Abstract
We report a pluripotent embryonic stem cell-like cell line designated as SBES from blastula stage embryos of Asian sea bass (Lates calcarifer), which is an economically important cultivable and edible marine fish species in India. The SBES cells were cultured at 28°C in Leibovitz L-15 medium supplemented with 20% fetal bovine serum without a feeder layer. The ES-like cells were round or polygonal and grew exponentially in culture. The SBES cells exhibited an intense alkaline phosphatase activity and expression of transcription factor Oct 4. The undifferentiated state of these cells was maintained at low seeding densities and the cells formed embryoid bodies when seeded in bacteriological plates. On treatment with all-trans retinoic acid, these cells differentiated into neuron-like cells, muscle cells, and beating cardiomyocytes, indicating their pluripotency. This embryonic ES-like cell line derived from an oviparous fish blastula conserved several peculiar features of viviparous mammalian embryonic stem cell lines. The present study highlights the importance and potential of piscine ES-like cell line for stem cell research without evoking ethical issues and invasive interventions sparing mammalian embryos.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammalian embryonic stem (ES) cell lines are undifferentiated long-term cell cultures derived from inner cell mass of developing embryos (Evans and Kaufman, 1981; Martin, 1981). These cells retain ES cell characteristics and can be induced to differentiate into a variety of cell types. When introduced into host embryo, the ES cells can participate in normal development and contribute to several tissues of the host, including cells of the germ line (Bradley et al., 1984). These characteristics make ES cells ideal experimental systems for in vitro studies of embryonic cell development and differentiation and a vector for the efficient transfer of foreign DNA into the germ line of an organism (Goossler et al., 1986). In addition, ES cells provide an attractive strategy for the preservation of biodiversity (Hong et al., 1996). ES cell lines have been first reported in mice (Evans and Kaufman, 1981; Martin, 1981; Bradley et al., 1984) and extended to medakafish (Hong et al., 1996, 1998) and human (Thompson et al., 1998). These cells have the potential to produce any type of cell of the body and can be propagated in unlimited quantities for clinical applications.
Most attempts to culture ES cells have been based on the original methods established for mice (Evans and Kaufman, 1981; Martin, 1981). To prevent differentiation in culture, mouse ES cells have been derived and maintained on feeder layers (Robertson, 1987), in conditioned media (Martin, 1981; Handyside et al., 1989), or in a medium supplemented with leukemia inhibitory factor (LIF) (Pease et al., 1990; Hasty et al., 1991; Wurst and Joyner, 1993) or with LIF-related cytokines (Conover et al., 1993; Nichols et al., 1994; Piquet-Pellorce et al., 1994; Yoshida et al., 1994). In mammalian species other than mice and rat, however, ES cells could be cultured only for a limited period (Sims and First, 1993) or their pluripotency could be maintained only partially after long-term culture (Sukoyan et al., 1992; Campbell et al. 1996). It has been suggested that the differentiation-inhibiting conditions suited for mice did not adequately prevent differentiation of ES cells in species other than rodents (Anderson, 1992). To develop ES cell lines and gene targeting techniques in fish, extensive studies have been done in small model fishes, such as zebrafish (Danio rerio) and medaka (Oryzias latipes), because they offer the possibility of combining embryological, genetic, and molecular analysis of vertebrate development. ES-like cell lines have been established in medaka (Wakamatsu et al., 1994; Hong et al., 1996) and zebrafish (Collodi et al., 1992; Sun et al., 1995). One medaka ES-like cell line, MES1, was shown to retain a diploid karyotype and the ability to form viable chimeras (Hong et al., 1998). Attempts toward the derivation of ES cell lines from the zebrafish (Collodi et al., 1992; Sun et al., 1995) and medakafish (Wakamatsu et al., 1994) by using feeder layer techniques have been partially successful. Bejar et al. (1999) have derived a long-term embryonic cell culture from Sparus aurata, and these cells have been characterized in vitro for totipotency and transfected with a GFP plasmid. Chen et al., (2003a, b) have established a pluripotent cell line, LJES1, from blastula-stage embryos of Lateolabrax japonicus and red sea bream blastulas. These cells were found to have differentiated into various types of cells after treatment with retinoic acid (RA). Chen et al., (2007) have developed pluripotency and chimera competence of an embryonic stem cell line from the sea perch (L. japonicus). Holen and Hamre (2003) have derived a long-term embryonic stem cell–like culture from a marine flatfish, Scophtalmus maximus, and these cells expressed Oct4 transcription factor.
