Abstract
Endophytes have been shown to play a crucial role in determining the fitness of host plant during their association, yet the cross-functional effect of endophytes of one plant on another plant remains largely uncharacterized. In this study, we attempt to analyze the effect of native endophytes of Coleus forskohlii (Phialemoniopsis cornearis (SF1), Macrophomina pseudophaseolina (SF2), and Fusarium redolens (RF1), isolated from stem and root parts) on plant growth and secondary metabolite enhancement in medicinal plant Andrographis paniculata, and aromatic plants Pelargonium graveolens and Artemisia pallens. Here, we report, endophytic treatments with SF2 (21%) and RF1 (9%) in A. paniculata resulted in significant enhancement of andrographolide along with plant primary productivity. Correspondingly, application of fungal endophytes RF1, SF1, and SF2 significantly improved the plant growth (11 to 40%), shoot weight (28 to 34%), oil content (44 to 58%), and oil yield (72 to 122%) in P. graveolens. Interestingly, treatment of A. pallens with three fungal endophytes resulted in significant enhancement of plant productivity and oil content (12 to 80%) and oil yield (32 to 139%). Subsequently, the endophyte treatments RF1 and SF1 enhanced davanone (13 to 22%) and ethyl cinnamate (11 to 22%) content. However, SF2 endophyte-treated plants did not show any improvement in ethyl cinnamate content but enhanced the content of davanone (10%), a signature component of davana essential oil. Overall, results depict cross-functional role of native endophytes of C. forskohlii and repurposing of functional endophytes for sustainable cultivation of economically important medicinal and aromatic crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant microbe interaction plays a beneficial role in growth and development. Endophytes are the least explored group of microbes for their beneficial effects in enhancing plant fitness and metabolism. Endophytes reside inside tissues without hurting the plant and have been isolated from the surface disinfected plant parts (Hallmann et al. 1997). Endophyte relation with plants is most common and has been identified in most vascular plant families. Major roles of endophytes include phosphate solubilization, nitrogen fixation, and plant hormone production (Costacurta and Vanderleyden 1995; Verma et al. 2001; Compant et al. 2005). Endophytes confer tolerance to heavy metals during stress conditions such as drought and salinity stress and assist plants in escaping stress by exhibiting ACC deaminase activity and subsequent siderophore formation (Ullah et al. 2015; Kong et al. 2015). Endophytes can also modulate the growth parameters of non-host plant irrespective of their original host. For instance, Piriformospora indica is a known fungal endophyte isolated from xerophytes of Indian desert (Verma et al. 1998). In vitro studies have shown that the fungus has broad host spectrum activities, for example, the fungus can enhance growth of Arabidopsis (Strehmel et al. 2016), Bacopa monnieri (Prasad et al. 2013), Hordeum vulgare (Schaefer et al. 2009), Lycopersicon esculentum (Fakhro et al. 2010), C. forskohlii (Das et al. 2012), Withania somnifera (Ahlawat et al. 2015), and Artemisia annua (Sharma and Agarwal 2013) among others. Considering this broad host spectrum of endophyte, in this study, we have analyzed the beneficial role of C. forskohlii endophytes on other economical crops like P. graveolens, A. pallens, and A. paniculata.
The aromatic plant, P. graveolens, typically known as rose-scented geranium, belonging to the family Geraniaceae, is native to Mediterranean region and is distributed in all parts of the world. The aerial parts of geranium, especially shoot, are rich in glandular trichomes in which various aromatic compounds are synthesized and accumulated. Geranium oil can be extracted from all parts of the plant such as leaves, flowers, and stalks, by steam or hydrodistillation (Boukhris et al. 2013). Essential oil of geranium has wide range of applications in pharmaceuticals for treatments of acne, bruises, burns, cuts, dermatitis, eczema, and hemorrhoids. Subsequently, it is used in cosmetic industries, for making of soaps and perfumes. A. pallens (davana) is an aromatic medicinal herb (Shukla et al. 2015). Lifetime of A. pallens is 3 months and during this time, the plant accumulates the highest concentration of aromatic oil in aerial part like leaf, stem, and flowers, which imparts a pleasant smell. In the earlier days, davana oil has been used as flavoring agent for cakes, pastries, tobacco, and some expensive beverages. Subsequently, various medicinal properties of davana oil were reported, such as being anti-pyretic, antioxidant, anti-inflammatory, anti-allergic (Mukherjee et al. 2017), anthelmintic (Nakhare and Garg 1991), anti-diabetic (Subramoniam et al. 1996), insect deterrent (Bhagavathy et al. 2015), and anti-malarial (Ruikar et al. 2011). Artesunate is a derivative of artemisinin, which is a potential anti-malarial compound but is present in very low concentrations in davana oil. Chemically, davana oil contains davanone as the major constituent, in addition to seven other sesquiterpene compounds (Mallavarapu et al. 1999). The medicinal plant, A. paniculata belongs to the family Acanthaceae and is the only source for andrographolide. The plant has especially been used for the treatment of malaria for centuries in Asia, America, and Africa continents and diabetes, cancer, high blood pressure, skin diseases, ulcer, leprosy, flatulence, bronchitis, colic, influenza, dysentery, and dyspepsia (Okhuarobo et al. 2014).
