Abstract
This study evaluated the degree of endodontic smear layer removal using the Er:YAG PIPS technique (2.94 μm) in comparison with different irrigants. Sixty-four single-rooted teeth were endodontically prepared up to size #40 and were divided into 8 groups (a–h) (n = 8). Groups a, b, c, and d were irrigated with (3 % NaOCl + 20 % EDTA), (0.9 % NaCl), (3 % NaOCl), and (20 % EDTA), respectively. Groups e, f, g, and h were treated with (3 % NaOCl + 20 % EDTA + PIPS), (0.9 % NaCl + PIPS), (3 % NaOCl + PIPS), and (20 % EDTA + PIPS), respectively. The settings of the Er:YAG PIPS technique were (0.3 W, 20 mJ, 15 Hz, 50 μs, no water and air). The root canals were examined under a profilometer to evaluate the degree of smear layer removal using Hülsmann scores. The smear layer was present in the coronal, middle, and apical thirds of groups b, c, f, and g. Groups a, d, e, and h exhibited open dentinal tubules in the coronal and middle thirds. However, none of the apical thirds showed open dentinal tubules. No significant difference was observed between the groups treated only with irrigants and those treated with Er:YAG PIPS and the same irrigants (p ≥ 0.0018). The Er:YAG PIPS technique did not show any improved results in removing the smear layer when compared to the irrigants alone. Moreover, the open dentinal tubules in some groups were a result of the chelating action of 20 % EDTA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The endodontic smear layer is a result of mechanical debridement using manual or rotary endodontic instruments [1–3]. This layer is composed mainly of inorganic materials such as hydroxyapatite, dentinal shavings, and odontoblastic fragments, in addition to organic materials comprised of pulp tissue remnants, bacteria, and their toxins and dead blood cells [1–5]. The thickness of the outer part of the smear layer ranges between 1 and 2 μm, whereas the smear layer material packed inside the dentinal tubules can reach a depth up to 40 μm [4, 6]. Due to its bacterial and toxic content, in addition to creating a barrier which prevents medicaments and sealers from penetrating into the dentinal tubules, the smear layer is considered to be a harmful element which requires complete removal [3, 4, 7, 8].
The use of chemical irrigants is one of the most commonly used smear layer removal methods, due to the ease of administration of these aqueous solutions, in addition to their different chemical properties. However, delivering aqueous solutions in the narrow apical third of the canal can be a challenge. Sodium hypochlorite is one of the most commonly used irrigants in dental clinics due to its disinfecting properties [9–12], in addition to its capability of dissolving organic materials [1, 4, 9–11]. However, its action in removing inorganic materials, such as those composing the inorganic part of the smear layer, is limited [2, 4, 8]. Therefore, chelating agents, such as EDTA, are used to remove the endodontic smear layer due to their strong capability of dissolving inorganic materials and minerals [13–15]. Nevertheless, EDTA has shown inadequacy in completely removing the endodontic smear layer, especially in the apical region of the root canal and the smear layer material packed inside the dentinal tubules [1, 4, 5, 16]. Furthermore, a certain degree of erosion to the dentinal walls has been associated with the use of EDTA for long periods [2].
The Er:YAG laser (2940 nm) has been used to clean and disinfect the endodontic root canal. This wavelength has demonstrated positive results in removing the endodontic smear layer when applied directly inside the root canal and without the use of chemical solution [17–19]. A recent technique called the “PIPS technique” utilizes an Er:YAG laser to remove the endodontic smear layer and disinfect the root canal system. The term PIPS stands for photon-induced photoacoustic streaming; this technology is based on the bubble cavitation mechanism, which is described as strong shockwaves resulting from collapsing bubbles in a fluid, and these shockwaves are supposedly capable of removing the smear layer from the surrounding walls [20]. A certain amount of energy applied in the fluid is mandatory to create the shockwaves and achieve the cavitation effect. To perform the endodontic PIPS technique, a specially designed radial emitting tip is placed stationary over the canal orifice after filling the canal with irrigating solutions (NaOCl and EDTA, in sequence), the Er:YAG laser energy then activates the solutions by creating bubbles, which burst strongly and rapidly creating shockwaves throughout the fluid, and in turn eliminate the smear layer from the canal walls [20–23].