Another important aspect of piscine cells is their lowest position in the ladder of evolution, which makes them vulnerable for xenotransplantation in mammals (Wright and Yang, 1997; Laue et al., 2001; Wright and Pohajdak, 2001). The islet tissue in certain teleost fish like tilapia called Brockmann bodies has been shown to restore normoglycemia on transplantation into diabetic nude mice (Wright and Pohajdak, 2001). Similarly, reversal of streptozotocin-diabetes has been achieved after transplantation of piscine islets to nude mice (Laue et al., 2001). These reports indicate potential of piscine cells for clinical applications.
In the present study, an attempt has been made to establish and characterize the embryonic stem–like cells from blastula stage of sea bass embryos and their in vitro differentiation into multiple cell lineages.
Materials and Methods
Sea Bass Embryo Cell Culture
To establish a sea bass embryonic stem cell culture, blastula-stage embryos were obtained from a sea bass fish hatchery, Central Institute of Brackish Water Aquaculture, Chennai and were transported to the lab in sterile sea water. The entire process of cell culture was completed within 6 h after fertilization. A group of 300 to 500 embryos was disinfected with 70% ethanol, washed three times with sterile phosphate-buffered saline (PBS, pH 7.2), and the chorion was torn with fine forceps. The cell mass was released and the chorion was removed. Single cells were obtained via gentle pipetting. After several washes with PBS, the cells were transferred to complete medium consisting of Leibovitz’s L-15 (Gibco) supplemented with antibiotics (penicillin, 100 U/ml; streptomycin, 100 μg/ml; Gibco BRL) and fetal bovine serum (FBS, 20%) in 25-cm2 tissue culture flasks. Cells were cultured at 28°C in an incubator and the medium was changed at an interval of 2 to 3 days. After reaching 80% confluency, the cells were subcultured at a ratio of 1:2 following the standard trypsinization protocols.
Alkaline Phosphatase (AP) Staining
To examine the activity of AP in the cells prepared from blastula-stage embryos, the cells were washed with PBS, fixed in glutaraldehyde solution (1%) for 10 min, washed twice with PBS, and then stained in dark with bromochloroindolyl phosphate/nitroblue tetrazolium (BCIP/NBT, Sigma-Aldrich) as substrate.
Chromosome Analysis
SBES cells at passage 30 were used for chromosome analysis. The cells were seeded in a 25-cm2 culture flask and incubated for 24 to 36 h. Colchicine (0.04%) (Sigma-Aldrich) was added to the cells and incubated for 2 h in culture flasks. Cells were removed from the flask surface and centrifuged at 500 × g for 5 min. The pellet was gently resuspended in 0.027 M KCl and incubated at 25°C for 30 min. Cells were then centrifuged again at 500 × g for 5 min. Supernatants were discarded and cells were resuspended. Freshly prepared mixture of cold methanol–acetic acid (3:1) fixative was added slowly while aspirating the cell suspension gently. These fixed cells were then washed three times with fresh fixative, and then resuspended in a small quantity of fixative. The suspension was subsequently dropped on glass slides, air dried, and stained with 5% Giemsa (pH 6.8) for 15 to 20 min. Chromosome counts were performed in more than 100 metaphase plates.
In Vitro Differentiation Potential
Differentiation potential of sea bass embryonic cells was examined by applying RA (Hong et al., 1996). The embryonic cells were inoculated at a density of 5 × 105 cells per well and then cultured for 30 days with regular medium change. Two days after seeding, the medium was replaced by another medium supplemented either with 2 μM all-trans RA (Sigma-Aldrich) or adipogenic medium containing 10 mg/ml of insulin, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), and 0.25 mM dexamethasone initially for 48 h, followed by insulin alone for 10 days. Control cultures were devoid of inducing agents. Alternatively, cells were seeded on different extracellular matrix (ECM)-coated plates i.e., collagen, gelatin, laminin (1 mg/ml each), fibronectin (10 μg/ml), and commercially available ECM (Sigma-Aldrich). The ES cells were seeded at a density of 103 cells/well/2 ml of medium on coated plates. The ES cells seeded on bovine serum albumin (BSA)-coated wells served as negative control. Morphological changes in cells were examined daily for a period of 25 days. In all cases, the medium was changed once in 3 days.
