Abstract
Yellow catfish has become one of the most important freshwater aquaculture species in China. The mono-sex male yellow catfish has important application value in aquaculture because the male grows generally faster than the sibling females under the same conditions. This study has screened YY super-male and YY physiological female yellow catfish by sex reversal, gynogenesis, and progeny testing, which can help to achieve the large-scale production of YY super-male and XY all-male. From 2008 to 2010, about 123,000 YY super-male were produced, and about 81 million XY all-male fry were produced with 100 % male rate by random sampling. Therefore, these results indicate that YY super-male and YY physiological female yellow catfish can be viable and fertile. We conclude that the mono-sex breeding technique by YY super-male yellow catfish is stable and reliable, which has great potential for application in yellow catfish aquaculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yellow catfish (Pelteobagrus fulvidraco Richardson) has become an important freshwater variety in Chinese aquaculture (Fishery Bureau of Ministry of Agriculture PRC 2010). Because of its tender flesh, few intermuscular spines, and delicious flavor, yellow catfish is quite popular with consumers in China. The output of yellow catfish reached respectively 134,448 and 163,556 tons in 2008 and 2009 in China. According to our survey, under the same breeding condition, the growth rate of the male yellow catfish is 30–50 % faster than that of the female sibling in the first year, and one to two times faster than the female sibling in the second year. Therefore, all-male yellow catfish aquaculture can dramatically increase the quality, output, and economic returns of fishery products by sex control. For this reason, we obtained XY physiological female fish by hormonal-induced sex reversal technique, and then produced YY super-male fish by gynogenesis of XY physiological female fish, and finally obtained all-male yellow catfish by mating of YY super-male yellow catfish and normal XX female yellow catfish (Liu et al. 2007). So it is very important how to realize the large-scale production of the YY super-male and the XY all-male.
Many researchers are suspicious of the survivability and reproductive capacity of YY super-male fish and YY physiological female fish. Major concerns are about the survival rate and fertility of YY super-male fish and its physiological female fish, which are the key points to realize the large-scale production of the YY super-male and the XY all-male. Yamamoto (1955) first confirmed the existence of YY super-male medaka (Oryzias latipes) through sex-reversed XY females mated with normal XY males. Then, YY super-male fish of the other species were obtained by hormonal-induced sex reversal, gynogenesis, or androgenesis. The YY super-males were also obtained in a variety of fishes, including guppy (Poecilia reticulate) (Kavumpurath and Pandian 1992), goldfish (Carassius auratus var.) (Yamamoto 1975), channel catfish (Ictalurus punctatus) (Goudie et al. 1985), Nile tilapia (Oreochromis niloticus) (Baroiller and Jalabert 1989; Scott et al. 1989; Mair et al. 1997) and tilapia (Oreochromis mossambicus) (Varadaraj and Pandian 1989), rainbow trout (Salmo gairdneri) (Chevassus et al. 1988), common carp (Cyprinus carpio L.) ( Bongers et al. 1999), golden rosy barb (Puntius conchonius) ( Kirankumar et al. 2003), which confirm that YY super-male fish can be viable and fertile. There are a few reports that YY super-male fish cannot survive as well, e.g., fighting fish (Betta splendens) (George et al. 1994) and zebra cichlid (Cichlasoma nigrofasciatum) (George and Pandian 1996).
Scientists hope to feminize YY super-male fish by hormonal-induced sex reversal technology to obtain YY physiological female fish. Then, the large-scale breeding of YY super-male can be achieved by mating small-scale YY super-male with small-scale YY physiological female, which can help to realize the large-scale production of XY all-male by mating the YY super-male with the normal XX female. Therefore, the feminization of YY super-male is the key step to achieve the large-scale breeding of YY super-male fish, and also the key point that constrains the large-scale production of XY all-male (Mair et al. 1997; Vera Cruz et al. 1996). The production of YY physiological female fish by hormone-induced sex reversal is rarely reported. One main reason may be that YY physiological females are not viable and fertile in some fishes, e.g., YY physiological female of guppy (Kavumpurath and Pandian 1993) and channel catfish (Davis et al. 1995). Davis et al. (1995) reported that YY female channel catfish could only produce <1 % viable eggs, perhaps due to the serious reproductive problems of YY physiological female. Although there are reports on YY super-male channel catfish afterwards, no report is available on YY female channel catfish (Smitherman et al. 1996; Davis et al. 2007). Vera Cruz et al. (1996) reported that YY physiological female Nile tilapia was obtained by sex reversal induced by diethylstilbestrol. But there was the relatively lower rate of sex reversal in YY super-male (<64 %) than that of normal XY male (>90 %). It is considered that YY juvenile fish is harder to feminize than normal XY male by estrogen-induced sex reversal, which may be related to the higher level of testosterone secreted in YY juvenile fish during sexual differentiation than that of normal XY male (Vera Cruz et al. 1996; Mair et al. 1997).