Despite their aforementioned side effects [1, 2, 4], chemical solutions are still being used in routine endodontic treatments, as well as with the Er:YAG PIPS technique. This study tests the effectiveness of the PIPS technique using different chemical irrigants, including normal physiological saline, sodium hypochlorite, and the chelating agent EDTA in comparison with the use of the same irrigating solutions alone.
Materials and methods
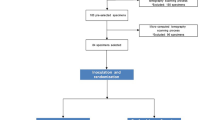
Sixty-four single-rooted human teeth extracted for periodontal or orthodontic reasons were endodontically prepared up to standardized size #40. The teeth were kept in 0.1 % thymol solution (0.01 % thymol + 0.09 % NaCl) to prevent bacterial growth. The apices of the teeth used for the Er:YAG PIPS technique were sealed with a wax layer covered by a layer of acrylic resin to prevent the irrigating solutions from escaping through the apical foramen.
The irrigating solutions used in this study were as follows:
-
0.9 % NaCl: Normal saline was used to offer continuous lubrication of the canal during preparation of some group samples and to provide the ability to view a complete picture of the endodontic smear layer.
-
3 % NaOCl: This irrigant was used alone and in combination with the Er:YAG PIPS to observe whether the laser energy can activate the solution.
-
20 % EDTA: was used alone and in combination with the Er:YAG PIPS technique to test if the laser is increasing the effect of EDTA.
An Er:YAG laser (2940 nm) device was used in this study (Lightwalker AT, Fotona, Lijubljana, Slovenia). The laser tip used was a 9-mm long, 600 μm in diameter, tapered with a stripped sheath PIPS tip (Fig. 1). The parameters used were as follows: average power = 0.3 W, pulse energy = 20 mJ, frequency = 50 Hz, pulse duration = 50 μs, water, and air off.
The teeth were divided into 8 groups where four groups (a–d) were treated with irrigating solutions alone, while the remaining four groups (e–h) were treated with a combination of the same irrigating solutions and the Er:YAG PIPS. Groups a and b were assigned as the positive and negative control groups, respectively. To perform the PIPS technique, each canal was filled with the assigned solution and subjected to two 30-s laser cycles during which the PIPS tip was kept stationary over the canal orifice, while continuous application of the irrigating solutions was maintained during laser application using a syringe to insure that the canal is always filled with the solution [20–23]. A 30-s rest period between the laser cycles was applied, during which the canals were rinsed with sterile water and dried with paper points. The canal was finally filled with the irrigating solution and subjected to the second Er:YAG laser cycle using the same settings.
The teeth were divided into 8 groups a–h (n = 8). The first four groups a–d were treated with the irrigating solutions alone, while groups e–h were treated with the same solutions and the Er:YAG PIPS technique as follows:
-
Group a (positive control): Irrigated with 3 ml of 3 % NaOCl after each file enlargement, followed by 3 ml of 20 % EDTA as a final flush
-
Group b (negative control): The samples of this group were irrigated with 0.9 % NaCl after each file enlargement, and 5 ml of the same solution was administered as a final flush.
-
Group c: 3 ml of 3 % NaOCl was used to irrigate the samples of this group after each file enlargement, with a 5-ml final flush.
-
Group d: 3 ml of 20 % EDTA was administered in the canal after each file enlargement. Five milliliters of the same solution was used at the end.
-
Group e: Each canal was filled with 3 ml of 3 % NaOCl and was subjected to a 30-s laser cycle. Continuous irrigation was maintained during the laser cycle using a syringe. The canal was then washed with normal saline and after a 30-s rest period, it was filled with 20 % EDTA and subjected to another 30-s laser cycle.
-
Group f: The canals were filled with 3 ml of 0.9 % NaCl in both laser cycles. Continuous irrigation was maintained during each 30-s laser cycle, with a 30-s rest period in between.
-
Group g: 3 ml of 3 % NaOCl was used to fill the canal and was continuously irrigated during both laser cycles. Each laser cycle lasted 30 s and was separated with a 30-s rest period.
-
Group h: Each canal was filled with 3 ml of 20 % EDTA during the laser cycles, while continuous irrigation was maintained. A 30-s rest interval was achieved between the laser cycles.