Formation of Embryoid Bodies
To induce the formation of embryoid bodies (EBs) cells were cultured in suspension. Five milliliters of cell suspension (106 cells/ml Leibovitz’s L-15 medium) was seeded on 6-cm bacteriological dishes. After 2 days, most of the cells participated in the process of growing aggregates, which further developed into three-dimensional structures. By day 5, the EBs were replated on gelatin-coated plates for attached growth. For drug-induced differentiation, all-trans RA was dissolved in dimethyl sulfoxide and added to cell cultures at a final concentration of l to 5 μM. Cell morphology was microscopically examined daily.
Teratoma Formation
To induce teratoma formation, the undifferentiated SBES cells were first injected (1 × 107 cells) into the hind limbs of two nude female mice (this was done at the National Centre for Cell Science, Pune) and then injected intramuscularly (1 × 107 cells) into five juvenile sea bass. The subjects were observed for 2 months for any tumor development.
Immunofluorescence Staining and Confocal Laser Scanning Microscopy
For immunophenotyping of the SBES cell line, the cells were grown on coverslips for 24 hours, fixed with 3.7% p-formaldehyde for 10 min at 4°C, washed with PBS, permeabilized with 0.1% Triton X-100, and blocked in PBS containing 1% bovine serum BSA. Mouse/rabbit preadsorbed monoclonal antibodies Actinin, Smad 4, Map2, Brachury, NKX2.5, GFAP and Oct 4 (Chemicon) were diluted 1:75 in PBS with 1% BSA and directly added to the fixed cells and left at room temperature for 2 h. The cells were then washed with wash buffer, followed by adding appropriate secondary antibody (Chemicon) for 45 min at RT in dark. The cells were again washed several times with wash buffer and were mounted in antifade mounting medium (Oncogene). The cells were observed by using a pinhole setting of 100 μm with CFLSM (Carl Zeiss, Jena, Germany). Images were captured via a CCD-4230 camera coupled with the microscope and processed using the computer-based programmable image analyzer KS300 (Carl Zeiss).
Results
Establishment of SBES Cell Line
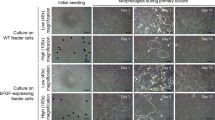
Cell cultures initiated from blastula stage embryos of sea bass are depicted in Figure 1A. After 3 days, the cells grew to confluence in the flask and were subcultured at an interval of 3 to 4 days. Six primary cultures were obtained from isolated blastomeres of sea bass blastula embryos. Five of the six primary cultures differentiated and gradually died, while the remaining culture developed into a cell line in Leibovitz’s L-15 medium supplemented with 20% FBS. This cell line was designated as SBES and was used for detailed characterization. Cells were seeded at low cell density of 2 × 104 cells/ml and were allowed to grow at not more than 70% confluence such that it remained in undifferentiated state. When these cells were left in the culture flask and attained more than 90% confluence, they spontaneously tended to differentiate into neuronal and muscular phenotypes. The SBES cells were either round or polygonal with a big nucleus and sparse cytoplasm and formed colonies (Figure 1B). The colony-forming ability is an important feature of ES-like cells. These colonies were tightly packed and uniform in morphology (Figure 1C). Most of the cells in the colony exhibited a strong alkaline phosphatase activity. After a culture period of around 230 days with more than 52 passages, the SBES cells displayed stable growth and ES-like morphology. During this period, SBES cell line did not show any detectable growth crisis or senescence. The population doubling time was found to be 48 h (data not shown).
Characterization of SBES cells (A) Blastula of sea bass embryo. (B) SBES cells at passage 25, showing round or polygonal cells with big nuclei and sparse cytoplasm. (C) ES-like cell colony of SBES cells at passage 30. (D) Alkaline phosphatase (AP) staining of SBES cells at passage 45, with almost all cells strongly AP positive. (E) Negative control for AP staining of SBES cells at passage 45. (F) Undifferentiated cells expressing transcription factor Oct 4 (green) at passage 40; nucleus stain with DAPI (blue). (G) Chromosome number distribution of SBES cells at 30th passage. One hundred metaphase plates were counted. (H) Metaphase of SBES cells at 30th passage.
SBES cells at different passages (10 and 45) were stained with BCIP-NBT, and most of the cells exhibited strong AP activity (Figure 1D). The negative control showed absence of AP (Figure 1E). The undifferentiated SBES cells showed the presence of pluripotency marker gene Oct 4 (Figure 1F), indicating that these cells are pluripotent in nature.