Although we had successfully bred the YY supper-male yellow catfish (Liu et al. 2007), it is very important to know how to achieve the large-scale production of the YY super-male and the XY all-male. For this purpose, we specifically test whether YY super-male yellow catfish could be successfully feminized by estrogen-induced sex reversal, and whether the YY physiological female could be viable and fertile in this study. Our data indicated that the large-scale breeding of YY super-male and XY all-male yellow catfish was realized because the YY super-male and YY physiological female were fertile.
Materials and Methods
Large-Scale Breeding Technology of YY Super-Male and XY All-Male Yellow Catfish
The large-scale breeding technology route of YY super-male and XY all-male yellow catfish is as follows (Fig. 1): Step 1, obtaining XY all-male by mating YY super-male with normal XX female; Step 2, obtaining XY physiologically female by estrogen-induced sex reversal of XY all-male fry; Step 3, conducting artificial gynogenesis on XY physiologically female yellow catfish, and selecting part of gynogenetic offspring for estrogen-induced sex reversal, while the others are cultured separately by conventional breeding methods; Step 4, the three progeny testing methods are simultaneously done from the gynogenetic offspring or their sex reversal offspring (Fig. 2). So the stable and reliable breeding system of YY super-male and YY physiological female is screened with 100 % male fish offspring in three progeny testing methods (Fig. 2); Step 5, small-scale YY super-male offspring is obtained by mating YY physiological female with YY super-male, and small-scale YY physiological females are obtained by estrogen-induced sex reversal. Step 6, the large-scale breeding of YY super-male was realized by mating small-scale YY super-male with small-scale YY physiological female, and which can help to realize the large-scale production of XY all-male by mating the YY super-male with the normal XX female.
Test Cross-Verification and Screening of YY Super-Male and YY Physiologically Female
In June 2005, XY all-male yellow catfish were obtained by mating YY super-male with normal XX female (Liu et al. 2007). The female parent was injected with the mixed spawning stimulation hormones (2,000 IU/kg HCG, 20 μg/kg LHRH-A, and 1 mg/kg carp pituitary), and the male parent was injected with the mixed spawning stimulation hormones (1,000 IU/kg HCG, 10 μg/kg LHRH-A, and 0.5 mg/kg carp pituitary). Then, the sperms were preserved in Hank’s balanced salt solution, which were derived from testicular homogenate extracts of YY super-male because sperm cannot be artificially extruded after induced spawning. And artificial insemination was done after the eggs were artificially extruded. On the fifth day of first feeding, the XY all-male fry were continuously fed by brine shrimp artemia immersed in 200 μg/L 17β-estradiol (E2) for 30 days, fed to satiation (eating up within half an hour) three times every day at 0800, 1300, and 1800 hours (Liu et al. 2007). So hundreds of XY physiological female were obtained by estrogen-induced sex reversal of XY all-male fry.
In June 2006, the sperms were preserved in Hank’s balanced salt solution, which were derived from testicular homogenate extracts of Chinese long-snout catfish (Leiocassis longirostris Günther). Meiotic gynogenetic diploids were obtained by fertilizing the eggs with UV-irradiated genetic inactivated sperms of Chinese long-snout catfish (764 μW/cm2) and subsequent cold shock (4 °C) for a duration of 20 min. Part of gynogenetic offspring was selected for estrogen-induced sex reversal, while the other was cultured separately by conventional breeding methods.
From June 1 to July 1 of 2007, the three progeny testing methods were simultaneously done from the gynogenetic offspring or their sex reversal offspring (Fig. 2). The offspring obtained by test cross was separately cultured according to different families. About 3 months later, the part of juvenile were dissected to identify the testis or ovary, record the gender proportion of testcross offspring of different families, and deduce the genotype of candidate male according to sex ratio of testcross offspring. So the stable and reliable breeding system of YY super-male and YY physiological female is screened with 100 % male offspring in three progeny testing methods (Fig. 2).