After completing the treatment procedures, the roots were longitudinally sectioned using a band saw. Moreover, the roots were polished to a thickness of 1–1.5 mm in order to fit under the microscope objective lens using a polisher (Saphir 360, ATM GmbH, Mammelzen, Germany). The microscope used to examine the canals was a scanning laser microscope (profilometer), manufactured by (Keyence, 3D laser scanning microscope, VK-X100K, Tokyo, Japan). This device is used to measure surfaces roughness and is also capable of developing images with high magnifications up to ×2000. It was chosen in this study as a new examination method in dental research due to its low cost, therefore serving as a suitable microscopic examination tool for large sample sizes.
Each sample section was examined in the coronal, middle, and apical regions. The images were captured with magnifications of ×400 and ×1000. The captured images were evaluated by 3 blinded calibrated observers using the Hülsmann scoring system [24]. The scoring index from 1 to 5 indicates the heaviness of the smear layer as follows:
-
Score 1: No smear layer, completely clean surface with open dentinal tubules
-
Score 2: Small amount of smear layer, many open dentinal tubules
-
Score 3: Homogeneous smear layer covering the root canal walls; only a few dentinal tubules open
-
Score 4: Complete root canal wall covered by a homogeneous smear layer; no dentinal tubules open.
-
Score 5: Heavy, nonhomogeneous smear layer completely covering root canal walls.
Statistical analysis
The Mann-Whitney U test was used to evaluate the differences between the groups pairwise, i.e., groups where the irrigating solutions were used with and without the PIPS technique. Due to the large number of samples in this study, the Bonferroni correction method was applied to determine the p value, where p < 0.001 was considered significant. The Bonferroni correction is applied in pairwise analyses to reduce the probability of obtaining a false-positive result on a single set of data, as in the case of the present study. Furthermore, the interclass correlation test (ICC) was performed to evaluate the consensus of the evaluators’ scores. The mean values of the ICC test are interpreted using the following scale:
-
Less than 0.40: Poor consistency in evaluators’ scores
-
Between 0.4 and 0.59: Fair consistency in evaluators’ scores
-
Between 0.60 and 0.74: Good consistency in evaluators’ scores
-
Between 0.75 and 1.00: Excellent consistency in evaluators’ scores.
Results
Microscopic images
Group a (3 % NaOCl + 20 % EDTA) (Fig. 2a,b, c), group d (20 % EDTA) (Fig. 3a,b, c), group e (3 % NaOCl + 20 % EDTA + PIPS) (Fig. 4a,b, c), and group h (20 % EDTA + Er:YAG PIPS) (Fig. 5a,b, c) revealed similar microscopic results, where the dentinal tubules in the coronal and middle regions of the root canal walls were almost completely uncovered. Whereas those in the apical region were mostly covered with a heavy smear layer.
Group b (0.9 % NaCl) (Fig. 6a,b, c), group c (3 % NaOCl) (Fig. 7a,b, c), group f (0.9 % NaCl + Er:YAG PIPS) (Fig. 8a,b, c), and group g (3 % NaOCl + Er:YAG PIPS) (Fig. 9a,b, c): In these four groups, the smear layer was present on the root surfaces of the coronal, middle, and apical thirds of the root canals.
Statistical results
The statistical results of the Hülsmann scores for all groups are presented in Figs. 10, 11, and 12.
In the coronal region, there was no significant difference between the Hülsmann scores of groups (a, e), (c, g), and (d, h) (p ≥ 0.001). However, a significant difference was found between groups (b, f) (p < 0.001), with a Hülsmann mean and SD of (4.8 ± 0.3) and (4.0 ± 0.8), respectively.
In the middle third region, a comparison between groups (a, e), (b, f), and (d, h) exhibited no statistically significant difference (p ≥ 0.0018). Nevertheless, a significant difference was observed between groups (c, g) (p < 0.001), with a Hülsmann mean and SD of (4.7 ± 0.6) and (4.0 ± 0.6), respectively.
The statistical results of the Hülsmann scores in the apical third did not show any significant difference between groups (a, e), (b, f), and (d, h) (p ≥ 0.0018). Whereas groups (c, g) significantly differ from each other (p < 0.0018) having a Hülsmann mean and SD of (4.5 ± 0.5) and (4.3 ± 0.7).
