Abstract
To access to the microbial genetic resources of deep-sea sediment by a culture-independent approach, the sediment DNA was extracted and cloned into fosmid vector (pCC1FOS) generating a library of 39,600 clones with inserts of 24–45 kb. The clone fss6 producing red-brown pigment was isolated and characterized. The pigment was identified as melanin according to its physico-chemical characteristics. Subcloning and sequences analyses of fss6 demonstrated that one open reading frame (ORF2) was responsible for the pigment production. The deduced protein from ORF2 revealed significant amino acid similarity to the 4-hydroxyphenylpyruvate dioxygenase (HPPD) from deep-sea bacteria Idiomarina loihiensis. Further study demonstrated that the production of melanin was correlated with homogentistic acid (HGA). The p-hydroxyphenylpyruvate produced by the Escherichia coli host was converted to HGA through the oxidation reaction of introduced HPPD. The results demonstrate that expression of DNA extracted directly from the environment might generate applicable microbial gene products. The construction and analysis of the metagenomic library from deep-sea sediment contributed to our understanding for the reservoir of unexploited deep-sea microorganisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oceans constitute more than 70% of the earth’s surface. Work from coastal marine sediments or water is highlighted to portend future direction for deep-sea studies. The deep sea (generally defined by depths >1,000 m) was thought to be a biological ‘desert’ until submarine hydrothermal systems were discovered along mid-ocean ridges at depths greater than 2,200 m (Nichols et al. 2005). The deep sea is usually characterized by extremely high salinity, darkness, high pressure, and high/low temperature (Deming 1998). Deep-sea environments are centers of unusual biological communities due to the selective pressures on the inhabitants of the ecosystem. Therefore, the deep-sea is considered to be an enormous source of genetic and metabolic microbial biodiversity. Innovative culturing techniques have been employed to gain insight into microbial biochemical process and microbial by-products used for growth and survival in deep sea (Deming 1998). However, the functional analysis and use of most microbial genes were excluded for the majority of the microbial community was not cultivated (Streit et al. 2004). The dilemma of being unable to cultivate the vast majority of the microorganisms present in most microbial niches has forced scientists to develop methods to exploit the genomes of these bacteria for biotechnology without prior cultivation. Given that the metagenomic approach clones the total microbial genome (the metagenome) in culturable bacteria (such as Escherichia coli) to discover novel microbial resources, it represents an efficient method of isolating novel and useful genes (Lim et al. 2005). Previous studies have shown that the metagenomic approach permits searches for various novel enzymes and metabolites (Lorenz and Eck 2005). However, novel microbial resources from deep-sea sediments have been little explored by metagenomic approach.
Melanins are polyphenolic heteropolymers with a wide range of colors and applications. Due to their chemical composition, melanins have physicochemical properties that allow them to act as ultraviolet absorbers, cation exchangers, drug carriers, amorphous semiconductors, X-ray and γ-ray absorbers, and in some instances, substrates with antioxidant and antiviral activity (Riley 1997; Lagunas-Muñoz et al. 2006). Bacteria are known to produce 3,4-dihydroxyphenylalanine-melanins, the pheomelanins or eumelanins, and melanin-like pigments that are derived from non-nitrogenous phenols (the allomelanins or pyomelanins; Gibson and George 1998). Thus, not all bacterial brown and black pigments are true melanins. Production of melanins by recombinant E. coli is useful in industrial processes. Thus, the construction of metagenomic libraries of microorganisms of deep-sea sediments for the identification of novel genes involved in melanin synthesis should be valuable.
In this study, we constructed a metagenomic fosmid library of high-molecular-weight microbial DNA from deep-sea sediment of South China Sea. The fosmid clone fss6 producing melanins was screened, and the genes involved in melanins synthesis were characterized.
Materials and Methods
Sediment Samples
Sediment samples for DNA extraction were collected from the subsurface sediments at water depths of 1,256 m (111°48.171′ E, 17°15.589′ N), 1,330 m (110°16.362′ E, 17°09.324′ N), 1,575 m (114°07.960′ E, 18°45.972′ N), and 2,893 m (118°44.856′ E, 20°10.074′ N) in South China Sea by the Department of Guangzhou Marine Geological Survey. The samples were frozen at −20°С before being processed. To minimize contamination, the superficial parts exposed to the air were discarded when processed.
