Abstract
Laser and LED phototherapies accelerate tissue repair. Mast cells induce the proliferation of fibroblasts and the development of local fibrosis. Increased numbers of myofibroblasts and mast cells are frequently found together in a normal wound repair, suggesting that mediators produced by the mast cells could play a role in the regulation of myofibroblast differentiation and function. The aim of this study was to analyze the involvement of mast cells on the synthesis of collagen and their influence on myofibroblast differentiation in the late phase of tissue repair on wounds treated with LLLT (λ 660 nm, 10 J/cm2, 40 mW, 252 s) or LED (λ 630 ± 10 nm, 10 J/cm2, 115 mW, 87 s). A 1 × 1-cm surgical wound was created on the dorsum of 30 rats divided into three groups of ten animals each: control, laser, and LED. The animals of each group were irradiated and sacrificed 7 and 14 days after injury. The statistical analysis was performed using the Mann–Whitney and Spearman correlation tests. Laser light improved the collagen deposition rate along the time points (p = 0.22), but when compared to the control groups during the periods studied, the number of mast cells decreased significantly (p ≤ 0.05). With respect to myofibroblasts, the results showed a trend to their reduction. No statistical significances were observed for LED light according to the parameters used in this study. It is concluded that the mast cell and myofibroblast population might participate in the collagen formation of irradiated wounds particularly in relation to laser phototherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mast cells are derived from the bone marrow and play an important role in inflammation, immune response, and tissue repair and are relevant to the genesis of tissue because potent granule-stored mediators are related to fibroblast proliferation and collagen synthesis [1]. Among these mediators are histamine, heparin, hyaluronic acid, proteoglycans, proteases, growth factors, and cytokines [2]. Histamine has been related to strong fibrogenic activity [1] and myofibroblast differentiation [3]. In addition, previous authors demonstrated a directly proportional relationship between the number of mast cells and myofibroblasts [4].
The photobiomodulation generated by the irradiation of low-level laser therapy (LLLT) has an important influence on increasing the rate of cell proliferation [5], reducing the inflammatory process, and promoting angiogenesis [6] by acting positively in different cells types such as fibroblasts [7], keratinocytes [8], osteoblasts [9], mesenchymal stem cells and cardiac cells [10], muscle cells [11], and endothelial cells [12]. Previous reports also showed that light-emitting diode (LED) plays a role in mast cell degranulation [13], fibroblastic proliferation [14, 15], and angiogenesis [16]. However, the influence of these phototherapies on mast cell and of myofibroblastic differentiation on the synthesis of collagen was minimally explored [17, 18].
The aim of this study was to analyze the involvement of mast cells on the synthesis of collagen and their influence on myofibroblast differentiation in the late phase of tissue repair on wounds treated with LLLT or LED.
Materials and methods
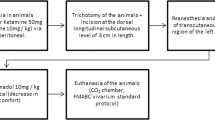
Following the approval by the Animal Experimentation Ethics Committee of the School of Dentistry of the Federal University of Bahia, 30 young adult male Wistar rats weighing 200–230 g were obtained from the vivarium of the Federal University of Bahia and were kept at the Animal Experimentation Laboratory of the Federal University of Bahia. The animals were kept in individual plastic cages lined with wood chips and were maintained at 23 °C in a 12/12-h day/night light cycle.
The animals were fed a standard laboratory diet and had water ad libitum. After a regular quarantine period, the animals were randomly distributed into three main groups and then subdivided into six groups according to the time of death (7 or 14 days) as shown in Table 1.
Under intramuscular general anesthesia (0.2 ml/100 mg of ketamine and 0.06 ml/100 g of xylazine), each animal had its dorsum shaven and skin cleaned with 2 % chlorhexidine solution. A 1 × 1-cm full-thickness excisional wound was created on the dorsum of each animal with a no. 15 scalpel blade. The depth of the wound was controlled by cutting to the depth of the bevel of the scalpel blade, which was 1 mm.
The laser and LED phototherapy treatments started immediately after surgery and were repeated at 48-h intervals for 7 and 14 days. The spatial average energy fluency (SAEF) per session was 10 J/cm2 [16] for the irradiated groups. When laser (GaAlAs, λ 660 nm, 40 mW, CW, spot 4 mm2, 252 s, Twin Flex Evolution, MMoptics®, São Carlos, São Paulo, Brazil) was used, the energy was delivered in four points around the wound (4 × 2.5 J/cm2). The LED probe (InGaAlP, λ 630 ± 10 nm, 115 mW, CW, spot 0.5 cm2, 87 s, Fisioled, MMoptics®, São Carlos, São Paulo, Brazil) was placed in a device in order to provide a focal distance that allowed the spot size to be 1 cm2. Thus, the energy was delivered in a single point over the wound. Both phototherapies were applied in a contact manner to the tissue. A summary of the parameters used on this study is presented in Table 2.