Feminization of YY Super-Male and Small-Scale Breeding of YY Super-Male Yellow Catfish
From June 1 to July 1 of 2008 and 2009, the YY super-male fry was obtained by mating YY super-male with YY physiological female screened by testcross verification. On the fifth day of first feeding, the YY super-male fry were continuously fed by brine shrimp artemia immersed in 200 μg/L E2 for 30 days, fed to satiation (eating up within half an hour) three times every day at 0800, 1300, and 1800 hours (Liu et al. 2007). So YY physiological female juvenile were obtained by estrogen-induced sex reversal of YY super-male fry. And YY physiological female juvenile and YY super-male juvenile were cultured separately to sexual maturity by conventional breeding methods.
Large-Scale Breeding of YY Super-Male and XY All-Male Yellow Catfish
The large-scale breeding of YY super-male was realized by mating small-scale YY super-male with small-scale YY physiological female, and the large-scale production of XY all-male was realized by mating the YY super-male with the normal XX female. Generally, large-scale breeding of yellow catfish adopts total artificial methods, i.e., artificial propagation, artificial fertilization, and artificial incubation. Male and female parents are artificially selected after enhanced cultivation. The female parent was injected with the mixed spawning stimulation hormones (2,000 IU/kg HCG, 20 μg/kg LHRH-A, and 1 mg/kg carp pituitary), and the male parent was injected with the mixed spawning stimulation hormones (1,000 IU/kg HCG, 10 μg/kg LHRH-A, and 0.5 mg/kg carp pituitary). Then, these parents were transferred into a pond after with the mixed spawning stimulation hormones. After courtship reaction, the sperms were preserved in Hank’s balanced salt solution, which were derived from testicular homogenate extracts of YY super-male, and artificial insemination was done after the eggs were artificially extruded. The sperm of one spawning male can match the egg of about five to seven females on average. After artificial insemination, hatch and management of fish fry follow routine methods. Postpartum female parent can be put back to special pond for culture and for reproduction the next year. The male rate (percentage) is one of the key indicators to evaluate the seeding quality of all-male yellow catfish during large-scale production, which can be identified by two gender identification methods by random sampling. The PCR detection by specific molecular markers linked to sexuality applies to more than 3-day-old fry (Wang et al. 2009). The gonad anatomy for gender identification applies to more than 30-day-old juvenile (Liu et al. 2007).
Data Processing and Analysis
If the testcross offspring between “YY” male yellow catfish candidate and normal XX female is 100 % male, the male parent can be confirmed as YY super-male yellow catfish. Likewise, if the testcross offspring between “YY” female yellow catfish candidate and normal XY male is 100 % male, the female parent can be confirmed as YY female yellow catfish. The frequency obtained from the testcross offspring analysis was subjected to descriptive statistics with STATISTICA 6.0.
Result
Progeny Testing, Screening, and Establishment of Breeding System for YY Super-Male and YY Physiological Female Yellow Catfish
The gynogenetic offspring of XY physiological female yellow catfish was used for sex reversal induced by 17β-estradiol to obtain YY physiological female and XX female. Thirty-three female parents were used for testcross with normal XY male, and 7 YY physiological females were screened with 100 % male offspring in 33 female parents, the proportion of which was 21.21 % (Table 1). Thirty-seven male parents were used for testcross with normal XX female, and 14 YY super-male were screened with 100 % male offspring in 27 male parents, the proportion of which was 51.85 % (Table 2). Fifty-six mating combinations between “YY” female yellow catfish candidate and “YY” super-male yellow catfish candidate had successfully been done, and the two mating combinations of YY super-male and YY physiological female are screened with 100 % male offspring in three progeny testing methods, the proportion of which was 3.57 % (Table 3). Forty YY super-male offspring reached sex maturity from the two mating combinations of YY super-male and YY physiological female, and the other 30 YY super-male offspring were successfully induced to YY physiological female by 17β-estradiol.
Influence of 17β-Estradiol on Sex Reversal of YY Super-Male
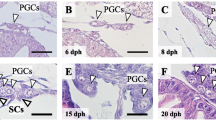
From 2008 to 2009, 5,220 YY super-male yellow catfish were used for hormone-induced sex reversal by 17β-estradiol (Table 4). And 4,568 YY physiologically female yellow catfish were obtained, with an average sex reversal rate of 96 % and an average survival rate of 86 % (Table 4). The successful sex reversal of YY super-male yellow catfish was displayed by H&E sections (Fig. 3). The testis of 131-day-old YY super-male was at stage I, and the ovary of 131-day-old YY physiological female was at stage II (Fig. 3).