Besides, there is an enormous demand for essential oil and secondary metabolites in market, compounded with limitation in agricultural land for cultivation of aromatic and medicinal plants being the major problems. To overcome these problems, alternative crop technologies like developing new cultivars that have high oil yield or identifying the endophyte that can enhance plant growth as well as in planta accumulation of essential oils need to be addressed. Research on developing new cultivars is time consuming and a genetically high limitation process. Hence, in this regard, the current article proposing a novel mechanism comprising identification of endophyte that can compensate the demand could be a sustainable approach in agriculture sector. Moreover, application of endophytes as bio-fertilizers is a part of green revolution, eco-friendly, and economically favorable for sustainable cultivation of commercial crops. For instance, in earlier studies, the application of bio-organics and Trichoderma harzianum was shown to lead to enhancement of plant biomass, flower bud, oil yield, and managing root knot causing nematode (Meloidogyne incognita) as well (Pandey 2005). In view of growing demand for medicinal and aromatic plants and its challenges for cultivation, the present study was aimed at repurposing three functional fungal endophytes F. P. cornearis (SF1), M. pseudophaseolina (SF2), redolens (RF1) of C. forskohlii for cultivation of other economically important medicinal and aromatic plants A. paniculata, P. graveolens, A. pallens. The study was also designed to understand the cross-functional role of three fungal endophytes, to accomplish in planta secondary metabolites enhancement in other medicinal and aromatic crops.
Materials and methods
Fungal endophytes and inoculum preparation
Fungal endophytes, RF1, SF1, and SF2, were isolated during our previous study from different parts of C. forskohlii plants (Suppl. Fig. 1) (Mastan et al. 2019a). The isolated endophytes were characterized by amplifying the internal transcribed spacer-1 (ITS1 and ITS 4). Three native fungal endophytes of C. forskohlii such as RF1, SF1, and SF2 were identified as Fusarium redolens, Phialemoniopsis cornearis, and Macrophomina pseudophaseolina and were deposited at National Collection of Industrial Microorganisms (NCIM), Pune with accession numbers NCIM-1402, NCIM-1404, and NCIM-1403, respectively. For the preparation of endophytic bio-inoculum, fungal cultures were inoculated in 500 ml of PDB broth and grown for 7 days at 28 °C on a shaker at 180 rpm min−1. Number of colonies was counted by diluting in PBS and normalized for 1 to 3 × 108 CFUmL−1.
Evaluation of fungal endophytes on A. paniculata under field conditions
The endophytes of C. forskohlii such as RF1, SF1, and SF2 were cross inoculated into medicinal plant A. paniculata, to understand their effect in enhancing plant growth and secondary metabolite content. Seeds of A. paniculata were obtained from the National Gene Bank for Medicinal and Aromatic Plants, CSIR-Central Institute of Medicinal and Aromatic Plants (CSIR-CIMAP), Lucknow, India, and seedlings were generated under greenhouse conditions. Healthy seedlings were transplanted to field conditions (designed as 3 × 3 m dimension at a spacing of 60 cm plant to plant) to inoculate the endophytes. In the field, each plant (16 plants plot−1) was treated with 30 mL of endophytic (RF1, SF1, and SF2) inoculums, and one plot without any endophyte treatment, which served as control. Plant growth parameters such as plant height and the number of branches were recorded. The plants of both endophyte treatments and control were harvested at 90 days post inoculum. After harvesting, total fresh shoot weight was measured and dry weight measured after 30 days of incubation at room temperature. Completely dried shoot samples were subjected to analysis of the variation of andrographolide content after treatment of endophytes.
