect vascular injury are present, including a complex, comminuted proximal femur fracture with the ballistic tract in very close proximity to the femoral artery and vein, and shrapnel less than 5 mm from the vessels (arrow)
Active arterial hemorrhage manifests as active contrast extravasation outside of the vessel lumen on CTA, usually into the surrounding soft tissues. Delayed phase images will demonstrate an increase in extraluminal contrast with time (Figs. 2 and 3). Management depends on the vessel involved, though typically surgical repair or endovascular treatment is employed for larger vessels [17].
Hematoma with active contrast extravasation and vasospasm. Patient with a gunshot wound to the right lower pelvis and upper thigh with gas and blood products in the subcutaneous tissues of the upper right thigh (a and b). Arterial phase imaging (a) demonstrates contrast layering dependently within the hematoma. Delayed image (b) performed 7 min later demonstrates diffusely increased attenuation within the hematoma consistent with active extravasation (arrows). 3D rendering (c) and maximum intensity projection (MIP) images (d) show subtle smooth narrowing of the upper superficial femoral artery (arrowheads) which resolved on imaging the following day, consistent with vasospasm
A pseudoaneurysm is an injury to the artery contained by fibrous tissue or adventitia, which is in contrast to true aneurysms which contain all three layers of the vessel wall [22]. On CTA, a pseudoaneurysm will appear as a focal contrast-filled outpouching (Figs. 3, 4, and 5). In contrast to active hemorrhage, pseudoaneurysms maintain their shape on delayed phase imaging, whereas contrast will increase and change shape in the setting of active hemorrhage [21]. Occasionally, the outpouching will not entirely fill with contrast owing to the presence of thrombus [23]. Sometimes pseudoaneurysm can have delayed presentation with atypical clinical symptoms such as compressive neuropathy or pulsatile soft tissue mass. Pseudoaneurysms may be treated by operative or endovascular management depending on the size, morphology, risk of rupture, and vessel involved [17].
Focal contrast-filled outpouching of the right axillary artery (arrowheads, a and b) without change on the delayed phase image (not shown) consistent with a pseudoaneurysm. CTA was performed in this patient who experienced a gunshot injury to the chest and was noted to have a bruit and thrill in the infraclavicular region on physical examination. Note the bullet within the superior aspect of the C5 vertebral body (white arrow, c). Right upper lobe patchy ground glass pulmonary opacities (black arrow, d) are consistent with a pulmonary contusion. Given the large size of aneurysm (3 cm), surgical exploration of both right supraclavicular and infraclavicular fossa with graft reconstruction of the axillary artery was performed
Ulnar pseudoaneurysm occurring secondary to penetrating trauma from windshield glass in the setting of a motor vehicle accident. Axial (a), sagittal (b), and 3D reformatted (c) images from a CTA image at the level of the proximal forearm demonstrates a focal contrast-enhanced outpouching with early opacification of the adjacent vein (arrows) consistent with both a pseudoaneurysm and an AVF
An AVF occurs when traumatic injury results in a direct communication between an artery and an adjacent vein without an intervening capillary bed. The exact site of communication may not be visible; however, early filling of a vein adjacent to an artery in the region of traumatic injury (in the absence of venous filling in the more distal extremity) is indicative of an AVF [21, 24] (Figs. 5 and 6). Treatment involves either operative repair, or more commonly endovascular repair with a stent-graft [17].
Iatrogenic traumatic arteriovenous fistula in a patient presenting 4 days after coronary catheter angiography via the right common femoral artery. Note increased attenuation of the right common femoral and right external iliac veins relative to the left (*, a and b) secondary to venous early filling in the setting of an AVF. MIP image demonstrates a communication between the right common femoral artery and vein (arrowhead, c). Stranding in the fat surrounding the right common femoral artery (arrow, a) is compatible with recent instrumentation
Complete transection occurs when all walls of an artery are entirely disrupted. This may present as an abrupt cessation of contrast opacification or caliber change with no flow in the arterial segment in the vicinity of soft tissue or osseous injury (Figs. 7, 8, and 9). Arteries distal to the occluded segment may opacify via collateral flow. Compared with chronic occlusion due to atherosclerosis, collaterals in the setting of acute injury may be smaller and more difficult to visualize [25]. Certain fracture and dislocation patterns are associated with an increased risk of vascular injury. These combined orthopedic and vascular injuries, specifically those involving the popliteal and tibial arteries, are associated with relatively high amputation rate [26]. Knee dislocations were reported to be associated with popliteal artery injury (Fig. 7) in 19–100% of cases prior to 1985 [4], and more recently described in approximately 17% of cases [27]. In the pediatric population, supracondylar humeral fractures are known to be associated with vascular injury, particularly to the brachial artery, with an increased incidence in fractures of a higher Gartland classification [28, 29].
