Abstract
Effective decontamination of biofilm and bacterial toxins from the surface of dental implants is a yet unresolved issue. This in vitro study aims at providing the experimental basis for possible use of diode laser (λ 808 nm) in the treatment of peri-implantitis. Staphylococcus aureus biofilm was grown for 48 h on titanium discs with porous surface corresponding to the bone-implant interface and then irradiated with a diode laser (λ 808 nm) in noncontact mode with airflow cooling for 1 min using a Ø 600-μm fiber. Setting parameters were 2 W (400 J/cm2) for continuous wave mode; 22 μJ, 20 kHz, 7 μs (88 J/cm2) for pulsed wave mode. Bactericidal effect was evaluated using fluorescence microscopy and counting the residual colony-forming units. Biofilm and titanium surface morphology were analyzed by scanning electron microscopy (SEM). In parallel experiments, the titanium discs were coated with Escherichia coli lipopolysaccharide (LPS), laser-irradiated and seeded with RAW 264.7 macrophages to quantify LPS-driven inflammatory cell activation by measuring the enhanced generation of nitric oxide (NO). Diode laser irradiation in both continuous and pulsed modes induced a statistically significant reduction of viable bacteria and nitrite levels. These results indicate that in addition to its bactericidal effect laser irradiation can also inhibit LPS-induced macrophage activation and thus blunt the inflammatory response. The λ 808-nm diode laser emerges as a valuable tool for decontamination/detoxification of the titanium implant surface and may be used in the treatment of peri-implantitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peri-implantitis is one of the most severe complications of implant therapy: It is defined as an inflammatory reaction associated with loss of supporting bone around an implant in function [1]. According to the Consensus Report of the Sixth European Workshop in Periodontology (2008), implant therapy is associated with up to 80 % incidence of mucositis and 28–56 % incidence of peri-implantitis [2].
A substantial body of literature has shown that bacterial pathogens and their pro-inflammatory products, such as the bacterial endotoxin lipopolysaccharide (LPS), play a central role in pathogenesis of peri-implantitis and failing implants [3–5]. Many studies have shown that Staphylococcus aureus is one of the most common bacterial pathogens involved in the early failure of dental implants [6–9] as it efficiently attaches onto different titanium surfaces [10]. LPS is also regarded as a key factor implicated in peri-implantitis, since it has been reported to adhere tenaciously to dental titanium implants and identified as a major factor of bacterial origin influencing bone resorption [11–13].
On these premises, an effective treatment of peri-implantitis should reach a dual goal: (i) remove bacteria and (ii) inactivate LPS present at the titanium interface with the host tissues [14]. Several methods of implant decontamination have been proposed for the treatment of peri-implantitis, including air-powered abrasive treatments, citric acid, mechanical cleaning with metal and plastic curettes or ultrasonic scalers [15–18], in combination with local and systemic antibiotic therapy [19, 20]. However, none of these methods gave satisfactory results in terms of eradication of bacteria from the contaminated implant surfaces [21, 22]. Paradoxically, a further release of LPS from dead bacteria might even enhance the inflammatory reaction around the dental implant. In the last decade, the positive effects of laser irradiation in cleaning and decontaminating the implant surfaces have been widely reported [23–26]. Various laser systems are in use in dental practice for different purposes: (i) Er:YAG laser was mainly used to ablate dental and bone hard tissues and to remove bacterial deposits and calculus from the dental and implant surfaces [23, 26]; (ii) Nd:YAG laser at low-power with high frequency irradiation was recognized to have bactericidal effects [27]; and (iii) diode laser was shown to be effective in the decontamination of implant surfaces with no side effects on the surrounding tissues [28]. In a previous study, we showed that irradiation with Nd:YAG laser, in addition to inducing bacterial implant decontamination, was capable of attenuating the LPS-induced inflammatory response [29]. Moreover, a recent study of our group has demonstrated that different titanium surfaces can greatly influence the temperature range measurable on the titanium target in response to laser diode (λ 808 nm) irradiation, highlighting the need to identify specific setting parameters to maintain the temperature below the tissue damage threshold, i.e. 50 °C [30]. Despite the numerous reports in the literature on the clinical use of lasers in implantology [31–34], little is known about the mechanisms by which lasers exert their effects on dental implant surfaces. Moreover, considering that the physical characteristics of implants vary greatly among different producers, there is no consensus on a standard laser-aided treatment for peri-implantitis.