In order to insure coherence of the reviewers’ scores, the interclass correlation (ICC) statistical test was performed. The mean values of the coronal, middle, and apical regions were 0.8, 0.7, and 0.8, respectively.
Discussion
The use of endodontic irrigants such as NaOCl and EDTA is arguably the most adopted smear layer removal method. The Er:YAG PIPS technique was introduced in an attempt to improve the effectiveness of these irrigating solutions. It would be expected that the Er:YAG laser energy can in fact improve the action of NaOCl and EDTA, especially in the apical third of the canal. However, the results of this study have shown that the PIPS technique did not enhance the outcome of the smear layer removal.
Results of the positive control group a (20 % EDTA + 3 % NaOCl) agree with those of Silveira et al. [25], which revealed that the alternate use of 17 % EDTA and 2.5 % NaOCl resulted in a good smear layer removal outcome in the coronal and middle thirds of the root canal. However, the apical third was still covered with a smear layer, and the irrigants were not capable of cleaning this part of the canal. This result was expected, since the irrigants cannot effectively clean the apical part due to the narrowness of the canal, in accordance with previous studies [1, 4, 5, 16].
Several studies have documented the success of removing the endodontic smear layer using the Er:YAG PIPS technique [20–23]. These studies confirmed that activating 5 % NaOCl and 17 % EDTA with the Er:YAG PIPS technique improved the irrigants’ action in removing the smear layer from the entire canal walls, including the apical third. However, contrary to those previously published studies [20–23], the apical third in group e was still covered with a smear layer, with an average mean Hülsmann value of 4.04, suggesting that there was no additional cavitation effect. There was no significant difference regarding the removal of the smear layer in all thirds of the canal walls between groups a and e (p ≥ 0.0018), indicating that the PIPS technique did not improve the irrigants’ action.
The use of Er:YAG PIPS technique with 0.9 % NaCl in group f generally did not show any morphological changes on the root canal surface, and the shockwave effect resulting from the collapsing bubbles could not be detected with normal saline, even though the cavitation effect is a physical effect that can be achieved in any fluid. However, three of the samples received a Hülsmann score 3 in the coronal region, and a statistically significant difference between groups (b and f) in the coronal third was noted (p < 0.001). The mean Hülsmann score and the mode (most occurring score) in the coronal third in group f were 4.0 and 3, respectively. These numbers indicate a level of variation regarding the results of smear layer removal in group f. This might be attributed to a slight insertion of the laser tip in the canal, resulting in a direct irradiation of the canal walls. Alternatively, it might suggest an actual laser-irrigant interaction. Further research is required in order to come with a definite conclusion.
Similarly to 0.9 % NaCl, the Er:YAG PIPS technique did not improve the smear layer removal results when used with 3 % NaOCl in group g. This result is comparable to those of Zhu et al. [24] who reported an inadequate smear layer removal when using the PIPS technique with 3 % NaOCl, especially in the apical third of the canal. Statistically, there was a significant difference between groups c and g in the middle third of the canal (p < 0.001). However, the mean and mode for both groups were 4.6 and 5 in group c, and 4.0 and 4 in group g, respectively. These numbers demonstrate that the middle thirds of both groups were both covered with a smear layer, where group g presents a more homogeneous layer.
The apical third of the root canal was completely covered with a smear layer in group h, where the Er:YAG PIPS technique was applied while irrigating with 20 % EDTA. It is well known that EDTA is a chelating agent which is capable of dissolving minerals and inorganic materials [2, 4, 5, 7, 14]. No statistically significant difference was observed when comparing groups d and h in all regions of the canal walls, implying that the PIPS technique did not enhance the action of 20 % EDTA.
In all 8 groups, there was a consensus in the Hülsmann scores given by the three evaluators. This test was performed to eliminate any possible significant variation between the evaluators’ scores. In all three thirds, the scores were between 0.7 and 0.8, indicating a very high level of agreement between the evaluators.
