Abstract
In some well-established laser applications where large spot sizes are used, an array of high-intensity light emitting diodes (LED) emitting at similar wavelength could potentially replace the laser. This situation applies for the photodynamic bleaching of stains in teeth. This study compared the relative efficacy of an array of visible green LED (535 nm ± 15 nm) with a KTP laser in photodynamic bleaching of tetracycline-stained dentine in human tooth roots. After establishing consistent staining in 96 roots using a validated method, the roots were sectioned into 2–3-mm thick horizontal slices that were treated with gels containing rhodamine B (Smartbleach® or Smartbleach® 3LT). Colour changes were tracked up to 1 month after treatment. While both systems were effective in bleaching the tetracycline-stained dentine, KTP laser activation gave greater bleaching efficacy than LED activation, enhancing the action of the gel. Use of the KTP laser would be preferable over an LED system when confronted with tetracycline staining. Use of this photodynamic bleaching method offers valuable means to reduce the severity of tetracycline staining.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For effects that require intense light in a large spot size, arrays of superluminous light emitting diodes (LED) may potentially replace lasers of similar wavelength. In dentistry, one such application is the bleaching of internal stains within tooth structure using bleaching gels which are activated by intense light, through photothermal, photochemical, photocatalytic or photodynamic processes. There is a growing interest in the use of intense visible light sources for enhancing the action of hydrogen peroxide-based bleaching gels, which can be applied to the tooth surfaces during a dental appointment.
Of the stains which occur in tooth structure, one of the most difficult groups to manage is that caused by tetracycline antibiotics, which readily bind to the dentine of teeth after systemic administration of these antibiotics during tooth formation [1], or when tetracycline-containing endodontic medicaments or irrigants are used in the root canal [2–6]. Traditionally, these stains could not be treated easily by conventional in-office or at-home bleaching treatments, necessitating the use of crowns and veneers to cover the affected brown and grey tooth structure [7]. Recently, photodynamic bleaching in the dental office has been viewed as an ideal treatment, being minimally invasive and relatively time efficient. The preferred approach uses the KTP laser to photooxidise the various coloured tetracycline compounds present directly, in addition to activating the sensitizer rhodamine B through photodynamic mechanisms to enhance the breakdown of coloured organic molecules even further [8, 9]. This allows greater effects to be achieved in a single dental visit than would be expected from several months of wearing bleaching trays [10–12].
Until recently, photodynamic bleaching employed the KTP laser [9, 13, 14], providing useful benefits for staining due to dental sclerosis from the normal aging process, without significant adverse effects on the oral soft tissues, dental pulp or tooth structure [15, 16]. With the advent of intense LED light sources, the question arises as to what extent could dental bleaching treatments use green LED arrays to substitute for the KTP laser. Already one manufacturer (Smartbleach International, Herzele, Belgium) has released a 535-nm green LED illuminator for photodynamic bleaching treatments. Given that past literature on using LEDs in dental bleaching has focussed on wavelengths other than in the visible green region, the comparability of these green LED arrays and the KTP laser has not been explored in detail. As both tetracycline-calcium chelate compounds and rhodamine absorb visible green light in the 525–540-nm range, one would anticipate that both light sources could be of value [9, 15, 17, 18]. Accordingly, the present study was undertaken to compare the effects of the two light sources on tetracycline-stained dentine. An established laboratory model of tetracycline staining of human tooth roots was used to provide a consistent starting point from which to measure the effects of the lights and bleaching gels alone and in combination.
Materials and methods
The intense light sources used were a frequency doubled Nd:YAG KTP laser (532 nm) (Smartlite, Deka, Florence, Italy) and the 3LT LED unit (Smartbleach International, Herzele, Belgium). The KTP laser had a maximum power output of 3.5 W in continuous wave mode but was used at a measured power output of 1.0 W (measured using a laser power metre) delivered through a 200-μm fibre, in line with the manufacturer’s recommendations. The 3LT has 75 LEDs arranged into three adjacent arrays of 25 LEDs each. The peak wavelength for the visible green emission from the LED array was 535 nm and the spectral bandwidth was 15 nm. The nominal optical output power of the 3LT unit as measured with a laser power metre was 3.5 W. The bleaching gels used were both based on rhodamine gel and were from the same manufacturer (Smartbleach International). The major differences between the two bleaching gels were that the gel intended for use with the KTP laser is prepared from a powder (containing the rhodamine) which is hand mixed with a solution of hydrogen peroxide and gives rapid release of hydrogen peroxide within a short time frame. The 3LT gel is delivered in a two-barrel syringe with automixing tips to combine the two liquid components in a preset ratio. This gel contains stabilisers to give gradual release of the hydrogen peroxide, so that only one gel application is needed for a treatment of 30 min versus three applications of 10 min each for the gel used with the laser. With both systems, the cumulative gel exposure time is 30 min.