In the present in vitro study, we investigated the effects of diode laser (λ 808 nm) in noncontact mode and in continuous or pulsed wave emission on both S. aureus biofilm and LPS adherent to titanium discs with rough titanium oxide coating reproducing the osseous interface of dental implants, in order to mimic the clinical conditions occurring at the implant/tissue interface.
Materials and methods
Materials
The experiments were performed on titanium discs, 6 mm in diameter, 2 mm thick, with the same surface finish as the osseous interface (TiUnite) of dental implants (Nobel Biocare Services AG, Zürich, Switzerland). The discs were ultrasonically cleaned in acetone, rinsed in distilled water, and autoclaved at 121 °C for 15 min.
Bacterial biofilm formation
Staphylococcus aureus strain ATCC 25923 was used for biofilm experiments. In a typical experiment, a suspension of about 3 × 105 CFU/ml was prepared in Tryptic Soy Broth (TSB, Oxoid, Milan, Italy) plus 0.5 % of d-glucose, in Tryptic Soy Agar (TSA, Oxoid). The bacterial suspension was dispensed in sterile 12-well polystyrene tissue culture plates (IWAKI microplate, Bibby Scientific Limited, Staffordshire, UK) (1.5 ml/well), and three discs were placed in each well. Biofilms were grown for 48 h at 35 °C (50 rpm in humid atmosphere), adding fresh medium (1.5 ml/well) after the first 24 h of incubation.
Laser treatment
Prior to laser treatment, planktonic or loosely adherent bacterial cells were removed by gently rinsing twice the discs with 1.5-ml sterile phosphate-buffered saline (PBS). After that, the samples of S. aureus biofilm grown on titanium discs were placed on sterile slides and irradiated. The treatment was carried out for 1 min with a diode laser emitting at λ 808 nm (Dental Laser System 4 × 4™, General Project Ltd., Montespertoli, Florence, Italy) in noncontact mode through a polyimide-coated silica fiber (Ø 600 μm) positioned perpendicular to the disc surface. The fiber tip was kept at a distance of about 2–5 mm from the disc surface. To minimize heating, the laser beam was continuously moved to draw adjacent horizontal lines over the whole disc surface at a speed of 2.5 mm/s, under airflow cooling. Respect to random movements on the surface, this procedure assures a systematic irradiation of the whole disc without untreated zones. The detailed laser specification and irradiation parameters are reported in Table 1. During the treatment, the temperature of the titanium surface was measured by a thermal probe included in the console of the laser and monitored by a thermal camera (Ti9, Fluke Corp., Everett, USA). The mean temperature was maintained below the 50 °C thermal damage threshold, as reported in preliminary experiments [30].
Viable cell count (CFU) after laser irradiation
Following the laser treatment, the discs were transferred in 1.5-ml sterile tubes (one disc/tube) containing 500 μl of TSB plus 0.1 % Tween 20 (Sigma-Aldrich, Milan, Italy) as recovery medium [35]. To allow biofilm disgregation, each tube was vortexed for 30 s and sonicated for 30 min at 30 KHz and 300 W (Elma Transsonic T 460, Singen, Germany). After the second cycle of 30-s vortexing, serial dilutions in sterile saline solution were prepared, and 100 μl of each dilution was plated onto TSA and incubated for 24 h at 35 °C for CFU count (limit of detection = 5.0 CFU/disc). The experiments were performed in triplicate. Controls consisted of biofilms processed in the same way, excluding laser irradiation.
Fluorescence analysis
Subsequent to the laser treatment, S. aureus biofilms formed on the titanium discs (n = 9) were stained with the fluorescent LIVE/DEAD BacLight™ (Invitrogen/Molecular Probes, Milan, Italy) solution for 2 min at 37 °C. The fluorescent kit contains a mixture of two dyes: SYTO 9 (green: excitation λ 488 nm, emission λ 500–540 nm) and propidium iodide (red: excitation λ 561 nm, emission λ 600–695 nm). SYTO 9 labels all bacteria, whereas propidium iodide enters only bacteria with damaged membranes. Thus, viable bacteria with intact cell membranes show green fluorescence, whereas dead bacteria with damaged membranes show red-yellow fluorescence. Labeled biofilms were washed twice with PBS, fixed with 0.5 % paraformaldehyde for 10 min and observed with a fluorescent microscope equipped with a digital camera (4000 B, Leica Microsystems, Milan, Italy). From each disc, three digital images (RGB color) from randomly selected microscopic fields were taken. Using ImageJ 1.42q software (http://rsb.info.nih.gov/ij), the green and red channels were split and the resulting two images were separately subjected to densitometry to evaluate the staining intensity of SYTO 9 and propidium iodide, corresponding to live and dead bacteria, respectively. Values were reported as means ± standard error of the mean (s.e.m.).