Frontal and lateral knee radiographs demonstrate lateral and posterior dislocation of the knee at the tibio-femoral joint (a and b). Lateral MIP (c) and 3D rendered CTA (d) images reveal lack of opacification of the popliteal artery below the knee due to occlusion (arrows) with reconstitution of the more distal tibial arteries
Complete transection of the ulnar artery. Axial CTA slice through the mid forearm demonstrates gas and bullet fragments along the volar aspect of the left upper extremity (arrow, a). Radial (white arrowhead, a) and interosseous arteries (black arrowhead, a) are visible; however, the ulnar artery is not opacified with contrast. Coronal MIP image of the forearm demonstrates absence of the proximal and mid ulnar artery, with filling of the ulnar artery distally (open arrowhead, b) via a patient radial artery (c) and palmar arches (not shown). There is an associated complex, comminuted fracture of the mid-distal ulna. Complete transection of the ulnar artery was confirmed at surgery
Frontal radiograph of the left shoulder demonstrates anterior dislocation of the humeral head relative to the glenoid with an associated depressed fracture of the humeral head (a). Just posterior to the pectoralis minor muscle there is narrowing and loss of contrast enhancement of the distal axillary artery (arrowheads, b and c), consistent with occlusion or severe stenosis. Soft tissue stranding is present surrounding the site of arterial injury
A decrease in vessel caliber may be the result of partial transection, dissection, vasospasm, or external compression [25]. Intimal injuries and partial transections may present as an intimal flap or wall defect (Fig. 10). Vessel contour irregularity with luminal narrowing may be present in the setting of superimposed thrombus. Dissection may be seen as a longitudinal linear hypoattenuating intimomedial flap when both true and false lumens are patent, or may present as a focal filling defect when one of the lumens (usually false) is thrombosed [25]. Vessels may also lack contrast opacification due to compression from surrounding hematoma.
Arterial vasospasm occurring in the setting of traumatic injury can be difficult to differentiate from dissection with a thrombosed false lumen. The etiology of vasospasm in the absence of visible endothelial injury is not entirely clear, but has been hypothesized to be related to mechanical stimulus from pressure waves transmitted from a high velocity penetrating object and/or release of vasoconstrictive substances [30]. Like other types of arterial injury, vasospasm is usually present adjacent to soft tissue injury or vessel segments along the path of the penetrating injury and appears as a narrowed segment of the artery (Figs. 2 and 11). Management depends on the severity and length of the narrowed segment, but may include conventional angiography, MR angiography, or clinical monitoring with follow-up imaging to ensure resolution and differentiate from a true arterial injury [21, 24].
Vasospasm and venous thrombus. Patient with gunshot wound to the upper-mid thighs. Asymmetric focal moderate-severe narrowing of the mid left superficial femoral artery is present on arterial phase images (arrow, a). Note lack of opacification of the adjacent femoral vein (arrowhead, a). Delayed phase axial slices through the mid thigh demonstrate asymmetric lack of opacification of the right femoral vein (arrowheads, b and c). Gas, fat stranding, and an intramuscular hematoma are present along the course of the projectile tract, extending from medial to lateral across the left thigh (b and c). 3D rendering demonstrates the length and severity of the superficial femoral artery stenosis with increased conspicuity (arrow, d). Subsequent imaging the following day confirmed the femoral vein thrombus, but showed resolution of the arterial stenosis, making vasospasm the most likely etiology
Manifestations of venous injury
The majority of peripheral extremity venous injuries are associated with arterial injuries [31]. In addition, venous injuries may not be visible on CTA when only arterial phase imaging is performed, and are only discovered at surgery [25]. Accumulation of contrast may occur in the soft tissues surrounding the injury, though should be absent on arterial phase imaging. Additionally, trauma is a known risk factor for the development of venous thrombus [32], and careful evaluation of the veins should be performed, especially when delayed phase imaging is obtained (Fig. 11). Additionally, expanding hematomas can increase risk of compartment syndrome which may lead to venous and arterial stenosis and occlusion.