A total of 160 human permanent tooth root slices were prepared for the experiment. Institutional ethics approval was gained for the collection of extracted teeth. The laboratory model for achieving consistent tetracycline staining of dentine has previously been described in detail [3]. In brief, all teeth had their crowns removed and then underwent standardised root canal treatment utilising rotary nickel titanium files, ProTaper Universal (Dentsply, Tulsa, OK, USA), up to size F3, extending 1–2 mm beyond the apex. Canal patency was verified using ISO #20/taper 0.04 hand files (Maillefer Colorinox K file; Dentsply, Tulsa, OK, USA). Irrigation using 4 % sodium hypochlorite (NaOCl) (Endosure Hypochlor 4 % Forte H4; Dentalife, Croydon, Australia) and 15 % ethylenediaminetetraacetic acid with cetrimide (EDTA/C) (EDTA/C E15; Dentalife, Croydon, Australia) delivered using a 25-gauge needle tip was undertaken throughout canal preparation to ensure absence of the smear layer. Prepared canals were then dried before a tetracycline (demeclocycline) paste (Ledermix®, Lederle Laboratories, Wolfratshausen, Germany) was placed into the canal with a spiral filler. The paste was left for 12 weeks to allow diffusion through dentine, with the roots stored in the dark at 37 °C in a fully humidified atmosphere. Subsequently, the Ledermix® paste was removed by thorough NaOCl and EDTA irrigation. A final rinse with distilled water was performed.
The stained roots were sectioned into 2–3-mm thick horizontal slices, from the crown down, using a fine diamond saw with copious distilled water irrigation. These thin slices were then divided in half using a smaller hand-held diamond disc. A bur mark was placed to aid orientation for achieving consistent photographic alignment. Slices of the same root were distributed randomly into all experimental groups. After allocation into groups, the slices were immersed in orthophosphoric acid (470 g/L) for 1 min followed by copious rinsing with distilled water, to remove the smear layer from the diamond saw cutting. The slices were then photographed and the images were used to match the intensity of staining, ensuring a consistent baseline.
There were 20 half slices in each of the eight experimental groups: control (1), 3LT light alone (2), Smartbleach® gel alone (3), 3LT light and Smartbleach® gel (4), KTP laser alone (5), KTP laser and Smartbleach® gel (6), 3LT light and 3LT gel (7) and 3LT gel alone (8). These slices were stored and immersed in distilled water in separate wells of a 96-well enzyme-linked immunosorbent assay (ELISA) plate (Optiplate™ 96, PerkinElmer, Waltham, MA, USA), with location mapping diagrams used for tracking of individual slices. Each ELISA plate was stored in a moist airtight container.
In the groups that had only light exposure with no gel, the slices were left immersed in distilled water during treatment. In the groups with bleaching gel, the slices were immersed fully in the gel. Each experimental group received treatment for 30 min. In the 3LT gel experiments, the gel was not replaced during the experiment. In the Smartbleach® gel experiments, the gel was replaced every 10 min, and thus, three times during each experimental run. For the 3LT light, all wells were irradiated simultaneously. With the KTP laser experiments, each well was irradiated individually for 3 cycles of 30 s, using 1 W in continuous wave mode. The KTP laser fibre was maintained at the same distance (12 mm) from each well of the ELISA plate, such that an individual well was completely illuminated, with a constant spot size of 6 mm. The fibre was kept stable during irradiation, with no movement. All irradiation procedures were performed by a single operator. The power density was 1.6 W/cm2 and the irradiance was 150 J/cm2. This resulted in comparable power density and irradiance between the two systems.