SEM
To evaluate the possible morphological changes of the titanium surface and the bacterial biofilm after laser treatment, biofilm-coated titanium discs (n = 9) were subjected (n = 6) or not (n = 3) to laser irradiation. The negative control was an uncoated, un-irradiated disc. Upon sputter-coating with platinum (10 nm), the discs were examined with a Supra 40VP scanning electron microscope (Zeiss, Oberkochen, Germany) by a trained observer blinded to the experimental conditions.
Experiments on LPS-coated titanium discs
In these experiments, we applied minor changes to the previously described method to study the effects of different laser treatments on LPS-induced inflammatory activation [5, 29]. Briefly, mouse RAW 264.7 macrophages obtained from the European Collection of Cell Cultures (ECACC, Salisbury, UK) were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10 % fetal bovine serum (FBS, Gibco Invitrogen, Milan, Italy), 100 U/ml penicillin-streptomycin, 200 mM l-glutamine (Sigma-Aldrich, Milan, Italy) and 4.5 g/l glucose, and cultured in 5 % CO2 atmosphere at 37 °C. In each set of experiments, macrophages were seeded on titanium discs subjected to different pretreatments:
-
1.
One disc was washed in sterile PBS, dried at 37 °C, and used as negative control.
-
2.
Two discs were coated with LPS via immersion in a solution of E. coli LPS (Sigma-Aldrich, 50 μg/ml in PBS) for 15 min and dried at 37 °C for 1 h (positive control).
-
3.
Six discs were LPS-coated, dried at 37 °C, and subjected to irradiation treatments detailed in Table 1.
The discs were then individually placed into the wells of a 96-well plate and RAW 264.7 macrophages (8 × 104 cells) were seeded on the discs and cultured in 250 μl of DMEM without phenol red, supplemented with 20 % of FBS, for 24 h. RAW 264.7 macrophages cultured alone in the same conditions without discs were used as basal controls. The generation of nitric oxide (NO), a typical reaction product of activated macrophages [36], was evaluated by measurement of nitrites, stable end-products of NO metabolism, in the conditioned medium of RAW 264.7 cells. The amounts of nitrites were determined spectrophotometrically by the Griess reaction, as previously described [37], and the optical density of the reaction product was measured at λ 546 nm with a Bio-Rad 550 microplate reader (Bio-Rad, Milan, Italy). Nitrite concentrations in the conditioned media were determined by comparison with standard concentrations of NaNO2 dissolved in culture medium and expressed as nMol/ml. To assess RAW 264.7 cell viability upon the different treatments, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Sigma-Aldrich) was used according to the manufacturer’s instructions. After 24-h incubation and removal of the conditioned medium, MTT stock solution was added to each well and incubated for 4 h at 37 °C. Dimethyl sulfoxide was added to each well to dissolve the formazan crystals. The plate was gently shaken for 10 min and read at λ 550 nm with the microplate reader (Bio-Rad). Optical density (OD) was assumed as indicator of cell viability. The MTT data were used to normalize the production of nitrites by the amount of viable RAW 264.7 cells.
Statistical analysis
The values are expressed as mean ± standard error of the mean (s.e.m.) of triplicate experiments. Statistical analysis of differences between the experimental groups was performed using one-way ANOVA followed by Newman-Keuls multiple comparison test, and results were considered statistically significant if p ≤ 0.05. Calculations were performed using the GraphPad Prism 4.0 statistical software (GraphPad, San Diego, CA, USA).
Results
Effect of laser treatment on S. aureus biofilm viability
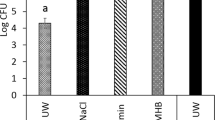
Adherent S. aureus biofilm onto TiUnite titanium discs (n = 27) was 7.3 ± 0.1 log CFU/disc (Fig. 1). The treatment with diode laser in both pulsed and continuous mode induced a statistically significant reduction of viable cell as compared with the untreated biofilm (1.5 and 2.2 log CFU/disc reduction, respectively: p < 0.01) (Fig. 1). In particular, the irradiation in continuous mode appeared to have a higher efficacy in terms of viability reduction, even if the comparison between the two treatments did not reach statistical significance (p > 0.05).
