Abstract
Surgery is still the first choice to treat oral cancer, where it is important to detect surgical margins in order to reduce cancer recurrence and maintain oral-maxillofacial function simultaneously. As a non-invasive and in situ imaging technique, optical coherence tomography (OCT) can obtain images close to the resolution of histopathology, which makes it have great potential in intraoperative diagnosis. However, it is not enough to find surgical margins accurately just observing OCT images directly and qualitatively. The purpose of this study is to identify oral cancer in OCT images by investigating the quantitative difference of cancer and non-cancer tissue. Based on an available optical attenuation model and the axial confocal PSF of a home-made swept source OCT system, by using fresh ex vivo human oral tissues from 14 patients of oral squamous cell carcinoma (OSCC) as the samples, diagnosis with sensitivity (97.88%) and specificity (83.77%) was achieved at the attenuation threshold of 4.7 mm−1, and the accuracy of identification reached 91.15% in our study. Our preliminary results demonstrated that the oral cancer resection will be guided accurately and the clinical application of OCT will be further promoted by deeply mining the information hidden in OCT images.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oral squamous cell carcinoma (OSCC) is one of the most common cancers in the oral cavity, and the 5-year survival rate of patients is still less than 50% (the mortality rate largely increases from ~ 17 to ~ 62% when the oral cancer progresses from local stage to distant stage) [1, 2]. The evidence suggests that the gross total resection (GTR) is the most important factor for delaying tumor recurrence and prolonging survival [3, 4].
In clinical practice, the GTR of oral cancer is usually determined by palpation of experienced clinicians. Due to the infiltration of cancer cells, if not being completely removed, it will lead to the recurrence of cancer. On the other hand, subjective enlarged resection will increase the patient’s recovery process. Accurate diagnoses of oral lesions still need to be confirmed by biopsy or histopathology now. However, this procedure is painful, invasive, and time-consuming. As a known real-time, non-invasive, and label-free imaging technique, the resolution of optical coherence tomography (OCT) is close to that of histopathological examination. If OCT can be used to accurately identify cancers from non-cancerous tissues, it will be helpful for surgeons to perform oral cancer resection.
OCT has been widely used in some clinical applications, such as ophthalmology [5, 6], cardiology [7], gastroenterology [8], and dermatology [9, 10]. In addition to high morphological resolution, rich information in OCT images can be deeply mined to analyze characteristics of bio-tissues or diagnose diseases quantitatively [11,12,13,14,15,16,17,18].
OCT has been used in oral tissue imaging [19,20,21,22,23,24,25,26,27,28,29]. However, quantitative analysis of OCT images of oral mucosal diseases was investigated by only a few papers in recent years. The group of Yang established the method based on three parameters for diagnosing oral mucosal tissue. Their study found that the standard deviation and fitted spatial frequency of an A-scan signal profile in OCT images are good indicators for the diagnosis of oral cancer [30]. Then, further study of this group referred to distinguishing OSCC by comparing the exponential decay constants of A-scan profiles, but no data about sensitivity and specificity were demonstrated [31]. Adegun et al. quantitatively differentiated dysplasia from normal control samples by using two-dimensional (2D)-OCT and histology images, and similar analysis on three-dimensional (3D)-OCT datasets resulted in the reclassification of biopsy samples into the normal/mild and moderate/severe groups [32].
If a quantitative diagnostic criterion to differentiate cancer from non-cancer can be obtained, it will play a great role in assisting surgeons to perform excision of oral cancers. In this study, an attenuation model, matching the confocal properties of OCT system, is established to calculate optical attenuation coefficients of oral tissues in OCT images, and an attenuation threshold is selected according to sensitivity and specificity to identify OSCC and non-cancer. 3D OCT dataset is used to display the visual distinction of cancerous regions. The research provides a real-time method to reconstruct pseudo-color maps and a high-resolution visual diagnostic tool to distinguish cancer from non-cancer, and demonstrates the potential of OCT in aided diagnosis and boundary localization in oral cancer surgery.