Each tooth slice was photographed from the coronal aspect with an identification tag using a digital camera (Coolpix 995, Nikon, Tokyo, Japan) attached to an Olympus BH-2 stereo dissecting microscope at a fixed final magnification of ×25. Consistent manual exposure settings (F3.3, 0.5 s exposure, infinity mode, no flash, fine image quality, 1600×1200 resolution) and sample illumination using a colour-corrected daylight LED lighting were employed, with colour calibration cards included in each image series. Images were taken at baseline, immediately following treatment, at 1 week and at 1 month following treatment. For analysis, GIMP® image processing software (v2.8.3; GNU Image Manipulation Program, http://www.gimp.org/) was used with the inbuilt histogram analysis function to determine mean red, green, blue and luminosity channel levels.
Analysis of colour change
The data analysis followed a repeated measures approach using the sequential images from each slice. InStat® software (GraphPad Software, La Jolla, CA, USA) was used to compare baseline data in each of the four channels (red, green, blue, luminosity) to immediate posttreatment values and those after the samples had been kept in water for a further 1 week or 1 month. Data sets from each group were assessed for normality using the Kolmogorov-Smirnov test, after which repeated measures analysis of variance was used, with post hoc Tukey-Kramer multiple comparison tests, examining the effects of treatment and time. The threshold for statistical significance was set at p < 0.05. The sample size of 20 per group was double the minimum number required to find a difference of 5 % in the channel data between treatment groups or time intervals, based on a formal power study conducted prior to the investigation.
Results
To confirm that the baseline level of staining in all eight experimental groups was comparable, the data for each of the four channels (red, green, blue, and luminosity) was compared between all groups. No significant differences were found, confirming the consistency of the groups at baseline and the effectiveness of the randomization process.
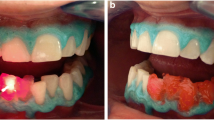
When data sets for all four channels were assessed, the greatest changes occurred in the blue channel, followed by the green channel, with luminosity and red channel data showing similar lesser changes. This corresponded to the samples appearing at the macroscopic level less brown and lighter in colour (Fig. 1).
Sequential images of the same root portion (half slice) treated with either the KTP laser with Smartbleach® gel (upper series) or the 3LT LED lamp and Smartbleach® 3LT gel (lower series). There is a clear improvement from the treatment, followed by slight relapse. From left to right, the images show the root slice at, baseline, immediately after treatment, at 1 week and after 1 month
The numerical results below summarise the changes that occurred following treatment through analysis of the blue channel data. In terms of the overall choice of bleaching system, both the KTP laser with Smartbleach® gel and the 3LT lamp with Smartbleach® 3LT gel gave a significant reduction in the intensity of stain compared with baseline (p < 0.0001 for both systems) and compared to the negative (water) control (p < 0.0001 for both systems). However, the KTP laser with Smartbleach® gel gave a significantly greater reduction in staining than the 3LT system, as seen by the larger mean change in blue pixel channel values (p < 0.0001) (Fig. 2).
Mean colour change in the blue channel at three different time periods after treatment (immediate, 1 week, 1 month), in each of the eight experimental groups (control, 3LT light only, Smartbleach® gel only, 3LT light and Smartbleach® gel, KTP laser only, KTP laser and Smartbleach® gel, 3LT light and 3LT gel, 3LT gel only). Bars show means and SD for N = 20 replicates
With both systems, the gel alone (without the activating light) provided a significant effect from both baseline (p < 0.0001 for both) and versus the water control (p = 0.0004 for Smartbleach® gel and p < 0.0001 for 3LT gel). When not activated by green light, both gels had a similar effect, and the difference between them was not significant (p = 0.6733). When activated by green light, the photodynamic activation efficacy of the KTP laser was much greater than that of the LED lamp (p < 0.0001). Combining the Smartbleach® gel and KTP laser was more effective than using the KTP laser alone without gel (p < 0.0001) or Smartbleach® gel alone (p < 0.0001).
The final combination scenario was to explore using the Smartbleach® bleaching gel, designed for use with the KTP laser, with the 3LT LED lamp instead. The combination of the Smartbleach® gel with the 3LT LED was less effective than combination of the Smartbleach® gel with KTP laser (p < 0.0001), or the combination of the 3LT gel with the 3LT LED lamp (p = 0.0356).