Effect of the different laser treatments on decontamination of the titanium surface coated with S. aureus biofilm evaluated by residual bacterial growth. The columns represent the number of postirradiation S. aureus colonies grown upon 24-h incubation in selective medium. Values are means ± s.e.m. *p < 0.05; **p < 0.01 vs. controls
Fluorescence analysis of S. aureus biofilm viability
The fluorescence analysis with BacLight staining method showed that, in comparison with the nonirradiated control discs, the diode laser used in pulsed or in continuous mode on S. aureus biofilm adherent to titanium discs caused a well-appreciable increase in the amount of red-stained dead bacteria in respect with green-stained viable ones (Fig. 2a). This visual finding was confirmed by densitometric analysis, which showed a remarkable, statistically significant increase (p < 0.01) in the percentage of dead over viable bacteria (Fig. 2b).
Effect of the different laser treatments on decontamination of the titanium surface coated with S. aureus biofilm evaluated by BacLight fluorescent vital stain. a Untreated control shows mostly viable bacteria (green). Upon irradiation in pulsed (b) or continuous (c) wave modes, most bacteria appear dead (red). Bars = 100 μm. In d, the corresponding densitometric analysis is shown. Values are means ± s.e.m. **p < 0.01 vs. controls
SEM examination of S. aureus biofilm morphology
The surface of the titanium discs, analyzed by scanning electron microscope, revealed the presence of a titanium oxide layer with numerous micro-cavities, designed to favor colonization by cells of the peri-implant tissues and hence to increase osteoconductivity (Fig. 3a). Analysis of the titanium discs with 48-h bacterial coating (Fig. 3b) revealed the presence of an adherent layer of closely packed bacteria with the typical features of a biofilm, which partially filled the cavities present on the titanium surface. The laser treatment both in continuous (Fig. 3c) and pulsed (Fig. 3d) wave mode caused a sharp reduction of the amount of adherent bacteria. No clear difference was evident between the continuous and pulsed wave mode of irradiation.
Representative scanning electron micrographs of uncoated (a) and biofilm-coated titanium discs from b untreated control, showing a dense bacterial layer masking the roughness of the titanium oxide surface, and c pulsed and d continuous wave laser irradiation, showing an almost complete removal of the biofilm coating and few residual bacteria. Bars = 10 μm
Effect of laser treatment on LPS-induced macrophage activation
Endogenous generation of NO/nitrites by activated RAW 264.7 macrophages was determined to investigate if the λ 808-nm diode laser was capable of inactivating the LPS adherent to titanium discs, thereby inhibiting macrophage activation (Fig. 4); the diode laser used in both continuous and pulsed wave mode caused a statistically significant decrease in nitrite production in comparison with the nonirradiated LPS-coated discs used as positive controls (p < 0.001).
Effect of the different laser treatments on detoxification of the titanium surface coated with bacterial LPS evaluated by RAW 264.7 macrophage activation. The columns represent the amount of nitrites, an index of LPS-induced iNOS activation and nitric oxide generation, in the cells’ conditioned medium, normalized by the amount of viable cells. Control indicates cell grown in standard conditions, Ti indicates cells grown on uncoated titanium discs, and Ti + LPS indicates cells grown on LPS-coated titanium discs. Values are means ± s.e.m. °°°p < 0.001 vs. the control; ***p < 0.001 vs. Ti + LPS
Discussion
Decontamination of bacteria and bacterial toxins from implant surfaces is a prerequisite to arrest the progression of peri-implantitis and for a successful treatment of failing implants. It has been shown that S. aureus, a Gram-positive bacterium, has an increased affinity to titanium substrates, being consistently found adherent to the surface of implants, particularly those with a rough surface finish [10, 38, 39]. S. aureus has been frequently isolated from failing dental implant sites and associated with poor clinical outcome [40]. LPS, the Gram-negative endotoxin, has received considerable attention because of its crucial role as a mediator of peri-implant inflammation [41]. Therefore, for a successful treatment of peri-implant disease, it is mandatory to eliminate bacteria and, in the meantime, remove all bacterial by-products and toxins which can hinder tissue regrowth on implant surfaces and osteo-integration.