Evaluation of fungal endophytes for cultivation of P. graveolens under field conditions
Beneficial effect of three fungal endophytes, RF1, SF1, and SF2, isolated from C. forskohlii was analyzed on P. graveolens under field conditions at CSIR-CIMAP, Research Center, Bengaluru, India. Plant material of P. graveolens was procured from plant material-stock of CSIR-CIMAP, Research Centre, Bangalore. One hundred twenty-day field-grown P. graveolens plants were selected from the stock, and 2–3 inter nodes containing stem cuttings were prepared using sterilized blade. The stem cuttings were transplanted to 15 × 6 cm polythene bags comprising 150 g of soil and 100 g of vermicompost and propagated under glass house conditions for further experiments. The developed nursery of geranium stem cuttings with established roots were transplanted to experimental plots (3 × 3 m dimension) at a spacing of 60 cm plant to plant and in a depth of 12–15 cm. Each treatment plot comprised four rows divided by a 50-cm guard ridge to reduce the effect of adjoining plots. Each bed was transplanted with 16 plants (12 peripheral and 4 central plants) and one complete bed without any treatment served as control. After 4 months of treatment, plant height and herbage yield were measured from each treatment plot. Two hundred grams of fresh geranium herbage from each treatment was weighed and transferred to Clevenger apparatus for hydrodistillation (Clevenger 1928). Distillation was performed at 50–60 °C temperature for 3 h to obtain complete oil from 200 g plant herbage. After distillation, the obtained essential oil was collected in 2 ml fresh microtubes and the oil yield (mL) and content (%) was calculated. To remove excess moisture, the distilled essential oil was treated with anhydrous sodium sulfate and the oil stored at 4 °C until experimental analysis.
Evaluation of fungal endophytes for cultivation of A. pallens under field conditions
Beneficial effects of three fungal endophytes, RF1, SF1, and SF2 was analyzed on A. pallens (local variety of Bangalore) under field conditions at CSIR-CIMAP, Research Center, Bengaluru, India. A. pallens seeds were sown at a depth of 2 cm and covered with thin layer of sand. Plants of four to five leaf stages were used for field experiments. Thirty milliliters of diluted endophyte suspension containing 1 × 108 CFU/mL was inoculated to each plant in the plot and one complete plot without any endophyte treatment served as control. Generally, after 60–70 days of transplantation, plants become ready to harvest, i.e., plants achieve maximum flowers in bloom stage. At this time, eight plants were randomly selected for the measurement of plant growth parameters from each treatment and control bed. Similarly, the herbage weight of plant from each treatment was measured. Since davana plant retains oil in its aerial parts, the complete aerial parts (leaf and stem) were harvested. Composite sample of 200 g herbage from each treatment bed was used for extraction of essential oil. Distillation of davana oil and calculation of yield (mL) and content (%) was performed similar to geranium experiment.
Secondary metabolite estimation and essential oil analysis
Andrographolide content was estimated in endophyte treated and control A. paniculata plants by TLC method. Completely dried leaves of A. paniculata from field experiments were powdered with mortar and pestle. One hundred milligrams crude powder was macerated in 1 mL of methanol and vortexed for 1 h. Supernatant was separated by centrifugation at 10,000 rpm for 4 min and the chlorophyll removed using activated charcoal. The final supernatant was concentrated in vacuum drier (V-AL mode) for 50 min at 45 °C. Concentrates were reconstituted in 100 μL methanol, from which 5 μL was used for analysis. For quantitative analysis, 98% andrographolide was purchased from Sigma-Aldrich and used as authentic standard. A. paniculata leaf samples along with standard andrographolide was loaded on aluminum pre-coated silica gel 60 F254 plates (Merck) by leaving 6 mm space between samples and plates were developed using mobile phase, chloroform, methanol, and ethyl acetate (8:1.5:1 ratio, respectively) (Rajani et al. 2000). The developed plates were exposed to UV light to analyze andrographolide spots, while the same plates were stained by anisaldehyde reagent (0.5 mL anisaldehyde, 85 mL methanol, 10 mL glacial acetic acid and 5 mL conc. H2SO4) and kept at hot air oven for 5 min at 110 °C. Both UV visualization and anisaldehyde treatment exposed andrographolide spots on TLC plates, and RF values and spot intensity were analyzed by referring to authentic andrographolide standard. For quantification, intensity level of the total spot size of andrographolide was measured using Spot Densitometric Analyzable Software AlphaEaseFC (Version 4.0.0, Alpha Innotec). Variation of andrographolide content between endophyte treatments and control A. paniculata plants was measured. Subsequently, essential oils were subjected to GC analysis to determine their constituents. The method was performed using Perkin-Elmer Gas Chromatograph system equipped with flame ionization detector (FID) as detection source (Saxena et al. 2007). Fused Silica capillary column BP-1 (25 m × 0.5 mm × 25 m film thickness) coated with polydimethylsiloxane was used as stationary phase. Nitrogen gas was used as the carrier at 10 psi inlet pressure and temperature ramp as follows: (initial 5 min) 220 °C at 5 °C/min and the final temperature was held for 5 min. The oil samples were injected by split mode (Split ratio 1:80); temperature of injector and detector was maintained at 250 °C and 300 °C, respectively. Compounds were identified by referring to standards retention times (procured from Sigma-Aldrich). The relative amounts of constituents were computed from peak areas without applying FID response factor correction and expressed as percentage.
Statistical analysis
For statistical difference, one-way ANOVA with Dunnett’s multiple test was calculated using GraphPad prism, version 6.0 (GraphPad Software, San Diego, CA). Statistically significant differences between control and endophyte treated samples were analyzed at different P values.