In terms of relapse, the samples treated with the KTP laser and Smartbleach® showed a small change over 1 month by visual examination (Fig. 1), with the greatest change again being in the blue channel (p < 0.001). The extent of change was less from 1 week to 1 month than from immediately postoperatively to 1 week (p < 0.05). In contrast, those treated with the LED lamp and 3LT gel showed most change between 1 week and 1 month (p < 0.01). Overall, more relapse was seen with the samples treated with the KTP laser than with the LED, but nevertheless they remained lighter than those treated with the LED system.
Lastly, the extent of change versus the original shade of the sample was examined. Again using the blue pixel channel data and plotting the baseline shade of individual slices against the percentage change in shade revealed that roots which were darker (more yellow, less blue) at baseline showed more positive shade change (Fig. 3). The linear correlation for this effect was stronger for the KTP laser plus Smartbleach® gel group (r = 0.835) than for the 3LT LED lamp plus 3LT gel group (r = 0.372).
Baseline blue channel mean versus percentage change in the blue channel, for the KTP laser plus Smartbleach® gel group and the 3LT lamp plus 3LT gel group. Individual data points show each of the 20 replicates in the two groups. The regression line shows the correlation between the data points, illustrating the association between more yellow (less blue) at baseline and an increased amount of change. The correlation (r) value for the KTP laser data set is 0.835, while the corresponding r value for the 3LT data set is 0.372
Discussion
This study provides insights into the comparative performance of photodynamic bleaching using laser or LED light sources, since several differences were found between the effects of the KTP laser and the 3LT LED lamp, despite their similarity in wavelength. The KTP laser gave a much greater green light-based photodynamic effect than the LED lamp, compared to the effects of gel alone. With both KTP laser and green LED approaches, the greatest changes in tetracycline stained dentine occurred in the blue channel, indicating a marked reduction in the yellow-brown shade.
Overall, the current results indicate that within the absorption spectrum of rhodamine, using an appropriate visible green wavelength will achieve photodynamic activation. The greater the power density, the more potent this activation becomes (up to a point), and thus the greater power density which can be achieved with the KTP laser compared to the LED lamp ensures its superior performance, despite a similar total irradiance, through better activation of the rhodamine photosensitizer. Moreover, the present results indicate that the two gels, when not exposed to green light, have similar effects. They both have similar levels of hydrogen peroxide, but their rates of release of radicals differ, being slower for the 3LT gel. This may explain in part why the 3LT gel did not perform as strongly as the Smartbleach® gel.
The experimental system used in the study provides an ideal contact interaction between the bleaching gel and the stained root dentine. While this would never be achievable in clinical practice, as enamel overlies the coronal dentine, the model nevertheless provides a valuable system for exploring the effects of various parameters under controlled conditions. By tracking individual slices from stained roots that are distributed evenly between treatment groups, a realistic assessment of the performance of the two systems can be made by comparing like with like. The observed visual reduction in tetracycline staining after the use of the KTP laser plus Smartbleach® gel aligns with more than a decade of positive clinical studies and case reports around its use for various forms of tooth discolouration, including confirmed tetracycline staining [8, 9, 13, 15]. Given that the KTP laser plus Smartbleach® gel was significantly more effective than the 3LT system, the former method would be preferred clinically for treating tetracycline staining.
A final interesting point from the present investigation is that a small degree of relapse was seen in all treatment groups. More relapse was seen in dentine that had undergone a greater level of change. Achieving a greater level of change in dentine with a darker initial shade is an aesthetically pleasing outcome for tetracycline stains, which are otherwise very difficult to bleach. Based on the present results, it would be prudent for patients to be warned of the likelihood of some relapse after treatment of intense tetracycline staining.
Conclusions
The KTP laser provides a greater effect than the LED array when used to activate a photodynamic bleaching gel system. The more intense light from the laser, coupled with readily available hydrogen peroxide from the gel, achieves useful benefits in reducing the intensity of tetracycline staining. Using such methods offers a promising minimally invasive treatment alternative for managing the challenges posed by tetracycline-stained tooth structure.