SBES Cells Form EBs and Retain Differentiation Potential
Embryoid body formation is one unique feature of mammalian ES cells. To determine the ability of SBES cells to form EBs, dissociated cells were cultivated in nonadhesive plastic dishes in the presence or absence of RA. After 2 days of suspension culture, the majority of cells formed aggregates and these aggregates became steadily larger, denser, and eventually developed into spherical structures called embryoid bodies. After 14 days, approximately 30% of EBs (Figure 2F) grew in the presence of RA. When 5-day-old EBs were transferred to gelatinized 24-well plates (5 to 20 EBs/well), melanocytes and neuron-like cells appeared 10 days after plating in more than 50% of the wells (Figure 2G and H). These results indicate that similar to mammalian ES cells SBES cells also form EBs that retain their potential to differentiate into melanocytes and neuron-like cells.
In vitro differentiation in SBES cells. (A) Melanocytes. (B) Neuron-like cells appeared in the culture 10 to 15 days after treatment with retinoic acid. (C) Network of muscle type cells appeared in the culture 3 weeks after treatment with retinoic acid. (D) Oligodendritic cells appeared in the culture 2 weeks after treatment with retinoic acid. (E) Formation of adipocytes after treating with insulin. Arrowheads show presence of oil droplets inside adipocytes. (F) Embryoid body. (G) Formation of melanin rich cells. (H) Nerve cells after RA treatment of embryoid body.
ES cells from the EBs were differentiated into neuronal lineages by adding RA (l to 5 μM) into culture medium. The neuronal differentiation was confirmed by immmunocytochemistry with Map2 antibodies (Figure 3A). Map2 is a marker for matured neuron. Positive cells indicated that the ES cells differentiated into neuronal lineages. Glial fibrillary acidic protein (GFAP) is a marker for oligodendrocytes. It was observed that ES cells formed oligodendrocytes (Figure 3C) also. In the same culture a few cells were found to be positive for mesodermal marker brachyury (Figure 3D). It means that after addition of RA, cells have differentiated into neuronal lineages with more efficiencies. The expression of smad4 was also observed in SBES cells (Figure 3F). The subsets of mesodermal cells were also positive for sarcomeric actinin (Figure 3E). A few cells were positive for NKX 2.5 (Figure 3B), indicating that cardiac differentiation was not up to the extent of the neuronal differentiation after adding RA. MCF-7 (a breast cancer cell line) (Figure 3G) was taken as negative control. No positive signal was seen in MCF-7 cells.
Differentiation potential of SBES embryoid bodies following treatment with RA Confocal micrographs showing expression of lineage specific markers. (A) Map2 (green) nucleus stain with DAPI (blue). (B) NKX2.5. (C) GFAP. (D) Brachyury. (E) Actinin. (F) Smad 4. (G) MCF -7 (breast cancer cell line) as a negative control.
SBES Cells Are Pluripotent and Differentiate into Various Cell Lineages Under Appropriate Inducing Agents
Under normal culture conditions, a few SBES cells in either primary or early passage cultures have spontaneously differentiated into neuron-like cells, pigment cells, fibroblast-like cells, muscle cells, and other unidentified cell types. These differentiated cells died during subsequent cultivations. SBES cells also differentiated into a variety of cell types under the influence of all-trans RA. When SBES cells at passages 5 to 15 were treated with RA, most cells lost their stem cell like morphology and differentiated into various cell types, including neuron-like cells, oligodendritic cells, melanocytes, muscle cells, and unidentified cells. Melanocytes began to appear 12 to 15 days after induction (Figure 2A). Neuron-like cells began to appear 8 to 10 days after induction (Figure 2B). Network of muscle type cells appeared on the 20th day after induction (Figure 2C). The oligodendritic cells appeared 12 to 16 days after induction (Figure 2D). Cardiomyocytes-like cells appeared on 15th day after induction and these cells showed periodic pulsation (movie supplement). When exposed to adipogenic medium containing insulin, IBMX, and dexamethasone, the cells differentiated into oil droplets containing adipocytes (Figure 2E).
The cells of SBES were seeded on different ECM protein-coated plates. The cells when seeded on plates coated with collagen, differentiated predominantly into spread cells with large lamellae and prominent nucleus after 9 days of incubation (Figure 4A). The cells treated with extracellular matrix differentiated into elongated fibroblastic type of cells after 7 days of incubation (Figure 4B). The cells seeded on gelatin-coated plates remained undifferentiated and formed polygonal type cells and embryoid body type clusters after 25 days of incubation (Figure 4C and D). The cells treated with laminin and fibronectin differentiated into mixed population of cells even after 25 days of culture. The untreated tissue culture plates were used as controls for the study. In these plates, the number of differentiated cells was very less. Moreover, it took a longer time for different phenotypes to appear (figures not shown).