Results
Effect of endophytic fungi on plant growth and andrographolide content of A. paniculata
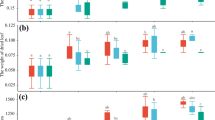
The endophytic fungal isolates viz., F. redolens (RF1), P. cornearis (SF1), and M. pseudophaseolina (SF2) showed differential enhancement in plant height, number of branches, and herbage yield of A. paniculata under field conditions (Fig. 1). In field experiment, the endophytic treatments RF1 and SF2 showed enhancement in plant height (12 and 11%) and number of branches (47 and 42%) compared to the other endophyte, SF1 treated and untreated control plants. However, the plants treated with SF1 under field conditions did not show significant effect on plant height but showed significant increase of branch number (28%) compared to control plants. In addition, the endophytic treatments, RF1, SF1, and SF2 contributed to significant enhancement in fresh shoot weight (60, 10, and 26%, respectively) and dry shoot weight (54, 47, and 55%, respectively) compared to uninoculated control plants. The effect of endophytes on andrographolide content of A. paniculata under field conditions was analyzed. Interestingly, the SF2 endophyte treatment showed significant enhancement of andrographolide (21%) content compared to control plants. Further, the plants treated with RF1 showed enhancement in andrographolide (9%) compared to control plants (Fig. 2). However, SF1 endophyte treated plants did not show significant difference in andrographolide content.
Evaluation of three fungal endophytes on Andrographis paniculata under field conditions. a Height of the plant, b branches, c fresh shoot weight, and d dry shoot weight. Vertical bars indicate S.D. of mean (n = 4). Asterisks above the error bars represents a significant difference between control and endophyte treated plants (*p < 0.05; **p < 0.01)
Relative yield of andrographolide after endophyte treatments under field conditions and analyzed by TLC method. The intensity of total andrographolide spot size was measured using AlphaEaseFC 4.0 software. a TLC pate visualized under UV at 254 nm before staining the plates. b Anisaldehyde-stained TLC plate. c Graphical representation of andrographolide relative yield. AG: andrographolide and Con: control plants. Vertical bars indicate standard deviation (SD) of mean (n = 3). Asterisks above the bars represent a significant difference between control and endophyte treated plants (*p < 0.05; **p < 0.01)
Effect of endophytic fungi on plant growth and oil yield of P. graveolens
Screening of three non-native fungal endophytes (RF1, SF1, and SF2) on P. graveolens under field conditions exhibited differential enhancement of plant height, number of branches, biomass, and oil yield, compared to uninoculated plants (Suppl Fig. 2). At 120 days post treatment, the plants treated with RF1, SF1, and SF2 showed enhancement of plant height by 22, 40, and 11%, respectively, compared to control plants, while significant enhancement of branch number by 88, 102, and 48% was noticed in endophyte treatments RF1, SF1, and SF2, respectively, compared to control plants (Fig. 3). Further, increment in the herbage yield was observed in the fungal endophytic treated plants over control plants. SF1 treatments significantly improved shoot weight by 34% followed by RF1 (30%) and SF2 (28%). Moreover, the application of endophytes RF1, SF1, and SF2 significantly enhanced the oil content (44, 58, and 56%, respectively) compared to control plants, thereby enhancement of oil yield by 0.7-, 1.2-, and 1.1-fold, respectively. The essential oil of P. graveolens (CIM—Pawan) contains geraniol and citronellol as major monoterpenes, in addition to linalool and another phenyl propanoids. The plants treated with SF2 enhanced citronellol content (11%) and geraniol content (4%) compared to control plants, while the plants treated with RF1 and SF1 did not show significant difference in citronellol and geraniol content compared to control plants. However, all three fungal endophyte treatments maintained the C/G (Citronellol/Geraniol) ratio more or less equal to one, which is considered as a good quality index for geranium essential oil (Table 1; Suppl. Fig. 3).