References
Sanchez AR, Rogers RS, Sheridan PJ (2004) Tetracycline and other tetracycline-derivative staining of the teeth and oral cavity. Int J Dermatol 43(10):709–715. doi:10.1111/j.1365-4632.2004.02108.x
Chen BK, George R, Walsh LJ (2012) Root discolouration following short-term application of steroid medicaments containing clindamycin, doxycycline or demeclocycline. Aust Endod J 38(3):124–128. doi:10.1111/aej.12000
Thomson AD, Athanassiadis B, Kahler B, Walsh L (2012) Tooth discolouration: staining effects of various sealers and medicaments. Aust Endod J 38(1):2–9. doi:10.1111/j.1747-4477.2011.00339.x
Kim ST, Abbott PV, McGinley P (2000) The effects of Ledermix paste on discolouration of mature teeth. Int Endod J 33(3):227–232
Ahmed HM, Abbott PV (2012) Discolouration potential of endodontic procedures and materials: a review. Int Endod J 45(10):883–897. doi:10.1111/j.1365-2591.2012.02071.x
Krastl G, Allgayer N, Lenherr P, Filippi A, Taneja P, Weiger R (2013) Tooth discoloration induced by endodontic materials: a literature review. Dent Traumatol 29(1):2–7. doi:10.1111/j.1600-9657.2012.01141.x
Abou-Rass M (1982) The elimination of tetracycline discoloration by intentional endodontics and internal bleaching. J Endod 8(3):101–106. doi:10.1016/S0099-2399(82)80243-4
Lin LC, Pitts DL, Burgess LW Jr (1988) An investigation into the feasibility of photobleaching tetracycline-stained teeth. J Endod 14(6):293–299. doi:10.1016/S0099-2399(88)80029-3
Kuzekanani M, Walsh LJ (2009) Quantitative analysis of KTP laser photodynamic bleaching of tetracycline-discolored teeth. Photomed Laser Surg 27(3):521–525. doi:10.1089/pho.2008.2332
Haywood VB, Leonard RH, Dickinson GL (1997) Efficacy of six months of nightguard vital bleaching of tetracycline-stained teeth. J Esthet Dent 9(1):13–19
Tsubura S (2010) Clinical evaluation of three months’ nightguard vital bleaching on tetracycline-stained teeth using Polanight 10% carbamide gel: 2-year follow-up study. Odontology 98(2):134–138. doi:10.1007/s10266-010-0130-7
Leonard RH Jr, Van Haywood B, Caplan DJ, Tart ND (2003) Nightguard vital bleaching of tetracycline-stained teeth: 90 months post treatment. J Esthet Restor Dent 15(3):142–152
Walsh LJ, Liu JY, Verheyen P (2004) Tooth discolouration and its treatment using KTP laser-assisted tooth whitening. J Oral Laser Appl 4:7–21
Luk K, Tam L, Hubert M (2004) Effect of light energy on peroxide tooth bleaching. J Am Dent Assoc 135(2):194–201
Goharkhay K, Schoop U, Wernisch J, Hartl S, De Moor R, Moritz A (2009) Frequency doubled neodymium:yttrium-aluminum-garnet and diode laser-activated power bleaching—pH, environmental scanning electron microscopy, and colorimetric in vitro evaluations. Lasers Med Sci 24(3):339–346. doi:10.1007/s10103-008-0567-x
Wetter NU, Barroso MC, Pelino JE (2004) Dental bleaching efficacy with diode laser and LED irradiation: an in vitro study. Lasers Surg Med 35(4):254–258. doi:10.1002/lsm.20103
Dominguez A, Garcia JA, Costela A, Gomez C (2011) Influence of the light source and bleaching gel on the efficacy of the tooth whitening process. Photomed Laser Surg 29(1):53–59. doi:10.1089/pho.2009.2751
Zhang C, Wang X, Kinoshita J, Zhao B, Toko T, Kimura Y, Matsumoto K (2007) Effects of KTP laser irradiation, diode laser, and LED on tooth bleaching: a comparative study. Photomed Laser Surg 25(2):91–95. doi:10.1089/pho.2006.2025
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bennett, Z.Y., Walsh, L.J. Efficacy of LED versus KTP laser activation of photodynamic bleaching of tetracycline-stained dentine. Lasers Med Sci 30, 1823–1828 (2015). https://doi.org/10.1007/s10103-014-1675-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-014-1675-4