SBES Cells Exhibit Diploidy
The results of chromosome counts of 100 metaphase plates from SBES cells at passage 30 showed a modal peak at 48 chromosomes (Figure 1G and H), exhibiting their diploid nature.
Nontumorigenic Nature
Undifferentiated SBES cells injected into the nude mice and juvenile sea bass did not show any sign of tumor formation for more than 60 days. This result indicates the nontumorigenic nature of SBES cells.
Discussion
The piscine ES cell has attracted the attention of fish breeders and molecular biologists owing to its possible importance in producing transgenic fish with site-directed integration of foreign gene and in studying gene function in fish (Ma et al., 2001; Wakamatsu et al., 2001; Chen et al., 2002; Rocha et al., 2004). Establishment of ES gene targeting techniques in cultured marine fish provides a novel approach for genetic improvements; developmental biology, and analysis of gene function in fish. Thus ES cell lines are urgently desired for use in basic and applied research.
The establishment and characterization of an ES-like cell line, designated as SBES (sea bass embryonic stem-like cells) derived from the blastula stage embryos of sea bass (Lates calcarifer), is described in this article. This cell line is well adapted to grow at 28°C in Leibovitz’s L-15 medium supplemented with FBS. The SBES cells showed typical ES-cell like morphology, which closely resembled that of mouse and human ES cells. The SBES displayed stable growth over 240 days of culture with more than 52 passages in the absence of feeder layer similar to that reported in the case of medaka fish (Hong et al., 1996). The feeder free culture cells retained their stem-ness by seeding cells at low cell density of 2 × 104 cells/ml. However, when these cells were left in the culture flask and attained more than 90% confluence, they tend to differentiate spontaneously into neuronal and muscular phenotypes. Because differentiated cells constitute only a minor population under normal culture conditions and have a limited life span, they do not affect their in vitro manipulation. Cellular alkaline phosphatase activity is a well-established indicator of pluripotency in mouse ES cells (Wombus et al., 1984; Pease et al., 1990). In SBES culture, most of the cells exhibited intensive alkaline phosphates staining (Figure 1D), which is similar to reports of Wakamatsu et al., (1994) and Hong et al., (1996), thus supporting our data and indicating their undifferentiated state.
The chromosome analysis of SBES cells at passage 30 showed modal chromosomal number of 2n = 48 (Figure 1G and H), which was identical with the diploid number of chromosome of this species. This diploid karyotype stability is of high importance for ES cells to enable them to form functional germ line chimeras (Bradley et al., 1984).
The determination and maintenance of the cell fate are ultimately due to differential gene activity. In the mouse, expression of the transcription factor Oct 4 was high in totipotent inner cell mass, germ cells and undifferentiated embryonic stem (ES) cells, but was dramatically reduced or lost upon differentiation. Interestingly, when SBES cells were tested with mammalian specific Oct 4 preadsorbed antibody and analyzed under confocal microscope, found to express Oct 4 in the fraction of cells (Figure 1F) localized in cytoplasm instead of its usual localization in the nucleus. Because expression of Oct 4 is restricted to mammalian ES cells, further experiments in SBES cells to confirm the expression of RNA and as well as protein level are in progress.
The potential to differentiate into various cell lineages is one of the important criteria for evaluation of pluripotency in ES cells in other animal species including fish (Martin, 1981; Thompson et al., 1998). The SBES cells were found to be pluripotent as evidenced by their ability to undergo differentiation in vitro into muscle cells, melanocytes, neuron-like cells, cardiomyocytes, and oligodendritic cells, in response to all trans RA induction. Similar observations were made in mouse and medaka fish (Nakano et al., 1994; Wakamatsu et al., 1994; Hong et al., 1996). Formation of EBs is another important characteristic of ES cells. One of the most impressive phenotypes which develop in vitro, is clusters of spontaneously beating cardiomyocytes followed by the formation of skeletal muscle and neuronal cells. However, lineage-specific differentiation occurs only after adding specific differentiation markers in ES cells and EBs (Wombus, 2001). SBES cells also exhibited EB formation by hanging drop culture. Interestingly, when these EBs were plated in the presence of RA they differentiated into melanocytes and neuron-like cells, indicating retention of their differentiation potential (Figure 2G and H). The neuronal differentiation was confirmed with a known lineage specific marker Map2 (Figure 3A). Similarly, the presence of oligodendrocytes was confirmed via GFAP marker (Figure 3C). In the same culture few cells were positive for mesodermal marker brachyury (Figure 3D). Thus addition of RA to SBES cells facilitated and enhanced the efficacy of differentiation into neuronal lineages. As smad4 nuclear localization has been observed (Figure 3F) along with some brachyury-positive cells, it indicates that mesoderm specification requires Bone Morphogenetic Protein (BMP) signaling. The subsets of mesodermal cells were also positive for sarcomeric actinin (Figure 3E). Very few cells were positive for NKX 2.5 (Figure 3B), indicating that cardiac differentiation was not up to the same extent as the neuronal differentiation after addition of RA. Furthermore, when SBES cells were exposed to adipogenic agent insulin, they exhibited typical adipocyte differentiation characterized by the formation of oil droplets in the cytoplasm (Figure 2E).