Extraction and Purification of Environment DNA
DNA extraction was performed using combined physical and chemical treatments described previously (Lai et al. 2007).
The crude DNA fragments of 30–50 kb (size-fractionated by pulsed-field gel electrophoresis, PFGE) were further purified using GELase™ Enzyme Preparation (Epicenter, Madison, WI, USA) according to the manufacturer’s protocol. High-molecular-weight DNA was precipitated by adding 1/10 volume of 3 M sodium acetate (pH 7.0) and 2.5 volumes of ethanol. Then, the pellets were dissolved in Tris–ethylenediaminetetraacetic acid buffer (pH 8.0) for metagenomic library construction.
Metagenomic Library Construction
The size-separated DNAs of 30–50 kb from different locations were pooled equally, and the genomic DNA was end-repaired to blunt end and cloned into the pCC1FOS fosmid vector using the Copycontrol Fosmid Library Production kit (Epicenter), according to the manufacturer’s instructions. The ligation mixture was then packed into lambda phages using MaxPlax™ Lambda Packaging Extracts. The packaged DNA was transformed into E. coli EPI300™-T1R. The E. coli transformants were diluted and plated on Luria–Bertani (LB) agar containing chloramphenicol (12.5 μg/ml). About 250–300 colonies were visible on each plate after incubating overnight at 37°С. The metagenomic library that consisted of 39,600 colonies was constructed by this method and stored at −80°С. Seventy randomly selected fosmid clones were used for determining the average insert size of the library and the polymorphism of the inserts. The fosmid DNA was isolated using alkaline minipreps. Then, the isolated DNA was digested with BamHI (Promega, Madison, USA) and examined by PFGE (Bio-Rad CHEF-DR II) under the conditions of 1% agarose in 1 × Tris–acetate-μ (TAE) buffer, 6 V/cm, 5–30 s swith for 16 h.
Subcloning a Pigment-Producing Clone
During the library storage process, we have selected metagenomic clones that altered the phenotype of E. coli cloning. The clone, designated fss6, producing red-brown pigment on LB medium was further analyzed by subcloning the responsible genes and identifying the pigment. The fss6 fosmid was extracted using QIAprep Spin Miniprep Kit (Qiagene) and partially digested with EcoRI, HindIII, KpnI, SalI, and SacI, respectively. Then, the fragments were size-separated by 1% agarose TAE gel electrophoresis. The fragments (1–10 kb) were collected and further purified using Qiaex П Gel Extraction Kit (Qiagene). The purified DNA fragments were ligated into pUC19 (Epicenter) with T4 DNA ligase (New England Biolabs). The ligation products were further transformed into E. coli DH5α. The transforments were grown overnight at 37°C on tyrosine medium (LB containing 0.1% tyrosine) to confirm whether the pigment belongs to melanins. The cloned DNA fragment of one clone (clone fss61) producing red-brown pigment was selected for sequencing using the universal M13-forward and M13-reverse primers. Sequences were analyzed using Editseq, Megalign, and Seqman programs of the DNAStar Packages. Open reading frames (ORFs) were identified using the ORF Finder program available at the National Center for Biotechnology Information. ORF-conferring pigment generation was further identified by subcloning. Putative complete ORFs (ORF2, ORF3, ORF4, and ORF3 together with ORF4) of fss61 were amplified using KOD-plus DNA polymerase (Toyobo) with corresponding primers, respectively. These polymerase chain reaction products were purified by the Qiaex П Gel Extraction Kit (Qiagene), digested with BamHI and SacI (Promega), and ligated into pUC19. The ligation products were transformed into E. coli cells and screened for pigment production on tyrosine medium.
Pigment Preparation
Purification of melanin produced in liquid cultures of recombinant E. coli was performed following a modified procedure from David et al. (1996). One liter of culture broth containing melanin was centrifuged (8,000×g, 15 min, 4°C). The cell pellets were discarded, and the melanin present in the supernatant was precipitated by adjusting the pH to 2.0 with 6N HCl. The precipitated melanin was concentrated by centrifugation (12,000×g, 30 min). Then, the melanin was dialyzed against ddH2O (8–10 kDa, 24 h) and air-dried. For use in experiment, dried melanin was dissolved in ddH2O at pH 12. Melanin concentration was determined spectrophotometrically at a wavelength of 400 nm.