Large-Scale Breeding of YY Super-Male and XY All-Male Yellow Catfish
From 2008 to 2010, the output of YY super-male yellow catfish was accumulated to 123,000, while the output of all-male yellow catfish was accumulated to 81 million. The male rate was stably 100 % by two gender identification methods during large-scale breeding of the all-male yellow catfish (Table 5). The YY super-male and YY physiological female were viable and fertile. The mono-sex breeding technique by YY super-male yellow catfish was stable and reliable during the large-scale breeding of YY super-male and XY all-male yellow catfish.
Discussion
Sex-Determining Mechanism of Yellow Catfish
The breeding technique of YY super-male yellow catfish is closely related to the sex-determining mechanism. Our lab found that the proportion of female offspring was 97 % (n = 346/357), and that the proportion of male offspring was only 3 % (n = 11/357) through the gynogenetic experiment on normal female yellow catfish (Liu et al. 2007). So it was concluded that the sex-determining mechanism of yellow catfish is XY male heterogamety (Liu et al. 2007). So the male in gynogenetic offspring of XY physiological female should theoretically be YY genotype according to the laws of Mendelian inheritance, which could be verified by testcross mating with the normal female. But it was found out that the male rate of testcross offspring was between 34.8 and 100 % in this study, and the individuals with male rate at 100 % in testcross offspring accounted for 40 % of all the testcross offspring. A similar phenomenon has been found in a large number of studies on fish mitotic gynogenesis and meiotic gynogenesis. It is inferred that the sex-determining mechanism of fish is quite complicated, controlled by multiple factors such as XY or ZW sex-determining system, autosomal genetic factor, or environmental factors, e.g., white sturgeon (Acipenser transmontanus Richardson) (Van Eenennaam et al. 1999), common carp (Komen et al. 1992; Cherfas et al. 1994), yellow perch (Esox masquinongy) (Dabrowski et al. 2000) channel catfish (Liu et al. 1996), Nile tilapia (Mair et al. 1991a), blue tilapia (Oreochromis aureus) (Mair et al. 1991b), etc. Therefore, we deduced that the sex-determining mechanism of yellow catfish is XY male heterogamety, possibly with some autosomal gene involved in sex determination and differentiation, while the impact of environmental factors such as temperature on sex determination of yellow catfish still needs further studies.
Mair et al. (1997) reported the realization of large-scale breeding of YY super-male tilapia and its application in aquaculture. In this report, the average male rate of offspring of YY super-male tilapia and normal female tilapia is 95.6 %, and the male rate of offspring of partial YY super-male tilapia and normal female tilapia can reach as high as 100 % (Mair et al. 1997). But the large-scale breeding of all-male yellow catfish has been realized by mating YY super-male with normal female yellow catfish in this study, and it is found that the average male rate of offspring of YY super-male and normal female yellow catfish is 100 % (So far, no female fish has been found in the sampling inspection). These difference between all-male tilapia study and all-male yellow catfish study may result from the following reasons: this study has conducted a large number of progeny tests, selected YY super-male yellow catfish and YY physiologically female yellow catfish with 100 % male fish in their offspring, and then preserved the pairing group of YY super-male yellow catfish and YY physiologically female yellow catfish with 100 % male fish in their offspring, realized the large-scale production of XY all-male yellow catfish (the male rate is 100 %), and ensured the stable and reliable production of XY all-male yellow catfish. The mechanism may be that the YY super-male yellow catfish breeding system has minimized the impact of autosome on sex in this study, or that YY super-male yellow catfish is homozygous dominant, and its genes concerning sex differentiation on autosome and sex chromosome are two same dominate allelic genes at the same locus, so the average male rate of offspring of YY super-male yellow catfish and normal female yellow catfish reached 100 %.
YY Super-Male and YY Physiological Female Yellow Catfish Can Be Viable and Fertile
Whether YY super-male and YY physiological female yellow catfish can be viable and fertile is the core element that restrains the large-scale production of YY super-male and XY all-male yellow catfish. The relevant research about YY super-male fish can relate to the origin and evolvement of sex chromosome. Scott et al. (1989) believed that X and Y sex chromosomes of tilapia have low differentiation degree, which may result in the survival of YY super-male tilapia. Meanwhile, different fish species have various modes of evolution during different stages of sex chromosome differentiation. Y chromosome of fish is still in the primitive stage, carrying a large number of functional genes (Charlesworth et al. 2005), which may also result in the fact that YY super-male fish and YY physiological female fish can be viable and fertile.