Materials and methods
Swept source OCT system
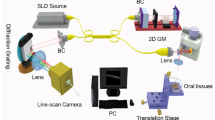
A portable home-made swept source OCT (SS-OCT) system was used to image the samples, whose swept source, with a central wavelength of 1310 nm and a tunable spectral range of 87 nm, has 20 mW average output power and 100 kHz sweep rate. The measured axial and lateral resolutions are 14 μm (in air) and 17 μm, respectively.
Sample preparation
Fresh oral tissues were obtained from the Tianjin Stomatological Hospital, Tianjin, China. The protocol was approved by Ethics Committee of Tianjin Stomatological Hospital. Ex vivo tissues were scanned by the home-made SS-OCT system immediately after surgery resection. Each 2D OCT image consists of 1024 × 1000 pixels in the axial and lateral directions; each 3D image contains 1000 2D images. 2D and 3D OCT datasets were stored digitally in a computer for post-processing and quantitative analysis. Images collected by OCT were separated into two data sets, training dataset, and validation dataset. The training dataset is used to build the optical attenuation threshold and the validation dataset is used to test and evaluate the identification result based on the threshold. 3D data will be processed to obtain en-face color map images.
After OCT scanning, the tissues within imaging range were successively fixed, embedded, sliced, and stained. Finally, a specialized pathologist at the Tianjin Stomatological Hospital inspected histology slides independently and assessed whether the slices contained cancerous tissue, and generated the final pathological reports. In addition, histology slides were digitized with a microscope for corresponding to OCT images.
Optical attenuation model
When the axial confocal PSF of OCT system is introduced into Beer’s law, a model of optical attenuation in tissue based on the OCT system can be obtained, whose signal profile in depth can be described in the following formula [12, 33]:
where I(z) is the averaged signal profile versus depth z (in mm), zcf is the position of the focal plane, μt is the attenuation coefficient, and zR is the Rayleigh length. By introducing two constants of multiplier A1 and offset A2, the fitting equation is changed as follows:
Then, A1, A2, and μt are the three fitting parameters. For quantitative analysis of depth-dependent OCT signals, each 2D OCT image is divided into sections as regions of interest (ROIs) according to the texture features of the tissue (non-cancer, cancer), so that each section is roughly in the same tissue region. The above boundary of the ROI is specified to be 20 μm below the sample surface, which can alleviate the influence of a strong entrance signal on the attenuation curve. The depth-dependent signal values of each region are averaged along the transverse direction, then I(z) is fitted based on Eq. 2.
Evaluation parameters and statistical analysis
The data from the training dataset are used to establish an optimal attenuation threshold to distinguish cancer from non-cancer. The distribution of attenuation coefficients is shown in a histogram of 0.5 mm−1 per bin. Sensitivity and specificity are assessed in order to select the optimal attenuation threshold, whose values are obtained by varying attenuation coefficients (from 0 to 8 mm−1 at 0.5 mm−1 intervals). In our study, the optimal attenuation threshold is defined as maximum sensitivity with at least 80% specificity like the studies [16, 34]. The receiver operating characteristic (ROC) curve is used to evaluate the true-positive (sensitivity) and false-positive (1-specificity) rates of different tissue types in the training dataset, which can indicate the effectiveness of using the optical attenuation coefficients for OSCC diagnosis based on OCT images.
Statistical differences are assessed with two-sample and one-tailed unequal variance Student’s t test. During evaluation, we assumed that the attenuation of non-cancer oral tissues is higher than that of cancer. P ≤ 0.05 was regarded as statistically significant.
Results
Samples from 14 patients with OSCC were collected for quantitative analysis. The demographic data of patients are given in Table 1. In order to establish an optimal optical attenuation threshold to distinguish between cancer and non-cancer based on optical attenuation characteristics, 3174 2D images (1034 non-cancers, 2140 cancers) from non-cancerous sites and cancerous sites are used as the training dataset. ROI (600 μm in transverse) was depicted from each 2D image. Then, the sensitivity and specificity of the attenuation threshold were tested and evaluated with 638 images (322 cancers, 316 non-cancers) as the validation dataset.