At the end of each experimental period, the animals were killed with an overdose of general anesthetics. The specimens were taken and fixed for 24 h on a 10 % formalin solution, routinely processed with wax, cut, stained with hematoxylin–eosin, and analyzed by light microscopy (AxiolabTM, Zeiss, Germany) at the Laboratory of Surgical Pathology of the School of Dentistry of the Federal University of Bahia.
To assess the intensity of collagen deposition in the tissue samples, cuts of 5-μm thick were stained using Picrosirius. The sections were examined using an optical microscope (Zeiss) and classified according to the following scores: (+) fibroplasia ≤ 50 % and (++) fibroplasia > 50 %.
Sections of 5-μm thick were also stained with toluidine blue for the visualization and semiquantitative analysis of mast cells in five microscopic fields (×400). Using the AxionVisionTM REL 4.8.6 2009 program (Zeiss), the images were captured and subjected to the counting of mast cells. Then, the average was obtained regarding the five fields of each sample for each group.
Immunohistochemistry was performed on paraffin wax-embedded sections (3-μm thick). The tissue sections were routinely deparaffinized and rehydrated. Endogenous peroxidase activity was blocked using hydrogen peroxide. Polyclonal antibody anti-SMA (Clone 1A4, Dako, Glostrup, Denmark; dilution 1:200) was used with the EnVision™ System (Dako, Carpinteria, CA, USA). The sections were incubated with the antibody (dilution 1:200) for 12 h at room temperature. The immunohistochemical reactions were developed with diaminobenzidine as the chromogenic peroxidase substrate, and the slides were counterstained with Harris's hematoxylin. Histological sections of the uterine leiomyoma were used as positive controls for the myofibroblasts. The samples were evaluated in five different representative fields identified by light microscopy at × 400 magnification. The myofibroblasts were counted using the AxionVisionTM program, and the results were expressed as the median number of myofibroblasts per field.
The results were statistically analyzed using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). The statistical analysis was carried out using Mann–Whitney and Spearman's tests. The significance level was accepted at p < 0.05.
Results
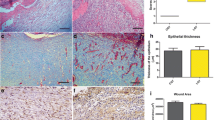
For all experimental groups, histological observations demonstrated that the mast cells were on the edge of the excisional wounds. Morphologically, the mast cells were elongated, oval, or round in shape. However, the myofibroblasts were found over all of the wound areas, showing spindle- to oval-shaped forms.
After 7 days, the following median numbers of mast cells were 26.00 (control), 22.4 (laser), and 12.0 (LED). Lower values were found after 14 days to be 22.00 (control), 8.0 (laser), and 10.0 (LED). The laser and LED groups showed reduced number of mast cells throughout the experiment. After 7 days, a statistically significant difference was only observed between the groups control and LED (p ≤ 0.05), while after 14 days, the statistical analysis showed significant difference when the control group was compared with the laser (p ≤ 0.05) and LED (p ≤ 0.05) groups. When the group laser 7 days and laser 14 days were compared, a significant difference was found between them (p ≤ 0.05) (Fig. 1a, b). However, no significant association was observed when the control group (7 days) was compared to the control (14 days) (p = 0.83) and laser (7 days) (p = 0.53) groups. Similarly, no significant association was observed when LED 7 days was compared to LED 14 days (p = 0.67) and laser 7 days (p = 0.14). This occurred also after 14 days when the laser group was compared to the LED group (p = 0.83).
Toluidine blue staining: a laser group at 7 days: focal accumulation of mast cells on dermis. b Laser group at 14 days: a greater number of mast cells scattered on the dermis compared to laser group at 7 days. EnVision™ polymer: c Laser group at 7 days: higher number of spindle-shaped myofibroblasts on dermis. d Laser group at 14 days: lower number of spindle-shaped and oval myofibroblasts compared to the laser group at 7 days. Picrosirius red: e deposition of collagen fibers over 50 % on laser group at 14 days
The median values of the myofibroblast on the healing area after 7 days of experiment were 21.00 (control), 46.00 (laser), and 19.00 (LED). After 14 days, lower values were observed 19.00 (control), 10.00 (laser), and 15.00 (LED). In general, no statistically significant difference was observed between the groups for all time points (p > 0.05). However, a trend in the reduction of myofibroblasts in all of the groups was observed throughout the experiment, which was more evident on the laser group (Fig. 1c, d).