Creating a bubble cavitation effect depends on several factors, such as the length of the tube holding the liquid, the viscosity of the liquid, and the circulation created in the liquid, in addition to the gravitational force [26]. In case of the PIPS technique, the tube holding the liquid is the root canal, which varies in length according to the tooth. The viscosity of the liquid plays a role in creating bubble cavitation, i.e., if the liquid is more viscous, more energy from the Er:YAG laser will be required to create a shockwave resulting from a bubble collapse. Gopikrishna et al. [27] reported that the viscosity coefficients of 5.25 % NaOCl and 17 % EDTA in 25 °C are 1.36 and 1.53 Centipoise (Cps), respectively. However, changing the percentage of the irrigating solutions creates a difference in the viscosity coefficient, which might play a role in the outcome of the bubble cavitation effect [28]. Some samples in group f where the laser was used with 0.9 % NaCl exhibited a few uncovered dentinal tubules in the coronal third. Moreover, a few open dentinal tubules could be detected in the coronal third of one sample in group g, irrigated with 3 % NaOCl while applying the Er:YAG laser. The presence of these results could be a starting point to understand the behavior of the PIPS technique with different solutions viscosities.
Combining these factors together, it is reasonable to point out that creating a cavitation effect cannot be achieved by using the same laser parameters with different root lengths and different irrigants. Longer roots will need more energy input to create strong pressure amplitudes. The physics background of the cavitation effect on which the PIPS technique is based is ought to be further researched and understood before applying the concept in endodontic treatments.
In this in vitro study, teeth that were treated with the Er:YAG PIPS technique were all positioned as those in the mandible, so as to keep the irrigating solutions inside the root canals while applying the laser cycles. However, applying this technique on the maxillary teeth in a clinical situation can be impractical, since the solutions will naturally flow downwards.
Conclusion
Within the limitations of this study, it can be concluded that removing the endodontic smear layer cannot be accomplished with the Er:YAG PIPS technique, since the desired cavitation effect cannot be attained by applying the same laser settings with different anatomically structured teeth, and different solution viscosities. From this study, it could be concluded that the exposed dentinal tubules on the root canal walls were only a result of the chelating action of EDTA, while no further noticeable laser activation of the different irrigants could be detected.
References
Garberoglio R, Becca C (1994) Smear layer removal by root canal irrigants. Oral Surg Oral Med Oral Pathol 78(3):359–367
Zivkovic S, Brkanica T, Dacic D, Opacic V, Pavlovic V, Medojevic M (2005) Smear layer in endodonics. Serb Dent 52:7–19
Paula D, Gomes Moura CC (2006) Smear layer: a brief review of a general concept. part i charachteristics, compounds, structure, bacteria and sealing. Rev Fac Odontol - UPF 11(2):96–99
Violich DR, Chandler NP (2010) The smear layer in endodontics—a review. Int Endod J 43(1):2–15
Mahajan V, Kamra A, Dahiwale S (2010) The effect of 17% EDTA and MTAD on smear layer removal and on erosion of root canal dentin when used as final rinse: an in vitro SEM study. J Int Clin Dent Res Org 2(3):113–118
Mader CL, Baumgartner JC, Peters ODD (1984) Scanning electron microscopic investigation of the smeared layer on root canal walls. J Endod 10(10):477–483
Ajwani P, Saini N (2010) The influence of the smear layer on dentinal tubule penetration depth by different root canal sealers—an in vitro SEM study. Endodontology 22(2):19–26
Prado M, Gusman H, Gomes BPFA, Simao RA (2010) Scanning electron microscopic investigation of the effectiveness of phosphoric acid in smear layer removal when compared with edta and citric acid. J Endod 37(2):255–258
Haapasalo M, Shen Y, Qian W, Gao Y (2009) Irrigation in endodontics. Dent Clin North Am 54(2):291–312