OCT images of OSCC
A typical example of OCT images of OSCC is shown in Fig. 1. The scanning position is shown by the black line in Fig. 1a. OSCC is epithelial invasive carcinoma with varying degrees of squamous differentiation, and consists of irregular squamous epithelium of nest- or cord-like hyperplasia. As shown in Fig. 1b, the boundary between the epithelial layer (EP) and the lamina propria (LP) is unclear, and the basement membrane is destroyed in the left side. As indicated by the black arrows, squamous cell carcinoma is shown as nested structures, and the cord-like structures (Fig. 1b). The boundary of squamous cell carcinoma and non-squamous cell carcinoma can be seen. The green dashed curve points out the approximate boundary. The right side is the keratinized layer (K), the EP and LP, and the white-dashed curve indicates the border between EP and LP (Fig. 1b). The corresponding histopathological image of OSCC is shown in Fig. 1c. Figure 1 d shows an OCT image of non-cancer tissue, where EP and LP can be clearly distinguished, and the basement membrane is intact indicated by a white-dashed curve. There are blood vessels in the submucosa as shown by the yellow arrow.
Images of OSCC versus non-cancerous oral tissue: a the photo of excised tissue specimen; b an OCT image scanned along the black line of a; c the corresponding histopathological image; d an OCT image of normal mucosa. SCC, squamous cell carcinoma. K, keratinized layer. EP, epithelium layer. LP, lamina propria. Scale bars are 1 mm
Optical attenuations of different tissues
Based on the OCT images obtained from fresh ex vivo human oral tissues in the training dataset, optical attenuation coefficients of cancer and non-cancer tissues were calculated, which are shown in Fig. 2. Slight overlap exists in the frequency distribution of optical attenuation coefficients between non-cancer and cancer tissues (Fig. 2a). Compared with non-cancer mucosa, OSCC has lower attenuation coefficients. The average attenuation of non-cancer (5.65 ± 0.82 mm−1) is significantly higher than that of OSCC (3.11 ± 0.85 mm−1).
Distribution of optical attenuation coefficients of different tissues in the training dataset. Histogram distribution of optical attenuation coefficient (a), sensitivity and specificity distribution (b), and scattering diagram (c) of different tissues with the optimal optical attenuation threshold for cancer and non-cancer
The corresponding curves of diagnostic sensitivity and specificity with attenuation coefficients are shown in Fig. 2b. Scattering diagram of different tissues is shown in Fig. 2c. To ensure the accuracy of the optical attenuation coefficients, all diagnoses of cancer and non-cancer tissues were firstly confirmed by an experienced pathologist based on histopathological analysis.
Statistical difference in the training dataset (cancer versus non-cancer) is assessed with two-sample, one-tailed unequal variance Student’s t test based on the hypothesis that non-cancer has higher attenuation. P < 0.001 was found between non-cancer and cancer.
The optimal optical attenuation threshold is defined as the attenuation coefficient of the maximum sensitivity with at least 80% specificity. According to the frequency distribution histograms, diagnostic sensitivity and specificity curves and scattering diagrams, the optimal attenuation threshold is selected as 4.7 mm−1.
ROC curve was drawn in Fig. 3. We can see that the curve is very close to the top left corner, so the obtained optical attenuation coefficients can be regarded as a good indicator in distinguishing cancer from non-caner tissues.
Evaluation by using the validation dataset
The validation dataset, consisting of an independent subset of tissues, was obtained for evaluation of sensitivity and specificity for the chosen optical attenuation threshold (4.7 mm−1). The histological diagnosis was made by an experienced pathologist who knew nothing about the OCT results. By using the established optical attenuation threshold of 4.7 mm−1, the specificity, sensitivity, and accuracy of identification are 83.77%, 97.88%, and 91.15%, respectively.