Pigment Characterization
Solubility of purified melanin was tested with water, methanol, methanal, acetone, ethanol, chloroform, butanol, ethyl acetate, toluene, and petroleum ether. Reactivity to FeCl3, KMnO4, H2O2, and NaClO were tested.
The ultraviolet-visible (UV-Vis) absorption spectrum and infrared spectrum were recorded on Shimadzu UV-visible spectrophotometer (UV-2501PC) and Bruker Fourier transformation infrared spectrometer (Equinox 55), respectively.
The free-radical nature of the pigment was investigated by the use of electron paramagnetic resonance (EPR) spectrometer (JES-FE1XG, Jeol, Tokyo, Japan). The parameters for EPR were set as follows: modulation frequency, 100 kHz; modulation amplitude, 10 × 100; center field, 3,360.0 G; sweep width, 500.0 G; microwave frequency, 9.45 GHz; and microwave power, 4 mW.
Detection of Homogentistic Acid by High-Performance Liquid Chromatography
Exponential phase cultures of recombinant E. coli without melanin production in tyrosine medium was used to produce cell-free filtrates. The filtrates (1 ml) were immediately acidified with 40 μl glacial acetic acid and stored at −20°C until assayed. An aliquot of 5-μl samples was injected onto C18 reverse-phase column (250 × 4.6 mm) in a HP1100 high-performance liquid chromatography (HPLC; Hewlett-Packard) and UV detector. The pump maintained a flow rate of 0.8 ml/min of the eluting solvent (10% methanol plus 90% 0.01 M KH2PO4 solution, pH 3.0). The homogentistic acid (HGA) was detected at 265 nm. Homogentistic acid (Sigma Chemical Co.) was used as standards.
Nucleotide Sequence Accession Number
The nucleotide sequence reported here has been deposited at GenBank under accession number EU196552.
Results
Construction of the Metagenomic Fosmid Library
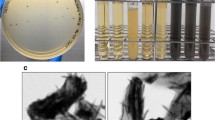
To obtain highly diverse genes from deep-sea sediments, samples isolated from different sites were pooled with equal quantity and cloned into the fosmid vector. The library that consisted of 39,600 clones was constructed from the deep-sea sediments and maintained for activity-based screening. The average DNA insert size was estimated at 33 kb (ranging from 24 to 45 kb) by analyzing 70 randomly selected clones via PFGE after BamHI digestion (examples are shown in Fig. 1). Restriction analysis of the clones revealed various restriction digestion patterns without any redundant pattern, indicating a high polymorphism of the cloned genes. Given the presence of color is an indication of biosynthetic genes for natural products, the red-brown clone fss6 was selected for further characterization.
Cloning and Sequence Analysis of Genes for Pigment Production
Since clone fss6 carried a relatively large insert DNA (approximately 36 kb), the DNA was fragmented by different restriction enzymes and cloned into pUC19 to construct a subsequent library. One clone, designated fss61, produced red-brown pigment as clone fss6 on solid and in liquid tyrosine medium. The inserted DNA of clone fss61 was further digested with SacI to determine the size of inserted DNA (Fig. 2). The insert DNA of approximately 4 kb was sequenced and analyzed for genetic information. The sequence analysis indicated that fss61 contains a 4.4-kb insert DNA with 51.8% G + C content. ORF analysis suggested that the fss61 harbors three complete ORFs (ORF2, ORF3, and ORF4) and one partial ORF (ORF1). The putative proteins encoded by these ORFs are shown in Fig. 3. Subcloning studies indicated that the recombinant clone carrying ORF2 can produce an identical level of pigment comparing with that of the original clones (fss6 and fss61; Fig. 4). The result suggested that ORF2 is responsible for melanin production.