Potential pitfalls
The radiologist should be aware of several potential pitfalls when interpreting upper and lower extremity CTAs. Beam hardening artifact from metal or dense contrast may obscure the arterial anatomy of interest. Multiplanar reconstructions and post-processing artifact reduction technology can be employed to help visualize the arterial lumen. In patients with severe peripheral vascular disease, calcium may cause blooming artifact, resulting in overestimation of the degree of stenosis. Vascular stents may also render evaluation of the underlying vascular lumen challenging. Using wider window and level settings and multiplanar reformatted areas can be helpful in troubleshooting these cases (Fig. 12). Performing a noncontrast CT prior to the CTA may be useful to delineate extent and burden of calcium; however, it does increase the radiation dose. In addition, maintaining a kV of greater than 100 in the setting of stents and the use of post-processing iterative reconstruction algorithms can help improve image quality [7]. Artifact from bright contrast opacification in the adjacent vein may occur when imaging the upper extremities (Fig. 13) and can be avoided by placing an IV in the contralateral arm or injecting via a lower extremity IV [24]. In the setting of low cardiac output, fast scanner technology, or injury to an upstream artery image acquisition of the distal portions of the extremities may outpace contrast transit to the arteries at this level. An immediate delay of the distal extremity can be obtained to differentiate occlusion from slow flow-related lack of opacification [25]; however, a delay performed too long after contrast injection can result in venous opacification (“contamination”) and difficulty visualizing the adjacent artery [7]. Patient immobilization and instructions to cease movement can decrease the chance of motion artifact compromising image quality.
Metallic artifact from bilateral knee arthroplasties in a patient with a left popliteal artery aneurysm. Axial images through the bilateral popliteal fossae at narrow window/level setting (a) and wider window/level setting (b) with improved visualization of the popliteal arteries. Coronal MPR image of the left popliteal artery reveals persistent artifact, though visualization of the dilated popliteal artery (arrows, b and c) is improved
CTA of the left upper extremity performed with IV contrast injection in the left antecubital fossa (a) results in dense contrast within the veins surrounding the less well opacified brachial artery (arrow). Subsequent contrast injection via a right antecubital fossa IV (b) results in a well opacified brachial artery without bright contrast in the surrounding veins compromising evaluation
Variant vascular anatomy can be a potential source of false positives results. If an artery does not arise at its conventional location, it may be mistaken for an occlusion. In a study of conventional arteriograms of the upper extremity, variants were noted in 9% of individuals, the most common being a high origin of the radial artery from the brachial artery, with high origins of the radial and ulnar artery from the axillary artery occurring less frequently [33, 34]. In the lower extremity, approximately 9% of individuals have variant popliteal and tibial artery branching patterns, with high origins of the anterior tibial artery being most common [35, 36]. In addition, the tibial arteries may be congenitally absent or hypoplastic, and in these cases should not be confused with occlusion. Findings that suggest a congenitally absent or hypoplastic tibial artery include robust remaining tibial arteries supplying the extremity in the region of the absent artery, finding present on a prior imaging study, lack of abrupt vessel cutoff, and extended distance from region of trauma.
Conclusion
CTA is a frequently used first-line imaging study for the assessment of peripheral vascular trauma. CTA is usually readily available in emergency settings where trauma protocol CTs of the chest and abdomen are performed and is often faster and prone to less iatrogenic complications than conventional angiography. Multiple specific manifestations of vascular trauma are readily demonstrated on CTA, which guide the next steps in patient management. Identification of these imaging signs is important to prevent devastating complications of vascular injury.
Data availability
Not applicable.