The statistical distribution of the validation dataset is shown in Fig. 4. Figure 4 a is the frequency distribution histogram of non-cancer versus cancer. Figure 4 b is the significant difference between cancer and non-cancer (P < 0.001). As shown in Fig. 4, optical attenuation coefficients between cancer and non-cancer tissues can be clearly separated based on the specified threshold.
Color map of the attenuation coefficient for 3D OCT dataset
In addition to these 2D data sets, we reconstructed en-faced color map visualization using the collected 3D data sets based on the optical attenuation threshold, whose typical results are shown in Fig. 5. For each imaging dataset, 840 attenuation data points were obtained by using a mask to translate horizontally in each 2D OCT image.
En-faced color maps based on attenuation coefficients of different tissues and their histopathological images. a and d, b and e, and c and f Color maps and their corresponding histopathological images from three different tissues, respectively. g 3D OCT data with color mapped attenuation coefficients. SCC, squamous cell carcinoma. EP, epithelium layer. LP, lamina propria. NC, non-cancer. Scale bars are 1 mm
Figure 5a–c and d–f are en-faced color maps and their corresponding histopathological images from three different tissues, respectively. Figure 5 a and d are non-cancer images (green). Figure 5 b and e are images of the boundary of non-cancer and cancer (yellow). Figure 5 c and f are images of OSCC (red). Non-cancer and cancer tissues can be clearly identified from the color maps.
In order to provide direct visual cues for cancer versus non-cancer areas, the color-coded optical property map is given based on the 3D dataset. 3D volumetric reconstructions are overlaid with en-faced color maps on the top surface. As shown in Fig. 5g, the range of OSCC is visual and the boundary between cancer and non-cancer in en-faced color map can be more clearly observed, which can help surgeons perform oral surgery effectively.
Discussions
Non-cancer tissues should be reserved as much as possible under the premise of complete removal of oral cancer. Therefore, surgical margin of cancer is an urgent topic in oral surgery. The increasing need for easy intraoperative identification of cancer has led to the development of different imaging surgical assistance, including ultrasound [35, 36], fluorescence [37], hyperspectral [38], Raman spectroscopy [39], and higher-harmonic generation microscopy [40]. These imaging patterns have contributed significantly to oral surgery studies. For example, ultrasound provides oral tissue imaging with good penetration depth and field of view, but it has limited spatial resolution and contrast for oral cancer detection. Fluorescence imaging is limited to two-dimensional imaging, resulting in the inability to obtain spatial information. Compared with other imaging methods, OCT is more suitable for clinical exploration because of its advantages of high-resolution, non-invasive, and 3D imaging.
We firstly analyze of the feasibility for accurate localization of OSCC boundary based on SS-OCT system quantitatively in this study. An available optical attenuation model is used to distinguish OSCC from non-cancer tissues with high sensitivity and specificity. This study shows that not only OCT imaging provides high-resolution oral histological microstructure, but also that quantitative analysis of OCT data and the establishment of color-coded maps based on different optical attenuation coefficients can provide important additional details. By combining structural imaging with optical coefficients, it is convenient for surgeons to visually evaluate diseased areas and distinguish cancer from non-cancer tissues.
OCT structure images with anatomical information like Fig. 1b and d can be used to identify different tissues, and the boundaries of non-cancer and cancer tissues can be clearly displayed by the optical attenuation information implied in OCT images, which is much easier for users unfamiliar with OCT images to interpret images and make decisions.
Based on the high-resolution images and the characteristics of optical attenuation of different tissues, OCT can accurately locate surgical margins of oral lesion, which provides a great potential tool in intraoperative diagnosis of oral tissues. It builds a bridge between the scientific research of OCT and the oral clinical application, which will delay tumor recurrence and improve survival and quality of life.