Therapeutic applications of ES cells aimed at regenerating damaged tissues and organs will require targeted differentiation of ES cells into specific progenitor cell types and an understanding of the factors that affect ES cell differentiation (Cool and Nurcombe, 2005). When SBES cells were seeded on plates coated with collagen, they differentiated predominantly and exhibited large lamellae with a prominent nucleus after 9 days of incubation (Figure 4A). These results are in agreement with the results of Chen et al., (2003), who got these types of cells in a 2D culture system. Similarly, gelatin-coated plates remained undifferentiated and formed polygonal type cells and EB type clusters after 25 days of incubation (Figure 4C and D). The cells seeded on uncoated plates retained the same morphology with less differentiation (figures not shown). The differentiation of SBES cells seem to be governed by the ECM used for coating the substratum.
The potential of SBES cells to differentiate into the above-mentioned cell types under specific conditions suggests that the sea bass ES-like cell line described in the present article remains pluripotent and in an undifferentiated state.
Pluripotent ES cells derived from mice (Evans and Kaufman, 1981) and human (Koestenbauer et al., 2006) exhibit several peculiar characteristics such as stable growth during long-term culture, small size, sparse cytoplasm and large nuclei, normal karyotype, high AP activity, the ability to form tightly compacted colonies, ability to grow in an undifferentiated state, and ability to differentiate into many cell types under defined conditions. In this article we have shown that fish embryonic stem-like cell line SBES derived from the sea bass shares all of these in vitro properties of rodent and human ES cells, indicating the prospect of using piscine SBES cell line for stem cell research without evoking ethical issues and invasive interventions sparing mammalian embryos.
Although in the previous reports on fish ES cells (Hong et al., 1996; Chen et al., 2003) the issue of tumorogenecity has not been dealt with, we made an effort to check the tumorogenic potential of SBES cells in vivo. We injected 1 × 107 cells intramuscularly into five juvenile sea bass and as well in two female nude mice and tumor development was observed for 2 months. Neither the mice nor fish showed any sign of tumor formation. However, in the case of fish, the cells migrated to distant locations. This indicates the nontumorigenic nature of SBES cells. This finding contradicts the studies in mouse and human ES cell lines, which are known to induce teratomas in nude mice (Thompson et al., 1998; Reubinoff et al., 2000). However, SBES cells appear to be similar to human adult bone marrow mesenchymal cells, which also do not induce tumors indicating their safety for transplantation purposes. One remarkable aspect of mesenchymal stem cell (MSC) physiology is that the cells may actually inhibit inflammation and immunological responses in the host. The immunomodulatory properties of MSCs can probably be explained by their lack of an HLA type II receptor and the secretion of cytokines (Blanc, 2003). Promising results have been obtained using human MSCs in clinical trials for osteogenesis imperfecta, metachromatic leukodystrophy, and Hurler syndrome. MSCs have also found a place in gene therapy as a powerful tool. In this context, SBES cell line seems to have tremendous therapeutic potential due to its nontumorigenic character. From the evolutionary point of view, because these cells are of piscine origin they would be an ideal candidate for xenotransplantation in higher mammals, as many studies on successful xenotransplantation have used fish islets viz., tilapia Brockmann bodies, to reverse diabetes (Wright and Yang, 1997; Laue et al., 2001; Wright and Pohajdak, 2001).
Taking into consideration the noninvasive and easy access to sea bass embryos due to its natural oviparous mode of reproduction as compared to the viviparous mode of mammalian reproduction, the sea bass embryos neither evoke ethical issues nor involve invasive interventions. Because of similarities between SBES and mouse ES cell lines, it is pertinent to mention here that SBES cells represent an ideal model for ES cell research with respect to propagation and differentiation. The ability of SBES cells to grow without feeder layers and to retain their pluripotency makes them an attractive model for stem cell research.