Schematic diagram of the DNA cloned in fss61. ORF1 partial similar to a gene encoding homogentisate 1,2-dioxygenase, ORF2 a gene-encoding putative 4-hydroxyphenylpyruvate dioxygenase characterized in this work, ORF3 similar to a transcriptional regulator gene, ORF4 similar to a gene encoding pterin-4-alpha-carbinolamine dehydratase
Sequence analysis of ORF2 (designated hppd) indicated that it encodes a protein of 354 amino acids with molecular weight of approximately 40 kDa and isoelectric point of 4.55. A putative purine-rich ribosome-binding site, CGAGG, was observed 5 bp upstream of the start codon. The BLASTP search showed that the deduced protein shared high identity with the 4-hydroxyphenylpyruvate dioxygenase from marine bacteria Idiomarina loihiensis (identities 81%, accession YP_155112), Vibrio parahaemolyticus (identities 75%, accession NP_797728), Listonella anguillarum (identities 73%, accession AAL59614), Aeromonas hydrophila subsp. (identities 72%, accession YP_857172), Chromobacterium violaceum (identities 69%, accession AAQ58643), and Hahella chejuensis (identities 67%, accession YP_432268). Multiple alignment of the 4-hydroxyphenylpyruvate dioxygenase (HPPD) with several Gram-negative bacteria and the very distantly related Gram-positive soil bacterium HPPD proteins was presented in Fig. 5. The multiple sequence alignment of HPPD with the homologous HPPDs revealed some of the highly conserved residues that are common even in distantly related bacterium. Base on the alignment, most of the conserved residues in the C-terminal domain are essential for catalytic function activity and the presence of four conserved residues, i.e., Pro215, Asn217, Phe334, and Phe338. Further analysis showed that HPPD is rich in tyrosine (17 residues) and histidine (11 residues). Probably, they are potential metal-binding sites of the protein.
Amino acid sequence blocks conserved in the deduced amino acid sequences of HPPD and its homologs. The amino acids of HPPD are aligned with the sequences of the HPPD proteins of Listonella anguillarum (accession AAL59614), Pseudomonas Fluorescens (accession 1CJX-C), Pseudomonas putida (accession AAO12525), Legionella pneumophila (accession P69053), Shewanella colwelliana (accession M59289), and Streptomyces avermitilis (accession U11864). The amino acid residues suggested to be active iron atom (His162, His240, and Glu319) and play an important role in substrate binding, positioning, and directing catalysis (Pro215, Asn217, Phe334, and Phe338) are boxed. The identical, strong similarity and weak similarity are marked by an asterisk, colon, and dot, respectively
Characterization of Melanin Pigment
The pigment produced by clone fss6 is dissolved in water at pH 10 and precipitated at pH 3. However, it is slightly soluble in either methanol or methanal, insoluble in acetone, ethanol, chloroform, butanol, ethyl acetate, toluene, and petroleum ether. It was retained in the dialysis tubing (8,000 molecular weight exclusion). The pigment was profoundly bleached by the action of H2O2 and NaClO. A brown flooculent precipitate was formed after FeCl3 was added. In the presence of KMnO4, the color of the pigment solution turned green from brown. The chemical characteristics of the extracted pigment showed similarities to those of melanin.
The absorption spectrum of the pigment revealed a featureless decreasing profile over the range 190–900 nm just like melanin. The Fourier transform infrared spectrum of the pigment showed the peaks at the following wave length: A peak at 3,400–3,300 cm−1 is ascribed to OH or NH group stretching; peak at 2,960–2,860 cm−1 ascribed to CH2, CH3, and NH group stretching; peak at 1,650–1,600 cm−1 ascribed to aromatic C=C conjugated with C=O and/or COO− group stretching; peak at 1,310–1,450 cm−1 ascribed to stretching of amino groups and CH2 groups of aliphatic radicals, and may be include phenolic C–O–H bending. Based on the EPR spectra of the pigment (data not shown), it is possible to state the stable free radical nature of paramagnetic centers responsible for the EPR signal of the pigment. Thus, the physico-chemical characteristics of the pigment are those typically described for melanins.
HPLC Analysis for HGA
HPLC analysis for HGA from the cell-free filtrate of cultures showed that HGA was produced by recombinant E. coli strains before melanin was produced (Fig. 6). Higher peak without shoulders was produced at the retention time point of standard HGA when standard HGA solution was added into the cell-free filtrate. E. coli harboring vector pUC19 (control) did not produce pigment and HGA. Therefore, HGA is the product of ORF2 and should be the precursor of melanin.
Discussion
In this study, a metagenomic library that consisted of 39,600 clones was constructed from deep-sea sediment samples (depths ranging from 1,256 to 2,983 m). The metagenomic library represented about 1,300 Mbp of genomic DNA in deep-sea sediments. Therefore, the construction and analysis of the metagenomic library from deep-sea sediment contributed to our understanding for the reservoir of unexploited deep-sea microorganisms.