The endophytic treatments of P. graveolens grown under feild conditions. a Plant height. b Shoot weight. c oil content. d Oil yield. Verticle bars represent standard deviation of four replicates. Asterisks above the bars represent a significant difference between control and endophyte treated plants (*p < 0.05; **p < 0.01)
Effect of endophytic fungi on plant growth and oil yield of A. pallens
Treatment of the three fungal endophytes significantly enhanced plant height and herbage weight of A. pallens under field conditions (Fig. 4). After 90 days of inoculum, the endophyte SF1-treated plants showed enhancement of plant height by 34% followed by the treatment of RF1 (26%) and SF2 (16%) compared to control plants. While the plants treated with SF2 showed significant result in enhancement of herbage weight (33%), followed by other endophytic treatments SF1 (22%) and RF1 (19%) compared to control plants. Further, the essential oil yield of A. pallens was differentially enhanced in endophyte-treated plants. Application of RF1, SF1, and SF2 augmented the essential oil content (12%, 52%, and 80%, respectively), and oil yield (33%, 52%, and 139%, respectively) compared to control plants. The essential oil of A. pallens contains davanone as major sesquiterpene followed by other chemical constituents such as ethyl cinnamate and bicyclogermacrene. Treatment of fungal endophytes differentially modulated the chemical constituents of davana essential oil (Table 2). Interestingly, SF2 endophyte treatment significantly enhanced the major chemical component, davanone content, by 22% followed by RF1 (13.9%) and SF1 (10%) when compared with control plants tested under field conditions. In addition, the plants treated with RF1 showed enhancement in ethyl cinnamate by 22% followed by treatment of SF1 (11%), while the fraction of ethyl cinnamate enhancement (5%) was noticed in SF2 endophyte treated plants compared to control. However, no enhancement of bicyclogermacrene was observed in any of the three endophyte treated plants. Overall, the application of C. forskohlii endophytes in cross-functionality approach significantly enhanced the plant height and herbage weight, thereby increasing production of essential oil through plant system.
Effect of three fungal endophytes on A. pallens grown under field conditions. a Plant height, b fresh shoot weight, c oil content, and d oil yield were analyzed after harvesting the plants. The error bars indicate standard deviation (SD) of four replicates for plant height and shoot weight, while eight replicated for oil yield. Asterisks above the bars represent a significant difference between control and endophyte treated plants (*p < 0.05; **p < 0.01)
Discussion
Various research groups have described endophytes of different species of medicinal and aromatic plants. However, in this study, we analyzed their beneficial effects on non-host plants, which include both medicinal (A. paniculata) and aromatic plants (P. graveolens and A. pallens) to improve plant growth and enhance in planta signature metabolites. Microorganisms as plant growth promoting (PGP) strains are now known to have various plant beneficial properties to assist plants in their metabolism and confer resistance under biotic and abiotic stress conditions, in which one of the classes of microbes termed endophytes reside inside the plant tissues and perform numerous beneficial functions in their host plants (Arnold 2007). Fungal endophytes are very common and greatly diverse microorganisms, and survive asymptomatically in the plant tissues (Faeth and Fagan 2002). Moreover, most of the fungal endophytes modulate plant growth and biomass by various mechanisms. Photosynthesis is very crucial process for generating energy molecules and carbon-containing compounds to perform biological functions within the plant cells (Tanaka and Makino 2009). Inoculation of endophytic fungi augments expression of genes and pigments of photosynthesis (Harman et al. 2019). Fortunately, endophytes (RF1, SF1, and SF2) of C. forskohlii have the ability to enhance photosynthetic pigments like chlorophyll a, chlorophyll b, and carotenoids and significantly enhance the photosynthetic process in their host plant (Mastan et al. 2019a). It has been stated that phosphate-solubilizing and plant-hormones-producing strains could help in increasing the plant growth and yield (Sharma et al. 2013; Khan et al. 2014). Inoculation of endophytic fungus Colletotrichum tofieldiae to Arabidopsis thaliana transfers the macronutrient phosphorus from soil to shoot system and thus promotes plant growth and increases fertility under phosphorus-deficient conditions (Hiruma et al. 2016). The endophytic fungi Phoma glomerata LWL2 and Penicillium sp. LWL3 significantly enhance shoot growth in gibberellins (GAs)-deficient dwarf mutant Waito-C and Dongjin-beyo rice by producing gibberellins and indoleacetic acid (Waqas et al. 2012).
In our previous studies, the fungal endophytes (RF1, SF1, and SF2) were identified as plant growth-promoting organisms for medicinal plant C. forskohlii, the host of the tested endophytes. Treatment of host plant C. forskohlii with fungal endophytes showed significant enhancement in plant height, number of branches, and shoot and root weight compared to untreated plants. PGP screening revealed that the endophytes RF1 and SF1 are positive for indole-3-acetic acid (IAA) production, while SF1 significantly solubilized the phosphate when tested using Pikovskaya’s broth and agar medium. The main limitation in cultivation of these crops is their high susceptibility to fungal diseases. Generally, P. graveolens is affected by fungal pathogens like F. oxysporum and Rhizoctonia solani, which causes wilt diseases that drastically affect the crop yield and quality of geranium oil (Prasad et al. 2008; Gautam 2014). In our previous studies, all the three endophytes (RF1, SF1, and SF2) displayed remarkable antagonistic activity against fungal pathogens like F. oxysporum, Colletotricum gloeosporioides, and Sclerotium rolfsii (Mastan et al. 2019a). Considering these important PGP properties of endophytes, we made an attempt to evaluate these beneficial effects of endophytes on other medicinal and aromatic plants. Surprisingly, application of endophytes enhanced plant height and herbage weight of aromatic plants, P. graveolens and A. pallens, under field conditions. Subsequently, the endophyte inoculated medicinal plant A. paniculata showed improved plant primary productivity.