References
Anderson GB (1992) Isolation and use of embryonic stem cells from livestock species. Anim Biotechnol 3, 165–175
Bejar J, Hong Y, Alvarez MC (1999) Towards obtaining ES cells in the marine fish species Sparus aurata; multipassage maintenance, characterization and transfection. Genet Anal Biomol Engng 15, 125–129
Blanc KL (2003) Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy 5, 485–489
Bradley A, Evans M, Kaufman MH, Robertson E (1984) Formation of germ-line chimeras from embryo derived teratocarcinoma cell lines. Nature 309, 255–256
Campbell KHS, McWhir J, Ritchie WA, Wilmut I (1996) Sheep cloned by nuclear transfer from a cultured cell line. Nature 380, 64–66
Chen S, Hong Y, Schartl M (2002) Development of a positive-negative selection procedure for gene targeting in fish cell. Aquaculture 214, 67–79
Chen SL, Sha ZX, Ye HQ (2003a) Establishment of a pluripotent embryonic cell line from sea perch (Lateolabrax japonicus) embryos. Aquaculture 218, 141–151
Chen SL, Ye HQ, Sha ZX, Hong Y (2003b) Derivation of a pluripotent embryonic cell line from red sea bream blastulas. J Fish Biol 63, 795–805
Chen SS, Revoltella RP, Papini S, Michelini M, Fitzgerald W, Zimmerberg J, Margolis L (2003) Multilineage differentiation of Rhesus monkey embryonic stem cells in three-dimensional culture systems. Stem Cells 21, 281–295
Chen SL, Sha ZX, Ye HQ, Liu Y, Tian YS, Hong Y, Tang QS (2007) Pluripotency and chimera competence of an embryonic stem cell line from the sea perch (Lateolabrax japonicus). Mar Biotechnol 9, 82–91
Collodi P, Kamei Y, Sharps A, Weber D, Barnes D (1992) Fish embryo cell cultures for derivation of stem cells and transgenic chimeras. Mol Mar Biol Biotechnol 1, 257–265
Conover JC, Ip NY, Poueymirou WT, Bates B, Goldfarb MP, DeChinara TM, Yancopoulos GD (1993) Ciliary neurotrophic factor maintains the pluripotentiality of embryonic stem cells. Development 119, 559–565
Cool SM, Nurcombe V (2005) Substrate induction of osteogenesis from marrow-derived mesenchymal precursors. Stem Cells Dev 14, 632–642
Evans MG, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156
Goossler A, Doetschman T, Korn R, Serfling E, Kembler R (1986) Transgenesis by means of blastocyst derived embryonic stem cell lines. Proc Natl Acad Sci USA 83, 9065–9069
Handyside AH, O’Neill GT, Jones M, Hooper ML (1989) Use of BRL-conditioned medium in combination with feeder layers to isolate a diploid embryonal stem cell line. Rouxs Arch Dev Biol 198, 48–55
Hasty P, Ramirez-Solis R, Krumlauf R, Bradley A (1991) Introduction of a subtle mutation into the Hox-2.6 locus in embryonic stem cells. Nature 350, 243–246
Holen E, Hamre K (2003) Towards obtaining long term embryonic stem cell like cultures from a marine flatfish, Scophtalmus maximus. Fish Physiol Biochem 29, 245–252
Hong Y, Winkler C, Schartl M (1996) Pluripotency and differentiation of embryonic stem cell lines from the medakafish (Oryzias latipes). Mech Dev 60, 33–44
Hong Y, Winkler C, Schartl M (1998) Production of medakafish chimeras from a stable embryonic stem cell line. Proc Natl Acad Sci USA 95, 3679–3684
Koestenbauer S, Zech NH, Juch H, Vanderzwalmen P, Schoonjans L, Dohr G (2006) Embryonic stem cells: similarities and differences between human and murine embryonic stem cells. Am J Reprod Immunol 55, 169–180
Laue C, Kaiser A, Wendl K (2001) Reversal of streptozotocin-diabetes after transplantation of piscine principal islets to nude mice. Transplant Proc 33, 3504–3510
Ma C, Fan L, Ganassin R, Bols N, Collodi P (2001) Production of zebrafish germ-line chimeras from embryo cell cultures. Proc Natl Acad Sci USA 98, 2461–2466
Martin GR (1981) Isolation of a pluripotent cell line from mouse embryo cultures in medium conditioned by tetracarcinoma stem cells. Proc Natl Acad Sci USA 78, 7634–7638
Nakano T, Kodama H, Honjo T (1994) Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science 265, 1098–1101