A summary of the statistical results described above is presented in Table 3. With respect to the correlation between the number of mast cells and myofibroblasts when collagen deposition achieved more than 50 % in the wound area (Fig. 1e), a statistically significant correlation between groups laser at 7 days and Laser at 14 days (p = 0.022) was observed. For the other experimental groups, there was no statistical difference throughout the experimental period (Table 4).
Discussion
Different wavelengths emitted by laser light can trigger mast cell degranulation in experimental models in animals [13, 19] and humans [20] because the chemical mediators released promote many different actions that are important steps in tissue repair [1]. In this study, because of distinct light beams, laser and LED protocols were adjusted to provide the same energy density, allowing the comparison of both devices. Similarly, an equal energy density (10 J/cm2) was used for both therapies, as recommended by Barolet [21].
The mechanisms that accounted for the increase of collagen and fibrosis mediated by mast cells are still controversial. This increase occurs because of the diversity of proinflammatory and fibrogenic factors synthesized and stored in mast cells that trigger different molecular pathways. Mast cells are believed to act on fibrotic processes from the content of their granules, are rich in proteases (tryptase and chymase), and in cytokines (IL-4, IL-6, IL-13, TGF-β, and TNF-α) and influence fibroblastic proliferation, production of extracellular matrix, and myofibroblast differentiation [3]. The myofibroblast capacity to possibly contribute to collagen deposition is generated by a cause–effect relationship between extracellular matrix deposition, inflammatory cytokines (chemokines and arachidonic acid metabolites) and growth factors (TGF-b, TGF-a, EGF, aFGF, and bFGF) [22]. Thus, this study might contribute to the knowledge on mast cell participation in collagen synthesis in irradiated wounds and their possible participation in myofibroblastic differentiation.
Laser and LED technologies have been shown to be effective alternatives for the treatment of cutaneous wounds [15]. When compared with lasers, LED technology generates negligible amounts of heat, has been clinically proven as safe, and has achieved non-significant risk status in human trials conducted by the Food and Drug Administration [21].
The results of this study showed a higher number of mast cells on the edges of wounds for all the experimental groups. When all three groups were compared, along with the experimental time points, a reduction in the median numbers of mast cells in both laser and LED groups occurred compared to the control group. This result is similar to those reported by Berbet et al. [17] who found a reduction of this cellular population in red laser and infrared laser groups compared to the control group when analyzing mast cells and the fibrosis index present in endodontic sealing material.
In the present study, regarding both periods of time, a comparison of mast cells in the specimens with more than 50 % collagen in wound healing showed that the mast cells in the laser group increased, contrary to what occurred in the LED group. This result could indicate the role of these cells in collagen synthesis when irradiated by different light sources. However, when comparing the median number of mast cells from day 7 to 14, a significant reduction in this cell population was observed on irradiated wounds using both phototherapies, whereas non-irradiated wounds showed no variation in their number of mast cells. Despite these aspects, Khoshvaghti et al. [19] demonstrated the same result when studying the laser treatment of burns with similar experimental periods, whereas Berbet et al. [17] found a lower number of mast cells after LLLT irradiation but no decrease in collagen deposition by Picrosirius. It is possible that the time used for these authors has influenced their results. We believe that a decrease in the mast cell population can prevent complications in wound healing, such as excessive collagen synthesis.
Previous studies showed that mast cells exert a strong influence over collagen production in different types of injury [17, 23] and repair processes [1, 24] considering their enzymatic content [1, 25]. Despite this participation, Kofford et al. [25] suggested that mediators such as chymase, also present in mast cell granules, directly transform procollagen in collagen fibrils in vitro and indirectly induce fibroblast proliferation through TGFB-1. Other authors, such as Garbuzenko et al. [1] showed that human mast cells directly modulate human skin fibroblast properties to a fibrotic phenotype by increasing the synthesis of fibroblasts, collagen, and TIMP-2 through the liberation of stored granules, also promoting tissue remodeling.