Essential oil of geranium is composed of various volatile terpenoids, which are especially rich in mono terpenoids like citronellal (33.6%), geraniol (26.8%), linalool (10.5%), citronellylformate (9.7%), and phenyl propanoids like isomenthone (6.0%), and these definite constituents-containing essential oil has wide range of applications in essential oil industry as well as in pharmaceuticals. Transcriptomic analysis of forskolin genes by quantitative real-time PCR (qPCR) revealed that inoculation of endophytes augmented the expression levels of forskolin diterpene synthase genes (Mastan et al. 2019a). Application of the fungal endophytes did not show any significant enhancement in oil components but enhanced the oil yield and maintained appropriate composition of geranium essential oil. In an earlier study, to increase artesunate production, Pala and group developed the hairy root culture approach form leaf and stem explants for maximum production of artesunate in in vitro conditions (Pala et al. 2016). In other modes of transgenic technology, Alok and group optimized in vitro regeneration and Agrobacterium mediated transformation of A. pallens (Alok et al. 2016). However, cell culture and transgenic technologies are more expensive and time-consuming processes. Hence, finding alternative strategies to augment the plant yield and secondary metabolites is the need of the hour. Inoculation of C. forskohlii with these three endophytes augmented the in planta forskolin at significant levels (Mastan et al. 2019b). In this study, application of endophytes on A. pallens showed differential effect in davana oil composition. RF1 and SF2 inoculation significantly induced davanone and ethyl cinnamate content in total oil, while SF2-treated plants exhibited increment in davanone content, which is main component of davana essential oil (Mallavarapu et al. 1999). In earlier studies, inoculation of both arbuscular mycorrhizal fungi (AMF) and endophyte enhanced plant growth as well as secondary metabolite content. Inoculation of Rhizophagus intraradices and AMF combination enhanced the content of hypericin and pseudohypericin in the shoots (Zubek et al. 2012), while colonization of AMF fungi in Curcuma longa enhanced its secondary metabolite content (Dutta and Neog 2016). Similarly, fungal endophytes of Catharanthus roseus were able to enhance vindoline content by modulating indole alkaloid pathway genes and various transcription factors (Pandey et al. 2016). The colonization of endophytic fungus, Trimmatostroma sp. promoted salidroside and tyrosol accumulations in Rhodiolacrenulate (Cui et al. 2017). Our endophyte-mediated secondary metabolite enhancement study revealed that the treatment of three endophytes differentially modulated the accumulation of andrographolide in leaves of A. paniculata; however, colonization of SF2 enhanced this metabolite content in significant amounts compared to untreated control and other endophyte treatments.
Conclusion
We have demonstrated cross-functionality of three fungal endophytes (RF1, SF1, and SF2), which are native to C. forskohlii, exhibit increased primary plant productivity, essential oil yield as well as enhanced secondary metabolite content in A. paniculata, P. graveolens, and A. pallens. Here, our results unambiguously demonstrate that these fungal endophytes could also be used as plant-probiotic bio-stimulants during cultivation of A. paniculata, P. graveolens, and A. pallens. It also provides novel strategies like deployment of non-native functional endophytes to improve plant productivity without compromising on quality of secondary metabolite content in medicinal and aromatic plants.
References
Ahlawat A, Saxena A, Abdin MZ (2015) Piriformospora indica elicitation of withaferin A biosynthesis and biomass accumulation in cell suspension cultures of Withania somnifera. Symbiosis 69:37–46
Alok A, Shukla V, Pala Z, Kumar J, Kudale S, Desai N (2016) In vitro regeneration and optimization of factors affecting Agrobacterium mediated transformation in Artemisia Pallens, an important medicinal plant. Physiol Mol Biol Plants 22:261–269
Arnold EA (2007) Understanding the diversity of foliar endophytic fungi: progress, challenges and frontiers. Fungal Biol Rev 21:51–66
Bhagavathy GV, Velazquez Nieves GM, Webb MZ, Chauhan KR (2015) Arthropod deterrents from Artemisia pallens (Davana oil) components. Nat Prod Commun 10:1335–1336
Boukhris M, Nasri-Ayachi MB, Mezghani I, Bouaziz M, Boukhris M, Sayadi S (2013) Trichomes morphology, structure and essential oils of Pelargonium graveolens L’Hér. (Geraniaceae). Ind Crop Prod 50:604–610