Since the sex reversal of teleost induced by female sex hormone initially successfully conducted by Yamamoto (1953), there are over 35 fish species that have gone through successful sex reversal from genetic male fish to phenotypic female fish induced by estrogen (Piferrer 2001). The feminization of YY super-male fish is the key step to establish the breeding stock of YY super-male fish, and also the key point that constrains the large-scale breeding of YY super-male fish, but it is generally believed that the feminization of YY juvenile fish is more difficult than that of normal XY male fish (Vera Cruz et al. 1996; Mair et al. 1997). At present, there are only two reports on successful sex reversal from YY super-male fish to fertile YY physiological female fish and large-scale production of YY super-male fish, e.g., YY physiological female medaka (Yamamoto 1967; Scholz et al. 2003) and YY physiological female tilapia (Vera Cruz et al. 1996) We feed the YY super-male yellow catfish on the fifth day after first feeding by brine shrimp immersed in 200 μg/L E2, and the average sex reversal rate reaches 96 %, the average survival rate reaches 86 %, the sex reversal efficiency is higher than that reported by Vera Cruz et al. (1996). It still needs further studies on whether the feminization of YY super-male yellow catfish juvenile is more difficult than that of normal XY male yellow catfish.
This study has reported that the breeding system of YY super-male yellow catfish is stable and reliable. And the large-scale breeding of YY super-male and XY all-male yellow catfish was realized, which indicates YY super-male yellow catfish and its physiological female can be viable and fertile. The feminization of YY super-male yellow catfish is of great importance to the large-scale production of YY super-male and XY all-male. Meanwhile, the reproductive capacity of YY super-male and its physiological female yellow catfish still needs further studies on the possibility of fertility degeneration of the breeding system caused by inbreeding of YY super-male yellow catfish and its physiological female.
Application Prospect of All-Male Yellow Catfish
Here are several alternatives to produce mono-sex male fish: artificial selection, sex reversal directly induced by androgen hormone, interspecific hybridization, temperature control, artificial androgenesis, YY super-male fish technology, triploidy, and transgenesis (Beardmore et al. 2001). Some catfish have such the physiological property that the male grows faster than the female. So it is of huge economic value to produce mono-sex male catfish. Park et al. (2004) obtained 90 % male bagrid catfish (Pseudobagrus fulvidraco (Richardson)) by hormone-induced sex reversal, but the method has the risk of hormone residue and water pollution. Lin and You (2004) reported that the proportion of male fish fry of Pseudobagrs vachelli (Richardson) could reach up to 78 % under high-temperature condition. But the proportion of male fish fry was only at 26.4 % in the other experiment with high-temperature-controlled method in the same lab (Cheng et al. 2007). Generally, fish under temperature-controlled condition are unstable, so it is unsuitable for large-scale production. Otherwise, some Chinese fish farmers adopt artificial selection or the method of keeping big fish while discarding small ones. Artificial selection can obtain a fairly high rate of mono-sex male, but both labor intensity and production costs are rather high. So it is hard to carry out large-scale commercial production of all-male fish. YY super-male fish technology can be without hormone residue, and be possible for large-scale production, but the developing process is time-consuming and labor-intensive (Mair et al. 1997). We have realized large-scale breeding of YY super-male and all-male yellow catfish, which is another successful case of sex control in fish. And YY super-male fish technology may be applied to other XY male heterogametic fish, which have the physiological property of the male growing faster than the female.