Similar to mast cells, the median number of myofibroblasts in the laser and LED groups reduced during the experimental time points. Importantly, no difference existed between the median numbers in both groups; this tendency for a reduction in myofibroblasts was observed specially in the laser group regardless of the experimental period. According to Desmouliére [26] and Gabbiani [27], this reduced number of myofibroblasts was the result of an apoptosis phenomenon, and this could be attributed to laser irradiation. Other authors found a reduction in myofibroblasts in the later experimental periods [28]. In addition, Karu [29] attributes this cellular reduction to an inhibitory effect of laser light on the cellular metabolism and apoptosis. Nevertheless, we demonstrated that the late phase, in the present study, showed an increase of collagen deposition.
For samples with more than 50 % collagen production, no significant difference was observed in the median of myofibroblasts for the control, laser, and LED groups. However, myofibroblast differentiation and its function are known to be regulated by mediators produced by mast cells [3] because serine proteases stimulate fibroblast proliferation and collagen synthesis [30]. This role seems to be in accordance with our results, as there was a significant correlation among mast cells, myofibroblasts, and collagen production over 50 % on laser-treated subjects throughout the experimental period, although we were not able to demonstrate a significant correlation for the other groups. This result might suggest the participation of mast cells on tissue remodeling promoted by the laser phototherapy used in this study because in addition to the mast cell contribution to collagen synthesis, other studies suggested their participation in differentiation of myofibroblasts [3, 4]. Further studies should be performed to clarify this matter.
It is important to state that LED is different from laser because the former is not a collimated light and non-coherent. The light emitted by LED is more divergent than that emitted by laser; thus, LED has less energy per spectral band as the photons spread over a larger surface. Although these differences exist, there is a similar energy concentration on the action area covered by both phototherapies, but their distribution remains different. All the aspects allow the photobiomodulation to act upon the photoreceptors that are present in the biological tissues [31] and probably on the different cellular populations such as mast cells and myofibroblasts.
Finally, the mast cell and myofibroblast population, in addition to their basic functions in wound tissue repair, might also participate in collagen formation and remodeling of irradiated wounds particularly in relation to laser light. Nevertheless, other methodologies and different parameters using both phototherapies could be performed in an attempt to contribute to the role of mast cells on the collagen synthesis and their participation in myofibroblastic differentiation.
References
Garbuzenko E, Nagler A, Pickholtz D, Gillery P, Reich R, Maquart FX, Levi-Schaffer F (2002) Human mast cells stimulate fibroblast proliferation, collagen synthesis and lattice contraction: a direct role for mast cells in skin fibrosis. Clin Exp Allergy 32:237–246
Caughey GH (2007) Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev 217:141–154
Gailit J, Marchese MJ, Kew RR, Gruber BL (2001) The differentiation and function of myofibroblasts is regulated by mast cell mediators. J Investig Dermatol 117:1113–1119
Mangia A, Malfettone A, Rossi R, Paradiso A, Ranieri G, Simone G, Resta L (2011) Tissue remodelling in breast cancer: human mast cell tryptase as an initiator of myofibroblast differentiation. Histopathology 58:1096–1106
Karu TI (1992) Local pulsed heating of absorbing chromophores as a possible primary mechanism of low power laser effects. In: Galetti G, Bolognani L, Ussia G (eds) Laser applications in medicine and surgery. Monduzzi, Bologna, pp 253–258
Salate AC, Barbosa G, Gaspar P, Koeke PU, Parizotto NA, Benze BG, Foschiani D (2005) Effect of In-Ga-Al-P diode laser irradiation on angiogenesis in partial ruptures of Achilles tendon in rats. Photomed Laser Surg 23:470–475
Kreisler M, Christoffers AB, Willershausen B, d’Hoedt B (2003) Effect of low-level GaAlAs laser irradiation on the proliferation rate of human periodontal ligament fibroblasts: an in vitro study. J Clin Periodontol 30:353–358