References
Perkins ZB, De’Ath HD, Aylwin C et al (2012) Epidemiology and outcome of vascular trauma at a British Major Trauma Centre. Eur J Vasc Endovasc Surg 44:203–209. https://doi.org/10.1016/j.ejvs.2012.05.013
White JM, Stannard A, Burkhardt GE, Eastridge BJ, Blackbourne LH, Rasmussen TE (2011) The epidemiology of vascular injury in the wars in Iraq and Afghanistan. Ann Surg 253:1184–1189. https://doi.org/10.1097/SLA.0b013e31820752e3
Hafez HM, Woolgar J, Robbs JV (2001) Lower extremity arterial injury: results of 550 cases and review of risk factors associated with limb loss. J Vasc Surg 33:1212–1219. https://doi.org/10.1067/mva.2001.113982
Lange RH, Bach AW, Hansen ST, Johansen KH (1985) Open tibial fractures with associated vascular injuries. J Trauma Inj Infect Crit Care 25:203–208. https://doi.org/10.1097/00005373-198503000-00006
DeBakey ME, Simeone FA (1946) Battle injuries of the arteries in World War II; an analysis of 2,471 cases. Ann Surg 123:534–579
Inaba K, Potzman J, Munera F, McKenney M, Munoz R, Rivas L, Dunham M, DuBose J (2006) Multi-slice CT angiography for arterial evaluation in the injured lower extremity. J Trauma - Inj Infect Crit Care 60:502–507. https://doi.org/10.1097/01.ta.0000204150.78156.a9
Ghouri MA, Gupta N, Bhat AP, Thimmappa ND, Saboo SS, Khandelwal A, Nagpal P (2019) CT and MR imaging of the upper extremity vasculature: pearls, pitfalls, and challenges. Cardiovasc Diagn Ther 9:S152–S173. https://doi.org/10.21037/cdt.2018.09.15
AbuRahma AF, Robinson PA, Boland JP et al (1993) Complications of arteriography in a recent series of 707 cases: factors affecting outcome. Ann Vasc Surg 7:122–129. https://doi.org/10.1007/BF02001005
Miller-Thomas MM, West OC, Cohen AM (2005) Diagnosing traumatic arterial injury in the extremities with CT angiography: pearls and pitfalls. RadioGraphics 25:S133–S142. https://doi.org/10.1148/rg.25si055511
Glass GE, Pearse MF, Nanchahal J (2009) Improving lower limb salvage following fractures with vascular injury: a systematic review and new management algorithm. J Plast Reconstr Aesthet Surg 62:571–579. https://doi.org/10.1016/j.bjps.2008.11.117
Green NE, Allen BL (1977) Vascular injuries associated with dislocation of the knee. J Bone Joint Surg Am 59:236–239
Patterson BO, Holt PJ, Cleanthis M, Tai N, Carrell T, Loosemore TM, on behalf of the London Vascular Injuries Working Group (2012) Imaging vascular trauma. Br J Surg 99:494–505. https://doi.org/10.1002/bjs.7763
Jens S, Kerstens MK, Legemate DA, Reekers JA, Bipat S, Koelemay MJW (2013) Diagnostic performance of computed tomography angiography in peripheral arterial injury due to trauma: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg 46:329–337. https://doi.org/10.1016/j.ejvs.2013.04.034
Busquéts AR, Acosta JA, Colón E et al (2004) Helical computed tomographic angiography for the diagnosis of traumatic arterial injuries of the extremities. J Trauma - Inj Infect Crit Care 56:625–628. https://doi.org/10.1097/01.ta.0000053546.28739.cf
Soto JA, Múnera F, Cardoso N, Guarín O, Medina S (1999) Diagnostic performance of helical CT angiography in trauma to large arteries of the extremities. J Comput Assist Tomogr 23:188–196. https://doi.org/10.1097/00004728-199903000-00005
Hogan AR, Lineen EB, Perez EA, Neville HL, Thompson WR, Sola JE (2009) Value of computed tomographic angiography in neck and extremity pediatric vascular trauma. J Pediatr Surg 44:1236–1241. https://doi.org/10.1016/j.jpedsurg.2009.02.039
Feliciano DV, Moore FA, Moore EE, West MA, Davis JW, Cocanour CS, Kozar RA, McIntyre RC Jr (2011) Evaluation and management of peripheral vascular injury. Part 1. Western trauma association/critical decisions in trauma. J Trauma - Inj Infect Crit Care 70:1551–1556. https://doi.org/10.1097/TA.0b013e31821b5bdd
Rubin G, Browne L, Fishman E et al (2016) ACR – NASCI – SIR – SPR practice parameter for the performance and interpretation of body computed tomography angiography (CTA). Am Coll Radiol Revis 1076(Resolution 16):1–15
Bozlar U, Ogur T, Norton PT, Khaja MS, All J, Hagspiel KD (2013) CT angiography of the upper extremity arterial system: part 1-anatomy, technique, and use in trauma patients. Am J Roentgenol 201:745–752. https://doi.org/10.2214/AJR.13.11207