As the aquaculture technology of yellow catfish becomes increasingly mature, yellow catfish culture fishery has achieved remarkable development. In 2009, the output of yellow catfish in China reached 163,556 t, with an output value of RMB 4.25 billion; the demand quantity across China was about 4.96 billion fish fry, and the production quantity of yellow catfish in China achieved an annual increasing rate of 21.65 %. Yellow catfish has become one of the key freshwater fish species in China (Fishery Bureau of Ministry of Agriculture PRC 2010). In 2008–2010, 81 million all-male yellow catfish fry was cultivated in Hubei, Hunan, Guangdong, Tianjin, Guangxi, Guizhou, Liaoning, and Xinjiang, with a culture area of 344.67 ha; the average growth rate is about 35 %. All-male yellow catfish became an officially recognized variety by the Ministry of Agriculture of the People's Republic of China in December 2010, which was named as “all-male ReleaseI yellow catfish” (no. GS-04-001-2010). All-male yellow catfish have some advantages for aquaculture, e.g., all male, tidy specifications, fast growth, high output, etc. The average yield of all-male yellow catfish is about 35 % higher than that of the normal catfish, which have preliminarily displayed excellent market application prospect. All-male yellow catfish is suitably cultivated in pond, cage, and rice field of most fresh waters. All-male yellow catfish is provided with better production performance and is expected to increase production yield in per unit water body as well as economic benefit. It is thus quite promising in market application.
References
Baroiller JF, Jalabert B (1989) Contribution of research in reproductive physiology to the culture of tilapias. Aquat Living Resour 2:105–116
Beardmore JA, Mair GC, Lewis RI (2001) Monosex male production in finfish as exemplified by tilapia: applications, problems, and prospects. Aquaculture 197:283–301
Bongers ABJ, Zandieh-Doulabi B, Richter CJ, Komen J (1999) Viable androgenetic YY genotypes of common carp (Cyprinus carpio L.). J Hered 90:195–198
Charlesworth D, Charlesworth B, Marais G (2005) Steps in the evolution of heteromorphic sex chromosomes. Heredity 95:118–128
Cheng XC, Lin DJ, You YL (2007) Influence of temperature on sex differentiation of teleost, Pseudobagrus vachelli. Zool Res 28(1):73–80
Cherfas NB, Gomelsky BI, Emelyanova OV, Recoubratsky AV (1994) Induced diploid gynogenesis and polyploidy in crucian carp, Carassius auratus gibelio (Bloch), X common carp, Cyprinus carpio L., hybrids. Aquac Res 25:943–954
Chevassus B, Devaux A, Chourrout D, Jalabert B (1988) Production of YY rainbow trout males by self-fertilization of induced hermaphrodites. J Hered 79:89–92
Dabrowski K, Rinchard J, Lin F, Garcia-Abiado MA, Schmidt D (2000) Induction of gynogenesis in muskellunge with irradiated sperm of yellow perch proves diploid muskellunge male homogamety. J Exp Zool 287:96–105
Davis KB, Goudie CA, Simco BA (1995) The plasticity of sex determining genotypes in channel catfish. In: Goetz F and Thomas P. The Fifth International Symposium on the Reproductive Physiology of Fish. The University of Texas at Austin, Texas, USA, pp. 93–95
Davis KB, Goudie CA, Simco BA (2007) Sex genotype and sex phenotype contribute to growth differences between male and female channel catfish. N Am J Aqualcult 69:324–329
Fishery Bureau of Ministry of Agriculture PRC (2010) China Fishery Statistical Yearbook. China Agriculture Press, Beijing
George T, Pandian TJ (1996) Hormonal induction of sex reversal and progeny testing in the zebra cichlid Cichlasoma nigrofasciatum. J Exp Zool 275:374–382
George T, Pandian TJ, Kavumpurath S (1994) Inviability of the YY zygote of the fighting fish, Betta splendens. Israel J Aquacult 46:3–8
Goudie CA, Khan G, Parker N (1985) Gynogenesis and sex manipulation with evidence for female homogameity in channel catfish (Ictalurus punctatus). Annual Progress Report, 1 Oct.–30 Sept., USFWS, Southeastern Fish Culture Laboratory, Marion, AL
Kavumpurath S, Pandian TJ (1992) Production of YY male in guppy (Poecilia reticulata) by endocrine sex reversal and progeny testing. J asian Fish Sci 5:265–276
Kavumpurath S, Pandian TJ (1993) Production of YY female guppy (Poecilia reticulata) by endocrine sex reversal and selective breeding. Aquaculture 116:183–189