The samples in our study were mainly acquired from gingival and tongue tissues, it is necessary to consider whether the tissues in different sites have an impact on the experimental results. Given this, we analyzed the differences of non-cancer tissue in different sites. Six hundred and two OCT images of gingival tissue were obtained from eight patients and 432 OCT images of tongue tissue from others (Table 1). By extracting attenuation coefficients, the mean and standard deviation of two kinds of data were calculated, and statistical analysis was assessed with two-sample and one-tailed unequal variance Student’s t test. The result showed that the average optical attenuations of gingiva (5.71 ± 0.83) and tongue (5.57 ± 0.69) were no significant difference (P = 0.1703). In other words, the different attenuation coefficients of gingival and tongue tissues can be neglected.
As shown in Fig. 1b and d, compared with non-cancerous oral mucosa, cancerous tissues have a more random distribution, whose composition scale in statistics becomes larger. It is proven that the fitting attenuation coefficients of cancerous tissue are smaller than those of non-cancerous tissue, which is the same as the study of Tsai [30].
Faber et al. verified the validity of the single scattering model with weakly scattering media, the attenuation coefficient of which is less than 8 mm−1 [33]. Zhang et al. applied this model to analyze rectal cancer and verified the feasibility of diagnosis [12]. Tes et al. also used the single scattering model to validate the application of skin tissue [17]. The single scattering model with OCT confocal properties was used for quantitative analysis of the OSCC in our study, and the high sensitivity (97.88%) and specificity (83.77%) of diagnosis were obtained.
Turani et al. [41] identified the melanoma by using a multilayer-scattering model which considered the so-called “shower curtain effect” and led to higher diagnostic sensitivity and specificity by incorporating the effects of single scattering and multiple scattering. Our study focuses specifically on the identification of OSCC from non-cancer in this paper. Oral benign and malignant tumors include pleomorphic adenoma, basal cell carcinoma, and adenoid cystic carcinoma [42]. The accurate classification and identification of common benign and malignant tumors is also our research focus. In the future, based on the OCT images for four salivary gland tumors we have obtained, we will further study the scattering model, especially the effect of the multiple scattering, in order to differentiate different tumors.
Image preprocessing and optimization of ROI are indispensable for improving the specificity and sensitivity. In addition, with the application of machine learning in various fields, the methods of identifying oral cancer tissues in OCT images by machine learning are our further research direction.
Conclusion
It was proven from the experimental results that the optical attenuation model and the established threshold had high sensitivity (97.88%) and specificity (83.77%) for identification of OSCC, which indicates the quantitative analysis method based on OCT images is effective to distinguish oral cancer from non-cancerous tissues. The accuracy of identification reached 91.15%. Based on the optical attenuation threshold (4.7 mm−1), the color-coded OCT maps can be obtained to locate the boundary of oral lesion accurately. Quantitative information hidden in OCT images exhibits great potential in oral surgery, in particular for the rapid and accurate intraoperative boundary detection of OSCC.