Almutairi A, Sun Z, Poovathumkadavi A, Assar T (2015) Dual energy CT angiography of peripheral arterial disease: feasibility of using lower contrast medium volume. PLoS One 10:1–15. https://doi.org/10.1371/journal.pone.0139275
Uyeda JW, Anderson SW, Sakai O, Soto JA (2010) CT angiography in trauma. Radiol Clin N Am 48:423–438. https://doi.org/10.1016/j.rcl.2010.02.003
Anderson SE, De Monaco D, Buechler U et al (2003) Imaging features of pseudoaneurysms of the hand in children and adults. Am J Roentgenol 180:659–664. https://doi.org/10.2214/ajr.180.3.1800659
Saad NEA, Saad WEA, Davies MG, Waldman DL, Fultz PJ, Rubens DJ (2005) Pseudoaneurysms and the role of minimally invasive techniques in their management. RadioGraphics 25:S173–S189. https://doi.org/10.1148/rg.25si055503
Nagpal P, Maller V, Garg G, Hedgire S, Khandelwal A, Kalva S, Steigner ML, Saboo SS (2017) Upper extremity runoff: pearls and pitfalls in computed tomography angiography and magnetic resonance angiography. Curr Probl Diagn Radiol 46:115–129. https://doi.org/10.1067/j.cpradiol.2016.01.002
Gakhal MS, Sartip KA (2009) CT angiography signs of lower extremity vascular trauma. Am J Roentgenol 193:49–57. https://doi.org/10.2214/AJR.08.2011
Wolma FJ, Larrieu AJ, Alsop GC (1980) Arterial injuries of the legs associated with fractures and dislocations. Am J Surg 140:806–809. https://doi.org/10.1016/0002-9610(80)90122-1
Miranda FE, Dennis JW, Veldenz HC, Dovgan PS, Frykberg ER (2002) Confirmation of the safety and accuracy of physical examination in the evaluation of knee dislocation for injury of the popliteal artery: a prospective study. J Trauma 52:247–252. https://doi.org/10.1097/00005373-200202000-00008
Campbell CC, Waters PM, Emans JB, Kasser JR, Millis MB (1995) Neurovascular injury and displacement in type III supracondylar Humerus fractures. J Pediatr Orthop 15:47–52. https://doi.org/10.1097/01241398-199501000-00011
Wegmann H, Eberl R, Kraus T, Till H, Eder C, Singer G (2014) The impact of arterial vessel injuries associated with pediatric supracondylar humeral fractures. J Trauma Acute Care Surg 77:381–385. https://doi.org/10.1097/TA.0000000000000306
Peach G, Antoniou GA, El Sakka K, Hamady M (2010) Traumatic arterial spasm causing transient limb ischaemia: a genuine clinical entity. Ann R Coll Surg Engl 92:1–2. https://doi.org/10.1308/147870810X1269966298091
Meyer J, Walsh J, Schuler J et al (1987) The early fate of venous repair after civilian vascular trauma. A clinical, hemodynamic, and venographic assessment. Ann Surg 206:458–464
Meissner MH, Wakefield TW, Ascher E, Caprini JA, Comerota AJ, Eklof B, Gillespie DL, Greenfield LJ, He AR, Henke PK, Hingorani A, Hull RD, Kessler CM, McBane RD, McLafferty R (2007) Acute venous disease: venous thrombosis and venous trauma. J Vasc Surg 46:S25–S53. https://doi.org/10.1016/j.jvs.2007.08.037
Uglietta JP, Kadir S (1989) Arteriographic study of variant arterial anatomy of the upper extremities. Cardiovasc Intervent Radiol 12:145–148. https://doi.org/10.1007/BF02577379
Haładaj R, Wysiadecki G, Dudkiewicz Z, Polguj M, Topol M (2018) The high origin of the radial artery (brachioradial artery): its anatomical variations, clinical significance, and contribution to the blood supply of the hand. Biomed Res Int 2018:1–11. https://doi.org/10.1155/2018/1520929
Öztekin PSÖ, Ergun E, Cıvgın E, Yigit H, Kosar PN (2015) Variants of the popliteal artery terminal branches as detected by multidetector ct angiography. Open Med 10:10–491. https://doi.org/10.1515/med-2015-0056
Day CP, Orme R (2006) Popliteal artery branching patterns-an angiographic study. Clin Radiol 61:696–699. https://doi.org/10.1016/j.crad.2006.03.014
Code availability
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors were involved with manuscript composition and figure contribution.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Walkoff, L., Nagpal, P. & Khandelwal, A. Imaging primer for CT angiography in peripheral vascular trauma. Emerg Radiol 28, 143–152 (2021). https://doi.org/10.1007/s10140-020-01826-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-020-01826-w