Kirankumar S, Anathy V, Pandian TJ (2003) Hormonal induction of super-male golden rosy barb and isolation of Y-chromosome specific markers. Gen Comp Endocrino 134:62–71
Komen J, Wiegertijes GF, Ginneken V, Van Muiswinkel WB, Huisman EA (1992) Gynogenesis in common carp (Cyprinus carpio L.): 3. The effects of inbreeding on gonadal development of heterozygous and homozygous gynogenetic offspring. Aquaculture 104:1–2
Lin DJ, You YL (2004) Exploration on artificial propagation and masculinizing technique of Pseudobagrus vachelli. J Fujian Fish 2:9–13
Liu HQ, Cui SQ, Hou CC, Xu J, Chen HX (2007) YY supermale generated gynogenetically from XY female in Pelteobagrus fulvidraco (Richardson). Acta Hydrobiol Sinica 31(5):718–725
Liu Q, Goudie CA, Simco BA, Davis KB (1996) Sex-linkage of glucose phosphate isomerase-B and mapping of the sex-determining gene in channel catfish. Cytogenet Cell Genet 73:282–285
Mair GC, Abucay JS, Skibinski DOF, Abella TA, Beardmore JA (1997) Genetic manipulation of sex ratio for the large scale production of all-male tilapia Oreochromis niloticus L. Can J Fish Aquat Sci 54(2):396–404
Mair GC, Scott AG, Penman DJ Beardmore JA, Skibinski DOF (1991a) Sex determination in the genus Oreochromis: I. Sex reversal, gynogenesis and triploidy in O. niloticus (L.). Theor Appl Genet 82:144–152
Mair GC, Scott AG, Penman DJ, Skibinski DOF, Beardmore JA (1991b) Sex determination in the genus Oreochromis: 2. Sex reversal, hybridisation, gynogenesis and triploidy in O. aureus Steindachner. Theor Appl Genet 82:153–160
Park IS, Kim JH, Cho SH, Kim DS (2004) Sex differentiation and hormonal sex reversal in the bagrid catfish Pseudobagrus fulvidraco (Richardson). Aquaculture 232:183–193
Piferrer F (2001) Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 197:229–281
Scholz S, RöSler S, Schäffer M, Hornung U, Schartl M, Gutzeit HO (2003) Hormonal induction and stability of monosex populations in the medaka (Oryzias latipes): expression of sex-specific marker genes. Biol Reprod 69:673
Scott AG, Penman DJ, Beardmore JA, Skibinsk DOF (1989) The ‘YY’ super-male in Oreochromis niloticus and its potential for aquaculture. Aquaculture 28:237–251
Smitherman RO, Dunham RA, Whitehead PK (1996) Selection, hybridization and genome manipulation in Siluroidei. Aquat Living Resour 9:93–102
Van Eenennaam AL, Van Eenennaam JP, Van Eenennaam JF, Medrano JF, Doroshov SI (1999) Evidence of female heterogametic genetic sex determination in white sturgeon. J Hered 90:231–233
Varadaraj K, Pandian TJ (1989) First report on production of super-male tilapia by integrating endocrine sex reversal with gynogenetic technique. Curr Sci 58:434–441
Vera Cruz EM, Mair GC, Marino RP (1996) Feminization of genotypically YY Nile tilapia Oreochromis niloticus L. Asian Fish Sci 9:161–167
Wang D, Mao HL, Chen HX, Liu HQ, Gui JF (2009) Isolation of Y- and X-linked SCAR markers in yellow catfish and application in the productionof all-male populations. Anim Genet 40:978–981
Yamamoto T (1953) Artificially induced sex-reversal in genotypic males of the medaka (Oryzias latipes). J Exp Zool 123:571–594
Yamamoto T (1955) Progeny of artificially induced sex-reversals of male genotype (XY) in the medaka (Oryzias latipes) with special reference to YY-male. Genetics 40:406–419
Yamamoto TA (1975) YY male goldfish from mating estrone-induced XY female and normal male. J Hered 66:2–4
Yamamoto TO (1967) Estrone-induced white YY females and mass production of white YY males in the medaka, Oryzias latipes. Genetics 55:329
Acknowledgments
This work was financially supported by the Special Fund for Agro-scientific Research in the Public Interest (grant no. 200903046), the Transformational Fund of Agricultural Scientific and Technological Achievements in China (grant no. 2010GB23320633), the National Natural Science Foundation of China (grant no. 31172400), and the Natural Science Foundation of Hubei Province of China (grant no. 2009CDA121, 2010CBB02101).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Authors Hanqin Liu and Bo Guan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, H., Guan, B., Xu, J. et al. Genetic Manipulation of Sex Ratio for the Large-Scale Breeding of YY Super-Male and XY All-Male Yellow Catfish (Pelteobagrus fulvidraco (Richardson)). Mar Biotechnol 15, 321–328 (2013). https://doi.org/10.1007/s10126-012-9487-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-012-9487-7