Clevenger JF (1928) Apparatus for the determination of volatile oil. J Am Pharm Assoc 17:345–349
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Costacurta A, Vanderleyden J (1995) Synthesis of phytohormones by plant associated bacteria. Crit Rev Microbiol 21:1–18
Cui JL, Wang YN, Jiao J, Gong Y, Wang JH, Wang ML (2017) Fungal endophyte-induced salidroside and tyrosol biosynthesis combined with signal cross-talk and the mechanism of enzyme gene expression in Rhodiola crenulata. Sci Rep 7:12540
Das A, Kamal S, Shakil NA, Sherameti I, Oelmüller R, Dua M, Tuteja N, Johri AK, Varma A (2012) The root endophyte fungus Piriformospora indica leads to early flowering, higher biomass and altered secondary metabolites of the medicinal plant, Coleus forskohlii. Plant Signal Behav 7:103–112
Dutta SC, Neog B (2016) Accumulation of secondary metabolites in response to antioxidant activity of turmeric rhizomes co-inoculated with native arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria. Sci Hortic 204:179–184
Faeth SH, Fagan WF (2002) Fungal endophytes: common host plant symbionts but uncommon mutualists. Integr Comp Biol 42(2):360–368
Fakhro A, Andrade-Linares DR, von Bargen S, Bandte M, Buttner C, Grosch R, Schwarz D, Franken P (2010) Impact of Piriformospora indica on tomato growth and on interaction with fungal and viral pathogens. Mycorrhiza 20:191–200
Gautam AK (2014) The genera Colletotrichum: an incitant of numerous new plant diseases in India. Journal on New Biological Reports 3:9–21
Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43:895–914
Harman GE, Doni F, Khadka RB, Uphoff N (2019) Endophytic strains of Trichoderma increase plants' photosynthetic capability. J Appl Microbiol. https://doi.org/10.1111/jam.14368
Hiruma K, Gerlach N, Sacristán S, Nakano RT, Hacquard S, Kracher B, Neumann U, Ramírez D, Bucher M, O'Connell RJ, Schulze-Lefert P (2016) Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell 165:464–474
Khan AL, Waqas M, Kang SM, Al-Harrasi A, Hussain J, Al-Rawahi A, Al-Khiziri S, Ullah I, Ali L, Jung HY, Lee IJ (2014) Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J Microbiol 52(8):689–695
Kong Z, Mohamad OA, Deng Z, Liu X, Glick BR, Wei G (2015) Rhizobial symbiosis effect on the growth, metal uptake, and antioxidant responses of Medicago lupulina under copper stress. Environ Sci Pollut Res 22:12479–12489
Mallavarapu GR, Kulkarni RN, Baskaran K, Rao L, Ramesh S (1999) Influence of plant growth stage on the essential oil content and composition in Davana (Artemisia pallens Wall.). J Agric Food Chem 47:254–258
Mastan A, Bharadwaj RKB, Kushwaha RK, Vivek Babu CS (2019a) Functional fungal endophytes in Coleus forskohlii regulate labdane diterpene biosynthesis for elevated forskolin accumulation in roots. Microb Ecol 78:914–926. https://doi.org/10.1007/s00248-019-01376-w
Mastan A, Rane D, Dastager SG, Vivek Babu CS (2019b) Development of low-cost plant probiotic formulations of functional endophytes for sustainable cultivation of Coleus forskohlii. Microbiol Res 227:126310. https://doi.org/10.1016/j.micres.2019
Mukherjee AA, Kandhare AD, Rojatkar SR, Bodhankar SL (2017) Ameliorative effects of Artemisia pallens in a murine model of ovalbumin-induced allergic asthma via modulation of biochemical perturbations. Biomed Pharmacother 94:880–889
Nakhare S, Garg SC (1991) Anthelmintic activity of the essential oil of Artemisia pallens Wall. Anc Sci Life 10:185–186
Okhuarobo A, Falodun JE, Erharuyi O, Imieje V, Falodun A, Langer P (2014) Harnessing the medicinal properties of Andrographis paniculata for diseases and beyond: a review of its phytochemistry and pharmacology. Asian Pac J Trop Dis 4:213–222
Pala Z, Shukla V, Alok A, Kudale S, Desai N (2016) Enhanced production of an anti-malarial compound artesunate by hairy root cultures and phytochemical analysis of Artemisia pallens Wall. 3 Biotech 6:182
Pandey K (2005) Management of Meloidogyne incognita in Artemisia pallens with bio-organics. Phytoparasitica 33:304–308
Pandey SS, Singh S, Babu CS, Shanker K, Srivastava NK, Shukla AK, Kalra A (2016) Fungal endophytes of Catharanthus roseus enhance vindoline content by modulating structural and regulatory genes related to terpenoid indole alkaloid biosynthesis. Sci Rep 6:26583
Prasad D, Singh KP, Kumar J (2008) Root rot and wilt complex of rose-scented Geranium (Pelargonium graveolens) in Uttarakhand. Indian Phytopath 61(3):365–366
Prasad R, Kamal S, Sharma PK, Oelmueller R, Varma A (2013) Root endophyte Piriformospora indica DSM 11827 alters plant morphology, enhances biomass and antioxidant activity of medicinal plant Bacopa monniera. J Basic Microbiol 53:1016–1024