The clone fss6 producing red-brown pigment was isolated and further characterized in the study. Based on the physico-chemical characteristics of the pigment, the red-brown pigment was identified as melanin. The cloned gene (ORF2) encoding putative 4-hydroxyphenylpyruvate dioxygenase (HPPD) was responsible for the production of melanin in E. coli. The HPPD catalyzes the conversion of 4-hydroxyphenylpyruvate to HGA. This reaction is the second step of a metabolic pathway that generates acetoacetate and fumarate from l-tyrosine (Knox and Edwards 1955). Given that ketogenic and glucogenic products have a direct energetic contribution, the transformation catalyzed by HPPD showed both agricultural and therapeutic significance (Keon and Hargreaves 1998). Although the HPPD genes have been cloned from the metagenomic library of soil microbial DNA, the gene isolated from deep-sea sediment showed no similarity to the reported soil HPPD genes (MacNeil et al. 2001; Gillespie et al. 2002). The deduced amino acid sequence of the HPPD shares high identity with that of deep-sea bacterium I. loihiensis. Probably, the insert DNA of clone fss6 originated from a member of this genus. The members of Idiomarina could tolerate different extreme marine environments and were isolated from deep-sea hydrothermal vents, deep-sea water, and hypersaline water of solar saltern; however, they do not produce melanin (Ivanova et al. 2000; Brettar et al. 2003; Donachie et al. 2003; Martínez-Cánovas et al. 2004; Choi and Cho 2005). The I. loihiensis genome revealed that the bacteria derive carbon and energy primarily from amino acid metabolism rather than sugar fermentation (Hou et al. 2004). The HPPD enzyme is likely to be involved in tyrosine metabolism.
The known pathway for the catabolism of tyrosine to HGA in mammals and bacteria is that tyrosine is converted to p-hydroxyphenylpyruvate (p-HPP) through a transamination reaction. Then, p-HPP is converted to HGA through the oxidation of HPPD (Lindstedt and Odelhög 1987; Lindstedt et al. 1977). In the study, the transaminase for the initial step should exist in clone fss6. Three aminotransferase capable of converting tyrosine into p-HPP have been identified in E. coli (Pittard 1987). We propose that tyrosine is first catabolized by aminotransferases of E. coli host, then, the produced p-HPP is converted to HGA by the introduced HPPD gene products. Lastly, the accumulation and polymerization of HGA results in the formation of melanin. The results demonstrate that the chimeric metabolic pathway could generate different chemical structures, and expression of DNA extracted directly from the environment might find previously uncharacterized natural products.
The general limitations of metagenomic cloning and heterogenous expression in E. coli have been found (Daniel 2005). Developing the technology such as expression in hosts with different expression properties should greatly enhance the ability to detect novel biocatalysts and natural products from unusual and extreme environments. The metagenomic approach should increase the likelihood of future discoveries of novel natural products.
References
Brettar I, Christen R, Höfle MG (2003) Idiomarina baltica sp. nov., a marine bacterium with a high optimum growth temperature isolated from surface water of the central Baltic Sea. Int J Syst Evol Microbiol 53:407–413
Choi DH, Cho BC (2005) Idiomarina seosinensis sp. nov., isolated from hypersaline water of a solar saltern in Korea. Int J Syst Evol Microbiol 55:379–383
Daniel R (2005) The metagenomics of soil. Nat Rev Microbiol 3:470–478
David C, Daro A, Szalai E, Atarhouch T, Mergeay M (1996) Formation of polymeric pigments in the presence of bacteria and comparison with chemical oxidative coupling-II. Catabolism of tyrosine and hydroxyphenylacetic acid by Alcaligenes eutrophus CH34 and mutants. Eur Polym J 32:669–679
Deming JW (1998) Deep ocean environmental biotechnology. Curr Opin Microbiol 9:283–287
Donachie SP, Hou S, Gregory TS, Malahoff A, Alam M (2003) Idiomarina loihiensis sp. nov., a halophilic gamma-proteobacterium from the Lō’ihi submarine volcano, Hawai’i. Int J Syst Evol Microbiol 53:1873–1879