The present findings offer evidence that the λ 808-nm diode laser can achieve an efficient reduction of S. aureus biofilm and detoxification of LPS on the tested titanium surface. In particular, 1-min irradiation of the biofilm-coated discs was able to reduce the number of colony forming units of 99 and 94 % for continuous and pulsed wave mode, respectively. Scanning electron microscopy (SEM) analysis of the titanium discs revealed that, with the adopted irradiation procedure, the λ 808-nm diode laser did not alter the original features of the titanium oxide surface layer, characterized by micro-roughness and micro-pores. However, despite the remarkable reduction of microbial load upon laser treatment, the present findings showed that a complete decontamination and cleaning of the titanium discs were not achieved, as can be argued by the detection of residual organic remnants of the bacterial biofilm on the targeted surfaces by SEM and fluorescence analyses. On the other hand, our data indicated that both continuous and pulsed wave laser irradiation were capable of inactivating the titanium-adherent LPS, as judged by failed induction of inflammatory activation of RAW 264.7 macrophages used as a probe to measure LPS bioactivity.
A decontaminating potential of λ 808-nm diode laser had been previously shown in vitro [42] as well as on intraoral biofilms grown on rough titanium surfaces [43]. The present findings confirm and extend this notion, although our results are difficult to compare with the previous ones because of substantially different irradiation parameters, treatment modalities and physical-chemical characteristics of the targeted titanium surfaces. It has been reported that Gram-negative species show immediate structural damage after treatment with λ 808-nm diode laser, whereas Gram-positive micro-organisms require repeated irradiations [44]. Our study provides experimental evidence that, with proper irradiation modes and parameters, the λ 808-nm diode laser is endowed with a reasonable activity against the S. aureus biofilm and pro-inflammatory bacterial by-products, such as LPS, adherent to the titanium surface. This effect was exerted in the absence of alterations of titanium surface morphology and below the threshold for thermal tissue damage. This decrease in bacterial load can reestablish a more favorable balance between peri-implant microbiota and host defenses, allowing a stable clinical improvement over time [45].
A limitation of the present study is the fact that the treatments were performed on new, clean discs in simplified and highly standardized laboratory conditions, which are substantially different from a real clinic situation of laser-aided implant surgery, where a dental implant has a markedly different mass and surface geometry, is interlocked with peri-implant bone, and can be covered by subgingival plaque, blood, inflammatory cells, proteins, and calculus. Another limitation comes from the fact that we did not compare this laser treatment with other decontaminating procedures. Therefore, caution is required when extrapolating the present findings to clinical practice. In addition, further studies are necessary to evaluate the effects of λ 808-nm diode laser on a broader spectrum of oral micro-organisms and for longer irradiation times.
In conclusion, our data suggest that the λ 808-nm diode laser can be considered a valuable tool for bacteria/LPS reduction adherent to the titanium oxide implant surface, which in turn may increase the odds of successful control of peri-implant disease.
References
Zitzmann NU, Berglundh T (2008) Definition and prevalence of peri-implant diseases. J Clin Periodontol 35:286–291
Lindhe J, Meyle J (2008) Group D of european workshop on periodontology. “Peri-implant diseases: consensus report of the sixth european workshop on periodontology”. J Clin Periodontol 35:282–285
Nouneh RA, Wataha JC, Hanes PJ, Lockwood PE (2001) Effect of lipopolysaccharide contamination on the attachment of osteoblast-like cells to titanium and titanium alloy in vitro. J Oral Implantol 27:174–179
Greenfield EM, Bi Y, Ragab AA, Goldberg VM, Nalepka JL, Seabold JM (2005) Does endotoxin contribute to aseptic loosening of orthopedic implants? J Biomed Mater Res B Appl Biomater 72:179–185