Clarkson RM, Moule AJ (1998) Sodium hypochlorite and its use as an endodontic irrigant. Aust Dent J 43(4):250–256
Spencer HR, Ike V, Brennan PA (2007) Review: the use of sodium hypochlorite in endodontics, potential complications and their management. Br Dent J 202(9):555–559
Siqueira JRJF, Machado AG, Silveira RM, Lopes HP, De Uzeda M (1997) Evaluation of the effectiveness of sodium hypochlorite used with three irrigation methods in the elimination of Enterococcus faecalis from the root canal, in vitro. Int Endod J 30:279–282
Baumgartner JC, Mader CL (1987) A scanning electron microscopic evaluation of four root canal irrigation regimens. J Endod 13(4):147–157
Goldman M, Goldman LB, Cavaleri R, Bogis J, Lin PS (1982) The efficacy of several endodontic irrigating solutions: a scanning electron microscopic study: part 2. J Endod 8(11):487–492
Grande NM, Plotino G, Falanga A, Pomponi M, Somma F (2006) Interaction between EDTA and sodium hypochlorite: a nuclear magnetic resonance analysis. J Endod 32(5):460–464
Peeters HH, Suardita K (2011) Efficacy of smear layer removal at the root tip by using ethylenediaminetetraacetic acid and erbium, chromium: Yttrium, scandium, gallium garnet laser. J Endod 37(11):1585–1589
Matsuoka E, Yonaga K, Kinoshita J, Kimura Y, Matsumoto K (2000) Morphological study on the capability of er:yag laser irradiation for root canal preparation. J Clin Laser Med Surg 18(4):215–219
Schoop U, Moritz A, Kluger W, Patruta S, Goharkhay K, Sperr W, Wernisch J, Gattringer R, Mrass P, Georgopoulos A (2002) The Er:YAG laser in endodontics: results of an in vitro study. Lasers Surg Med 30(5):360–364
Scaini F, Souza-Gabriel AE, Alfredo E, Da Cruz Filho AM (2008) Temperature variation on the external root surface during intracanal er:yag laser irradiation. Photomed Laser Surg 26(5):413–417
Olivi G, DiVito E (2012) Review: photoacoustic endodontics using pipsTM: experimental background and clinical protocol. J Laser Health Acad 2012(1):22–25
DiVito E, Peters OA, Olivi G (2012) Effectiveness of the erbium:yag laser and new design radial and stripped tips in removing the smear layer after root canal instrumentation. Lasers Med Sci 27(2):273–280
Sathe S, Hegde V, Jain P, Ghunawat D (2014) Effectiveness of Er: YAG (PIPS) and Nd: YAG activation on final irrigants for smear layer removal—SEM observation. J Dent Lasers 8(1):8–13
Olivi G, DiVito E, Peters O, Kaitsas V, Angiero F, Signore A, Benedicenti S (2014) Disinfection efficacy of photon-induced photoacoustic streaming on root canals infected with enterococcus faecalis: an ex vivo study. J Am Dent Assoc 145(8):843–848
Hulsmann M, Rummelin C, Schafers F (1997) Root canal cleanliness after preparation with different endodontic handpieces and hand instruments: a comparative SEM investigation. J Endod 23:301–306
Zhu X, Yin X, Chang JWW, Wang Y, Cheung GSP, Zhang C (2013) Comparison of the antibacterial effect and smear layer removal using photon-initiated photoacoustic streaming aided irrigation versus a conventional irrigation in single-rooted canals: an in vitro study. Photomed Laser Surg 31(8):371–377
Yang SH, Jaw SY, Yeh KC (2009) Single cavitation bubble generation and observation of the bubble collapse flow induced by a pressure wave. Exp Fluids 47(2):343–355
Gopikrishna V, Ashok P, Kumar AP, Narayanan LL (2014) Influence of temperature and concentration on the dynamic viscosity of sodium hypochlorite in comparison with 17gluconate: an in vitro study. J Conserv Dent 17(1):57–60
Barbier C, Chahine G (2009) Experimental study of the effects of viscosity and viscoelasticity on a line vortex cavitation. In: CAV2009 – 7th International Symposium on Cavitation, 16-20 August 2009, Ann Arbor, MI
Acknowledgments
The authors would like to thank Dr. Melanie Joseph for providing the Er:YAG Fotona laser device to complete this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nasher, R., Franzen, R. & Gutknecht, N. The effectiveness of the Erbium:Yttrium aluminum garnet PIPS technique in comparison to different chemical solutions in removing the endodontic smear layer—an in vitro profilometric study. Lasers Med Sci 31, 1871–1882 (2016). https://doi.org/10.1007/s10103-016-2063-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-2063-z