Gibson LF, George AM (1998) Melanin and novel melanin precursors from Aeromonas media. FEMS Microbiol Lett 169:261–268
Gillespie DE, Brady SF, Bettermann AD, Cianciotto NP, Liles MR, Rondon MR, Clardy J, Goodman RM, Handelsman J (2002) Isolation of antibiotics turbomycin A and B from a metagenomic library of soil microbial DNA. Appl Environ Microbiol 68:4301–4306
Hou S, Saw JH, Lee KS, Freitas TA, Belisle C, Kawarabayasi Y, Donachie SP, Pikina A, Galperin MY, Koonin EV, Makarova KS, Omelchenko MV, Sorokin A, Wolf YI, Li QX, Keum YS, Campbell S, Denery J, Aizawa S, Shibata S, Malahoff A, Alam M (2004) Genome sequence of the deep-sea γ-proteobacterium Idiomarina loihiensis reveals amino acid fermentation as a source of carbon and energy. Proc Natl Acad Sci U S A 101:18036–18041
Ivanova EP, Romanenko LA, Chun J, Matte MH, Matte GR, Mikhailov VV, Svetashev VI, Huq A, Maugel T, Colwell RR (2000) Idiomarina gen. nov., comprising novel indigenous deep-sea bacteria from the Pacific Ocean, including descriptions of two species, Idiomarina abyssalis sp. nov. and Idiomarina zobellii sp. nov. Int J Syst Evol Microbiol 50:901–907
Keon J, Hargreaves J (1998) Isolation and heterologous expression of a gene encoding 4-hydroxyphenylpyruvate dioxygenase from the wheat leaf-spot pathogen, Mycosphaerella graminicola. FEMS Microbiol Lett 179:5862–5868
Knox WE, Edwards SW (1955) Enzymes involved in conversion of tyrosine to acetoacetate. Methods Enzymol 2:287–300
Lagunas-Muñoz VH, Cabrera-Valladares N, Bolívar F, Gosset G, Martínez A (2006) Optimum melanin production using recombinant Escherichia coli. J Appl Microbiol 101:1002–1008
Lai X, Cao L, Tan H, Fang S, Huang Y, Zhou S (2007) Fungal communities from methane hydrate-bearing deep-sea marine sediments in South China Sea. ISME J 1:756–762
Lim HK, Chung EJ, Kim JC, Choi GJ, Jang KS, Chung YR, Cho KY, Lee SW (2005) Characterization of a forest soil metagenome clone that confers indirubin and indigo production on Escherichia coli. Appl Environ Microbiol 71:7768–7777
Lindstedt S, Odelhög B (1987) 4-Hydroxyphenylpyruvate dioxygenase from human liver. Method Enzymol 142:139–142
Lindstedt S, Odelhög B, Rundgren M (1977) Purification and some properties of 4-hydroxyphenylpyruvate dioxygenase from Pseudomonas sp. P. J. 874. Biochemistry 16:3369–3377
Lorenz P, Eck J (2005) Metagenomics and industrial applications. Nat Rev Microbiol 3:510–516
MacNeil IA, Tiong CL, Minor C, August PR, Grossman TH, Loiacono KA, Lynch BA, Phillips T, Narula S, Sundaramoorthi R, Tyler A, Aldredge T, Long H, Gilman M, Holt D, Osburne MS (2001) Expression and isolation of antimicrobial small molecules from soil DNA libraries. J Mol Microbiol Biotechnol 3:301–308
Martínez-Cánovas MJ, Béjar V, Martínez-Checa F, Páez R, Quesada E (2004) Idiomarina fontislapidosi sp. nov. and Idiomarina ramblicola sp. nov., isolated from inland hypersaline habitats in Spain. Int J Syst Evol Microbiol 54:1793–1797
Nichols MCA, Guezennec J, Bowman JP (2005) Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: a review. Mar Biotechnol (NY) 7:253–271
Pittard AJ (1987) Biosynthesis of the aromatic amino acids. In: Neidhardt FC (ed) Escherichia coli and Salmonella typhimurium: cellular and molecular biology. ASM, Washington, DC, pp 368–394
Riley PA (1997) Melanin. Int J Biochem Cell Biol 29:1235–1239
Streit WR, Daniel R, Jaeger KE (2004) Prospecting for biocatalysts and drugs in the genomes of non-cultured microorganisms. Curr Opin Biotechnol 15:285–290
Acknowledgment
This work was supported by a grant from the National High Technology Research and Development Plan (863) of China (2007AA091904) and China Ocean Mineral Resources R&D Association (DYXM-115-02.2.14).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Huang, Y., Lai, X., He, X. et al. Characterization of a Deep-Sea Sediment Metagenomic Clone that Produces Water-Soluble Melanin in Escherichia coli . Mar Biotechnol 11, 124–131 (2009). https://doi.org/10.1007/s10126-008-9128-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-008-9128-3