Rajani M, Shrivastava N, Ravishankara MN (2000) A rapid method for isolation of andrographolide from Andrographis paniculata nees (kalmegh). Pharm Biol 38:204–209
Ruikar AD, Misar AV, Jadhav RB, Rojatkar SR, Mujumdar AM, Puranik VG, Deshpande NR (2011) Sesquiterpene lactone, a potent drug molecule from Artemisia pallens wall with anti-inflammatory activity. Arzneimittelforschung 61:510–514
Saxena G, Banerjee S, Laiq-ur-Rahman et al (2007) Rose-scented geranium (Pelargonium sp.) generated by Agrobacterium rhizogenes mediated Ri-insertion for improved essential oil quality. Plant Cell Tiss Organ Cult 90:215
Schaefer P, Pfiffi S, Voll LM, Zajic D, Chandler PM, Waller F, Scholz U, Pons-Kuehnemann J, Sonnewald S, Sonnewald U, Kogel KH (2009) Manipulation of plant innate immunity and gibberellin as factor of compatibility in the mutualistic association of barley roots with Piriformospora indica. Plant J 59:461–474
Sharma G, Agarwal V (2013) Marked enhancement in the artemisinin content and biomass productivity in Artemisia annua L. shoots co-cultivated with Piriformospora indica. World J Microbiol Biotechnol 29:1133–1138
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus. 2:587. https://doi.org/10.1186/2193-1801-2-587
Shukla V, Pala Z, Alok A, Desai N (2015) Screening of different Artemisia spp. from western ghats of Maharashtra for an antimalarial compound-artemisinin. Am J Plant Sci 06:1619–1632
Strehmel N, Mönchgesang S, Herklotz S, Krüger S, Ziegler J, Scheel D (2016) Piriformospora indica stimulates root metabolism of Arabidopsis thaliana. Int J Mol Sci 17
Subramoniam A, Pushpangadan P, Rajasekharan S, Evans DA, Latha PG, Valsaraj R (1996) Effects of Artemisia pallens wall. on blood glucose levels in normal and alloxan-induced diabetic rats. J Ethnopharmacol 50:13–17
Tanaka A, Makino A (2009) Photosynthetic research in plant science. Plant Cell Physiol 50(4):681–683
Ullah A, Heng S, Munis MFH, Fahad S, Yang X (2015) Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. Environ Exp Bot 117:28–40
Verma S, Varma A, Rexer K, Hassel A, Kost G, Sarbhoy A, Franken P (1998) Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 90:896–903
Verma SC, Ladha JK, Tripathi AK (2001) Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J Biotechnol 91:127–141
Waqas M, Khan AL, Kamran M, Hamayun M, Kang SM, Kim YH, Lee IJ (2012) Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 17:10754–10773
Zubek S, Mielcarek S, Turnau K (2012) Hypericin and pseudohypericin concentrations of a valuable medicinal plant Hypericum perforatum L. are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza 22:149–156
Acknowledgments
The authors express their sincere thanks to the Director, CSIR- Central Institute of Medicinal and Aromatic Plants, Lucknow, India, for providing his support, encouragement, and necessary facilities during the course of investigation.
Funding
This work was supported by BSC0117 and BSC203 (XII Five-Year Plan Network Project) from Council of Scientific and Industrial Research (CSIR), India. AM greatly acknowledges to Department of Science and Technology (DST–INSPIRE) New Delhi, India, for providing the financial support in the form of fellowship.
Author information
Authors and Affiliations
Contributions
AM and CSV designed the research and performed the work. Data was analyzed by AM, CSV and wrote the manuscript. CH provided the seed material and analyzed field data. KVNSS, ANK, and JKK estimated the oil samples by GC analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This research article does not comprise any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
CIMAP Publication Communication Number
CIMAP/PUB/2019/Mar/12.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Suppl Fig. 1 Function-fungal endophytes (RF1, SF1, and SF2) of Coleus forskohlii. Suppl Fig. 2 Effect of fungal endophytes (RF1, SF1 and SF2) on plant growth response of P. graveolens under field conditions. The control plants in plot (a), RF1 (b), SF1 (c), and SF2 (d). The images (plots) represent the field view of endophytes treated P. graveolens plants (n = 16) at the time of harvesting. Suppl Fig. 3 GC analysis of geranium essential oil composition. The chromatograms represent control and endophyte treatments of P. graveolens. (a) Control, (b) RF1, (c) SF1, and (d) SF2. (DOCX 5265 kb)
Rights and permissions
About this article
Cite this article
Mastan, A., Vivek Babu, C.S., Hiremath, C. et al. Treatments with native Coleus forskohlii endophytes improve fitness and secondary metabolite production of some medicinal and aromatic plants. Int Microbiol 23, 345–354 (2020). https://doi.org/10.1007/s10123-019-00108-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-019-00108-x