Giannelli M, Pini A, Formigli L, Bani D (2011) Comparative in vitro study among the effects of different laser and LED irradiation protocols and conventional chlorhexidine treatment for deactivation of bacterial lipopolysaccharide adherent to titanium surface. Photomed Laser Surg 29:573–580
Shrestha B, Theerathavaj ML, Thaweboon S, Thaweboon B (2012) In vitro antimicrobial effects of grape seed extract on peri-implantitis microflora in craniofacial implants. Asian Pac J Trop Biomed 2:822–825
Cortizo AM, Fernández Lorenzo de Mele MA (2012) Chlorhexidine delivery system from titanium/polybenzyl acrylate coating: evaluation of cytotoxicity and early bacterial adhesion. J Dent 40:329–337
Zhao B, van der Mei HC, Subbiahdoss G, de Vries J, Rustema-Abbing M, Kuijer R, Busscher HJ, Ren Y (2014) Soft tissue integration versus early biofilm formation on different dental implant materials. Dent Mater 30:716–727
Thurnheer T, Belibasakis GN (2015) Incorporation of staphylococci into titanium-grown biofilms: an in vitro "submucosal" biofilm model for peri-implantitis. Clin Oral Implants Res. doi:10.1111/clr.12715
Harris LG, Richards RG (2004) Staphylococcus aureus adhesion to different treated titanium surfaces. J Mater Sci Mater Med 15:311–314
Nelson SK, Knoernschild KL, Robinson FG, Schuster GS (1997) Lipopolysaccharide affinity for titanium implant biomaterials. J Prosthet Dent 77:76–82
Dixon DR, Darveau RP (2005) Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid A structure. J Dent Res 84:584–595
Ozaki Y, Ukai T, Yamaguchi M et al (2009) Locally administered T cells from mice immunized with lipopolysaccharide (LPS) accelerate LPS-induced bone resorption. Bone 44:1169–1176
Renvert S, Samuelsson E, Lindahl C, Persson GR (2009) Mechanical non-surgical treatment of peri-implantitis: a double-blind randomized longitudinal clinical study. I: clinical results. J Clin Periodontol 36:604–609
Fox SC, Moriarty JD, Kusy RP (1990) The effects of scaling a titanium implant surface with metal and plastic instruments: an in vitro study. J Periodontol 61:485–490
Suarez F, Monje A, Galindo-Moreno P, Wang HL (2013) Implant surface detoxification: a comprehensive review. Implant Dent 22:465–473
Mettraux GR, Sculean A, Bürgin WB, Salvi GE (2015) Two-year clinical outcomes following non-surgical mechanical therapy of peri-implantitis with adjunctive diode laser application. Clin Oral Implants Res. doi:10.1111/clr.12689
Sahrmann P, Ronay V, Hofer D, Attin T, Jung RE, Schmidlin PR (2015) In vitro cleaning potential of three different implant debridement methods. Clin Oral Implants Res 26:314–319
Dörtbudak O, Haas R, Bernhart T, Mailath-Pokorny G (2001) Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis. Clin Oral Implants Res 12:104–108
Javed F, Alghamdi AS, Ahmed A, Mikami T, Ahmed HB, Tenenbaum HC (2013) Clinical efficacy of antibiotics in the treatment of peri-implantitis. Int Dent J 63:169–176
Mouhyi J, Sennerby L, Pireaux JJ, Dourov N, Nammour S, Van Reck J (1998) An XPS and SEM evaluation of six chemical and physical techniques for cleaning of contaminated titanium implants. Clin Oral Implants Res 9:185–194
Mouhyi J, Sennerby L, Van Reck J (2000) The soft tissue response to contaminated and cleaned titanium surfaces using CO2 laser, citric acid and hydrogen peroxide. An experimental study in the rat abdominal wall. Clin Oral Implants Res 11:93–98
Kreisler M, Götz H, Duschner H (2002) Effect of Nd:YAG, Ho:YAG, Er:YAG, CO2, and GaAIAs laser irradiation on surface properties of endosseous dental implants. Int J Oral Maxillofac Implants 17:202–211
Sculean A, Schwarz F, Becker J (2005) Anti-infective therapy with an Er:YAG laser: influence on peri-implant healing. Expert Rev Med Devices 2:267–276
Roncati M, Lucchese A, Carinci F (2013) Non-surgical treatment of peri-implantitis with the adjunctive use of an 810-nm diode laser. J Indian Soc Periodontol 17:812–815
Aoki A, Mizutani K, Schwarz F, Sculean A, Yukna RA, Takasaki AA, Romanos GE, Taniguchi Y, Sasaki KM, Zeredo JL, Koshy G, Coluzzi DJ, White JM, Abiko Y, Ishikawa I, Izumi Y (2015) Periodontal and peri-implant wound healing following laser therapy. Periodontol 68:217–269