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34. https://doi.org/10.3322/caac.21551
American Cancer Society (2017) Cancer facts & figures. American Cancer Society, Atlanta
Massano J, Regateiro FS, Januario G, Ferreira A (2006) Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102(1):67–76. https://doi.org/10.1016/j.tripleo.2005.07.038
Azzopardi S, Lowe D, Rogers S (2019) Audit of the rates of re-excision for close or involved margins in the management of oral cancer. Br J Oral Maxillofac Surg 57(7):678–681. https://doi.org/10.1016/j.bjoms.2019.05.006
Costello F (2017) Optical coherence tomography in neuro-ophthalmology. Neurol Clin 35(1):153–163. https://doi.org/10.1016/j.ncl.2016.08.012
Abdelazeem K, Sharaf M, Saleh MGA, Fathalla AM, Soliman W (2018) Relevance of swept-source anterior segment optical coherence tomography for corneal imaging in patients with flap-related complications after LASIK. Cornea 38(1):93–97. https://doi.org/10.1097/ico.0000000000001773
Yonetsu T, Bouma BE, Kato K, Fujimoto JG, Jang IK (2013) Optical coherence tomography- 15 years in cardiology. Circ J 77(8):1933–1940. https://doi.org/10.1253/circj.cj-13-0643.1
Tsai TH, Leggett CL, Trindade AJ, Sethi A, Swager AF, Joshi V, Bergman JJ, Mashimo H, Nishioka NS, Namati E (2017) Optical coherence tomography in gastroenterology: a review and future outlook. J Biomed Opt 22(12):1–17. https://doi.org/10.1117/1.jbo.22.12.121716
Adabi S, Fotouhi A, Xu Q, Daveluy S, Mehregan D, Podoleanu A, Nasiriavanaki M (2018) An overview of methods to mitigate artifacts in optical coherence tomography imaging of the skin. Skin Res Technol 24(2):265–273. https://doi.org/10.1111/srt.12423
Jerjes W, Hamdoon Z, Hopper C (2019) Structural validation of facial skin using optical coherence tomography: a descriptive study. Skin Res Technol 00:1–10. https://doi.org/10.1111/srt.12791
Van Soest G, Goderie T, Regar E, Koljenovic S, van Leenders GLJH, Gonzalo N, van Noorden S, Okamura T, Bouma BE, Tearney GJ, Oosterhuis JW, Serruys PW, van der Steen AFW (2010) Atherosclerotic tissue characterization in vivo by optical coherence tomography attenuation imaging. J Biomed Opt 15(1):011105. https://doi.org/10.1117/1.3280271
Zhang Q, Wu X, Tang T, Zhu S, Yao Q, Gao B, Yuan X (2012) Quantitative analysis of rectal cancer by spectral domain optical coherence tomography. Phys Med Biol 57(16):5235–5244. https://doi.org/10.1088/0031-9155/57/16/5235
Xu C, Schmitt JM, Carlier SG, Virmani R (2008) Characterization of atherosclerosis plaques by measuring both backscattering and attenuation coefficients in optical coherence tomography. J Biomed Opt 13(3):034003
Kah JCY, Chow TH, Ng BK, Razul SG, Olivo M, Sheppard CJR (2009) Concentration dependence of gold nanoshells on the enhancement of optical coherence tomography images: a quantitative study. Appl Opt 48(10):D96–D108. https://doi.org/10.1364/AO.48.000D96
Yuan W, Kut C, Liang W, Li XD (2017) Robust and fast characterization of OCT-based optical attenuation using a novel frequency-domain algorithm for brain cancer detection. Sci Rep 7:44909. https://doi.org/10.1038/srep44909
Kut C, Chaichana KL, Xi J, Raza SM, Ye X, McVeigh ER, Rodriguez FJ, Quiñones-Hinojosa A, Li XD (2015) Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci Transl Med 7(292):292ra100. https://doi.org/10.1126/scitranslmed.3010611
Tes D, Aber A, Zafar M, Horton L, Fotouhi A, Xu Q, Moiin A, Thompson AD, Moraes Pinto Blumetti TC, Daveluy S, Chen W, Nasiriavanaki M (2018) Granular cell tumor imaging using optical coherence tomography. Biomed Eng Comput Biol 9:1–9. https://doi.org/10.1177/1179597218790250