Nichols J, Chambers I, Smith A (1994) Derivation of germline competent embryonic stem cells with a combination of interleukin-6 and soluble interleukin-6 receptor. Exp Cell Res 215, 237–239
Pease S, Braghetta P, Gearing D, Grail D, Williams RL (1990) Isolation of embryonic stem (ES) cells in media supplemented with recombinant leukemia inhibitory factor (LIF). Dev Biol 141, 344–352
Piquet-Pellorce C, Grey L, Mereau A, Heath JK (1994) Are LIF and related cytokines functionally equivalent? Exp Cell Res 213, 340–347
Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A (2000) Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol 18, 399–404
Robertson EJ (1987) Embryo-derived stem cell lines in teratocarcinomas and embryonic Stem cells: a practical approach, Robertson EJ (ed). (Oxford: IRL Press), pp. 71–112
Rocha A, Ruiz S, Estepa A, Coll JM (2004) Application of inducible and targeted gene strategies to produce transgenic fish: a review. Mar Biotechnol 6, 118–127
Sims M, First NL (1993) Production of calves by transfer of nuclei from cultured inner cell mass cells. Proc Natl Acad Sci USA 90, 6143–6147
Sukoyan MA, Golubitsa AN, Zhelezova AL, Shilov AG, Vatolin SY, Maximovsky LP, Andereeva LE, McWhir J, Pack SD, Bayborodin SI, Kerkis AY, Kizilova HI, Serov OL (1992) Isolation and cultivation of blastocyst-derived stem cell lines from American mink (Mustela vision). Mol Reprod Dev 33, 418–431
Sun L, Bradford CS, Ghosh C, Collodi P, Barnes DW (1995) ES-like cell cultures derived from early zebrafish embryos. Mol Mar Biol Biotechnol 4, 193–199
Thompson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147
Wakamatsu Y, Ozato K, Sasado T (1994) Establishment of a pluripotent cell line derived from a medaka (Oryzias latipes) blastula embryo. Mol Mar Biol Biotechnol 3, 185–191
Wakamatsu Y, Ju B, Pristyaznhyuk I, Niwa K, Ladygina T, Kinoshita M, Araki K, Ozato K (2001) Fertile and diploid nuclear transplants derived from embryonic cells of a small laboratory fish, medaka (Oryzias latipes). Proc Natl Acad Sci USA 98, 1071–1076
Wombus AM (2001) Potential of embryonic stem cells. Mol Aspects Med 22, 149–164
Wombus AM, Holzhausen H, Jakel P, Schoneich J (1984) Characterization of a pluripotent stem cell line derived from a mouse embryo. Exp Cell Res 152, 212–219
Wright JR Jr, Pohajdak B (2001) Cell therapy for diabetes using piscine islet tissue. Cell Transplant 10, 125–143
Wright JR Jr, Yang H (1997) Tilapia Brockmann bodies: an inexpensive, simple model for discordant islet xenotransplantation. Ann Transplant 2, 72–75
Wurst W, Joyner AL (1993) Production of targeted embryonic stem cell clones. In Joyner AL (ed.), Gene Targeting (Oxford: IRL Press), pp 33–61
Yoshida K, Chambers I, Nichols J, Smith A, Saito M, Yasukawa K, Shoyab M, Taga T, Kishimoto T (1994) Maintenance of the pluripotential phenotype of embryonic stem cells through direct activation of gp130 signalling pathways. Mech Dev 45, 163–171
Acknowledgment
The authors thank the management of C. Abdul Hakeem College for providing the facilities to carry out this work. The authors also thank the Director, Central Institute of Brackishwater Aquaculture, Chennai, for providing the experimental animals. Thanks are also due to Director, NCCS, Pune, for providing excellent infrastructure facilities for this study. The financial assistance provided by the Department of Biotechnology, Government of India, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
movie supplement
(AVI 3.38 MB)
Rights and permissions
About this article
Cite this article
Parameswaran, V., Shukla, R., Bhonde, R. et al. Development of a Pluripotent ES-like Cell Line from Asian Sea Bass (Lates calcarifer)—An Oviparous Stem Cell Line Mimicking Viviparous ES Cells. Mar Biotechnol 9, 766–775 (2007). https://doi.org/10.1007/s10126-007-9028-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-007-9028-y