Giannini R, Vassalli M, Chellini F, Polidori L, Dei R, Giannelli M (2006) Neodymium:yttrium aluminum garnet laser irradiation with low pulse energy: a potential tool for the treatment of peri-implant disease. Clin Oral Implants Res 17:638–643
Romanos GE, Everts H, Nentwig GH (2000) Effects of diode and Nd: YAG laser irradiation on titanium discs: a scanning electron microscope examination. J Periodontol 71:810–815
Giannelli M, Bani D, Tani A, Pini A, Margheri M, Zecchi-Orlandini S, Tonelli P, Formigli L (2009) In vitro evaluation of the effects of low-intensity Nd:YAG laser irradiation on the inflammatory reaction elicited by bacterial lipopolysaccharide adherent to titanium dental implants. J Periodontol 80:977–984
Giannelli M, Lasagni M, Bani D (2015) Thermal effects of λ = 808 nm GaAlAs diode laser irradiation on different titanium surfaces. Lasers Med Sci 30:2341–2352
Romanos GE, Gutknecht N, Dieter S, Schwarz F, Crespi R, Sculean A (2009) Laser wavelengths and oral implantology. Lasers Med Sci 24:961–970
Subramani K, Wismeijer D (2012) Decontamination of titanium implant surface and re-osseointegration to treat peri-implantitis: a literature review. Int J Oral Maxillofac Implants 27:1043–1054
Romanos GE, Gupta B, Yunker M, Romanos EB, Malmstrom H (2013) Lasers use in dental implantology. Implant Dent 22:282–288
Yan M, Liu M, Wang M, Yin F, Xia H (2015) The effects of Er:YAG on the treatment of peri-implantitis: a meta-analysis of randomized controlled trials. Lasers Med Sci 30:1843–1853
Harrison JJ, Stremick CA, Turner RJ, Allan ND, Olson ME, Ceri H (2010) Microtiter susceptibility testing of microbes growing on peg lids: a miniaturized biofilm model for high-throughput screening. Nat Protoc 5:1236–1254
Moncada S, Palmer RM, Higgs EA (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43:109–142
Masini E, Nistri S, Vannacci A, Bani Sacchi T, Novelli A, Bani D (2004) Relaxin inhibits the activation of human neutrophils: involvement of the nitric oxide pathway. Endocrinology 145:1106–1112
Izquierdo-Barba I, García-Martín JM, Álvarez R, Palmero A, Esteban J, Pérez-Jorge C, Arcos D, Vallet-Regí M (2015) Nanocolumnar coatings with selective behavior towards osteoblast and Staphylococcus aureus proliferation. Acta Biomater 15:20–28
Aguayo S, Donos N, Spratt D, Bozec L (2015) Nanoadhesion of staphylococcus aureus onto titanium implant surfaces. J Dent Res 94:1078–1084
Lee A, Wang HL (2010) Biofilm related to dental implants. Implant Dent 19:387–393
Barão VA, Mathew MT, Assunção WG, Yuan JC, Wimmer MA, Sukotjo C (2011) The role of lipopolysaccharide on the electrochemical behavior of titanium. J Dent Res 90:613–618
Kreisler M, Kohnen W, Marinello C, Schoof J, Langnau E, Jansen B, d’Hoedt B (2003) Antimicrobial efficacy of semiconductor laser irradiation on implant surfaces. Int J Oral Maxillofac Implants 18:706–711
Sennhenn-Kirchner S, Klaue S, Wolff N, Mergeryan H, Borg von Zepelin M, Jacobs HG (2007) Decontamination of rough titanium surfaces with diode lasers: microbiological findings on in vivo grown biofilms. Clin Oral Implants Res 18:126–132
Schoop U, Kluger W, Moritz A, Nedjelik N, Georgopoulos A, Sperr W (2004) Bactericidal effect of different laser systems in the deep layers of dentin. Lasers Surg Med 35:111–116
Mombelli A (2002) Microbiology and antimicrobial therapy of peri-implantitis. Periodontol 28:177–189
Acknowledgments
The authors are grateful to Dr. Peter Schüpbach, from Schüpbach Ltd. (Thalwil, Switzerland) for conducting the SEM investigation and Dr. Waldemar Hoffmann, Nobel Biocare Services AG (Zürich, Switzerland) for reviewing the manuscript.
This study was supported in part by General Project Ltd. (Montespertoli, Florence, Italy).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Giannelli, M., Landini, G., Materassi, F. et al. The effects of diode laser on Staphylococcus aureus biofilm and Escherichia coli lipopolysaccharide adherent to titanium oxide surface of dental implants. An in vitro study. Lasers Med Sci 31, 1613–1619 (2016). https://doi.org/10.1007/s10103-016-2025-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-2025-5