Adabi S, Hosseinzadeh M, Noei S, Conforto S, Daveluy S, Clayton A, Mehregan D, Nasiriavanaki M (2017) Universal in vivo textural model for human skin based on optical coherence tomograms. Sci Rep 7(1):17912. https://doi.org/10.1038/s41598-017-17398-8
Jung W, Zhang J, Chung J, Wilder-Smith P, Brenner M, Nelson JS, Chen Z (2005) Advances in oral cancer detection using optical coherence tomography. IEEE J Sel Top Quantum Electron 11(4):811–817. https://doi.org/10.1109/JSTQE.2005.857678
Wilder-Smith P, Hammer-Wilson MJ, Zhang J, Wang Q, Osann K, Chen Z, Wigdor H, Schwartz J, Epstein J (2007) In vivo imaging of oral mucositis in an animal model using optical coherence tomography and optical Doppler tomography. Clin Cancer Res 13(8):2449–2454. https://doi.org/10.1158/1078-0432.CCR-06-2234
Jerjes W, Upile T, Betz CS, Abbas S, Sandison A, Hopper C (2008) Detection of oral pathologies using optical coherence tomography. Eur Oncol Haematol 4(1):57–59. https://doi.org/10.17925/eoh.2008.04.1.57
Davoudi B, Lindenmaier A, Standish BA, Allo G, Bizheva K, Vitkin A (2012) Noninvasive in vivo structural and vascular imaging of human oral tissues with spectral domain optical coherence tomography. Biomed Opt Express 3(5):826–839. https://doi.org/10.1364/BOE.3.000826
Hamdoon Z, Jerjes W, Upile T, McKenzie G, Jay A, Hopper C (2013) Optical coherence tomography in the assessment of suspicious oral lesions: an immediate ex vivo study. Photodiagnosis and Photodynamic Therapy 10(1):17–27. https://doi.org/10.1016/j.pdpdt.2012.07.005
Choi WJ, Wang RK (2014) In vivo imaging of functional microvasculature within tissue beds of oral and nasal cavities by swept-source optical coherence tomography with a forward/side-viewing probe. Biomed Opt Express 5(8):2620–2634. https://doi.org/10.1364/BOE.5.002620
Maslennikova AV, Sirotkina MA, Moiseev AA, Finagina ES, Ksenofontov SY, Gelikonov GV, Matveev LA, Kiseleva EB, Zaitsev VY, Zagaynova EV, Feldchtein FI, Gladkova ND, Vitkin A (2017) In-vivo longitudinal imaging of microvascular changes in irradiated oral mucosa of radiotherapy cancer patients using optical coherence tomography. Sci Rep 7(1):16505. https://doi.org/10.1038/s41598-017-16823-2
Tsai M-T, Chen Y, Lee C-Y, Huang B-H, Trung NH, Lee Y-J, Wang Y-L (2017) Noninvasive structural and microvascular anatomy of oral mucosae using handheld optical coherence tomography. Biomed Opt Express 8(11):5001–5012. https://doi.org/10.1364/BOE.8.005001
Maslennikova A, Sirotkina M, Sedova E, Moiseev A, Ksenofontov S, Gelikonov G, Matveev L, Kiseleva E, Zaitsev V, Zagaynova E, Feldstein F, Gladkova N, Vitkin A (2018) In vivo imaging of microvascular changes in irradiated oral mucosa by optical coherence tomography. Radiother Oncol 127:E586–E586
Surlin P, Camen A, Stratul SI, Roman A, Gheorghe DN, Herascu E, Osiac E, Rogoveanu I (2018) Optical coherence tomography assessment of gingival epithelium inflammatory status in periodontal - systemic affected patients. Ann Anat 219:51–56. https://doi.org/10.1016/j.aanat.2018.04.010
Heidari AE, Sunny SP, James BL, Lam TM, Tran AV, Yu J, Ramanjinappa RD, Uma K, Birur P, Suresh A, Kuriakose MA, Chen Z, Wilder-Smith P (2019) Optical coherence tomography as an oral cancer screening adjunct in a low resource settings. IEEE J Sel Top Quantum Electron 25(1):1–8. https://doi.org/10.1109/JSTQE.2018.2869643
Tsai M-T, Lee H-C, Lee C-K, Yu C-H, Chen H-M, Chiang C-P, Chang C-C, Wang Y-M, Yang CC (2008) Effective indicators for diagnosis of oral cancer using optical coherence tomography. Opt Express 16(20):15847–15862. https://doi.org/10.1364/OE.16.015847
Tsai M-T, Lee C-K, Lee H-C, Chen H-M, Chiang C-P, Wang Y-M, Yang CC (2009) Differentiating oral lesions in different carcinogenesis stages with optical coherence tomography. J Biomed Opt 14(4):044028. https://doi.org/10.1117/1.3200936
Adegun OK, Tomlins PH, Hagi-Pavli E, McKenzie G, Piper K, Bader DL, Fortune F (2012) Quantitative analysis of optical coherence tomography and histopathology images of normal and dysplastic oral mucosal tissues. Lasers Med Sci 27(4):795–804. https://doi.org/10.1007/s10103-011-0975-1
Faber DJ, van der Meer FJ, Aalders MCG, van Leeuwen TG (2004) Quantitative measurement of attenuation coefficients of weakly scattering media using optical coherence tomography. Opt Express 12(19):4353–4365. https://doi.org/10.1364/OPEX.12.004353
Adegun OK, Tomlins PH, Hagi-Pavli E, Bader DL, Fortune F (2012) Quantitative optical coherence tomography of fluid-filled oral mucosal lesions. Lasers Med Sci 28(5):1249–1255. https://doi.org/10.1007/s10103-012-1208-y
Fatakdawala H, Poti S, Zhou F, Sun Y, Bec J, Liu J, Yankelevich DR, Tinling SP, Gandour-Edwards RF, Farwell DG, Marcu L (2013) Multimodal in vivo imaging of oral cancer using fluorescence lifetime, photoacoustic and ultrasound techniques. Biomed Opt Express 4(9):1724–1741. https://doi.org/10.1364/BOE.4.001724
Izzetti R, Fantoni G, Gelli F, Faggioni L, Vitali S, Gabriele M, Caramella D (2018) Feasibility of intraoral ultrasonography in the diagnosis of oral soft tissue lesions: a preclinical assessment on an ex vivo specimen. Radiol Med 123(2):135–142. https://doi.org/10.1007/s11547-017-0817-8
Simonato LE, Tomo S, Miyahara GI, Navarro RS, Villaverde AGJB (2017) Fluorescence visualization efficacy for detecting oral lesions more prone to be dysplastic and potentially malignant disorders: a pilot study. Photodiagn Photodyn Ther 17:1–4. https://doi.org/10.1016/j.pdpdt.2016.10.010
Lu G, Wang D, Qin X, Muller S, Wang X, Chen AY, Chen ZG, Fei B (2018) Detection and delineation of squamous neoplasia with hyperspectral imaging in a mouse model of tongue carcinogenesis. J Biophotonics 11(3). https://doi.org/10.1002/jbio.201700078
Wang J, Zheng W, Lin K, Huang Z (2018) Characterizing biochemical and morphological variations of clinically relevant anatomical locations of oral tissue in vivo with hybrid Raman spectroscopy and optical coherence tomography technique. J Biophotonics 11(3). https://doi.org/10.1002/jbio.201700113
Tsai M-R, Shieh D-B, Lou P-J, Lin C-F, Sun C-K (2012) Characterization of oral squamous cell carcinoma based on higher-harmonic generation microscopy. J Biophotonics 5(5–6):415–424. https://doi.org/10.1002/jbio.201100144
Turani Z, Fatemizadeh E, Blumetti T, Daveluy S, Moraes AF, Chen W, Mehregan D, Andersen PE, Nasiriavanaki M (2019) Optical radiomic signatures derived from optical coherence tomography images to improve identification of melanoma. Cancer Res 79(8):2021–2030
Yang Z, Shang J, Liu C, Zhang J, Hou F, Liang Y (2020) Intraoperative imaging of oral-maxillofacial lesions using optical coherence tomography. J Innov Opt Health Sci:2050010. https://doi.org/10.1142/s1793545820500108
Funding
This study was supported in part by the National Natural Science Foundation of China (61875092 and 11374167), Science and Technology Support Program of Tianjin (17YFZCSY00740), and Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Ethics Committee of Tianjin Stomatological Hospital.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Z., Shang, J., Liu, C. et al. Identification of oral cancer in OCT images based on an optical attenuation model. Lasers Med Sci 35, 1999–2007 (2020). https://doi.org/10.1007/s10103-020-03025-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-03025-y