Grossman N, Schneid N, Reuveni H, Halevy S, Lubart R (1998) 780 nm low power diode laser irradiation stimulates proliferation of keratinocyte cultures: involvement of reactive oxygen species. Lasers Surg Med 22:212–218
Stein A, Benayahu D, Maltz L, Oron U (2005) Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg 23:161–166
Tuby H, Maltz L, Oron U (2007) Low-level laser irradiation promotes proliferation of mesenchymal and cardiac stem cells in culture. Lasers Surg Med 39:373–378
Gavish L, Perez L, Gertz SD (2006) Low-level laser irradiation modulates matrix metalloproteinase activity and gene expression in porcine aortic smooth muscle cells. Lasers Surg Med 38:779–786
Schindl A, Merwald H, Schindl L, Kaun C, Wojta J (2003) Direct stimulatory effect of low-intensity 670 nm laser irradiation on human endothelial cell proliferation. Br J Dermatol 148:334–336
Monteiro JSC, de Oliveira SC, de FaTima Ferreira Lima M, Sousa JA, Pinheiro AN, Dos Santos JN (2011) Effect of LED red and IR photobiomodulation in tongue mast cells in Wistar rats: histological study. Photomed Laser Surg 29:767–771
de Sousa AP, Santos JN, Dos Reis JA, de Souza J Jr, Cangussú MC, Pinheiro ALB (2010) Effect of LED phototherapy of three distinct wavelengths on fibroblasts on wound healing: a histological study in a rodent model. Photomed Laser Surg 28:547–552
Oliveira Sampaio SC, de C Monteiro JS, Cangussú MC, Pires Santos GM, dos Santos MA, dos Santos JN, Pinheiro AL (2013) Effect of laser and LED phototherapies on the healing of cutaneous wound on healthy and iron-deficient Wistar rats and their impact on fibroblastic activity during wound healing. Lasers Med Sci 28:799–806
de Sousa AP, Paraguassú GM, Silveira NT, de Souza J, Cangussú MC, Dos Santos JN, Pinheiro AL (2013) Laser and LED phototherapies on angiogenesis. Lasers Med Sci 28:981–987
Berbert FL, Sivieri-Araújo G, Ramalho LT, Pereira SA, Rodrigues DB, de Araújo MS (2011) Quantification of fibrosis and mast cells in the tissue response of endodontic sealer irradiated by low-level laser therapy. Lasers Med Sci 26:741–747
Medrado AR, Pugliese LS, Reis SR, Andrade ZA (2003) Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med 32:239–244
Khoshvaghti A, Zibamanzarmofrad M, Bayat M (2011) Effect of low-level treatment with an 80-Hz pulsed infrared diode laser on mast-cell numbers and degranulation in a rat model of third-degree burn. Photomed Laser Surg 29:597–604
Sawasaki I, Geraldo-Martins VR, Ribeiro MS, Marques MM (2009) Effect of low-intensity laser therapy on mast cell degranulation in human oral mucosa. Lasers Med Sci 24:113–116
Barolet D (2008) Light-emitting diodes (LEDs) in dermatology. Semin Cutan Med Surg 27:227–238
Hebda PA, Collins MA, Tharp MD (1993) Mast cell and myofibroblast in wound healing. Dermatol Clin 11:685–696
Rojas IG, Boza YV, Spencer ML, Flores M, Martínez A (2012) Increased fibroblast density in actinic cheilitis: association with tryptase-positive mast cells, actinic elastosis and epithelial p53 and COX-2 expression. J Oral Pathol Med 41:27–33
Dong X, Geng Z, Zhao Y, Chen J, Cen Y (2013) Involvement of mast cell chymase in burn wound healing in hamsters. Exp Ther Med 5:643–647
Kofford MW, Schwartz LB, Schechter NM, Yager DR, Diegelmann RF, Graham MF (1997) Cleavage of type I procollagen by human mast cell chymase initiates collagen fibril formation and generates a unique carboxyl-terminal propeptide. J Biol Chem 272:7127–7131
Desmoulière A (1995) Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol Int 19:471–476
Gabbiani G (2003) The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 4:500–503
Ribeiro MA, Albuquerque RL Jr, Ramalho LM, Pinheiro AL, Bonjardim LR, Da Cunha SS (2009) Immunohistochemical assessment of myofibroblasts and lymphoid cells during wound healing in rats subjected to laser photobiomodulation at 660 nm. Photomed Laser Surg 27:49–55
Karu TI (1987) Photobiological fundamentals of low-power laser therapy. IEE J Quantum Electron 23:1703–1717
Abe M, Kurosawa M, Ishikawa O, Miyachi Y, Kido H (1998) Mast cell tryptase stimulates both human dermal fibroblast proliferation and type I collagen production. Clin Exp Allergy 28:1509–1517
Karu TI (2003) Low-power laser therapy. In: Vo-Dinh T (ed) Biomedical photonics handbook. CRC, Boca Raton, pp 79–95
Acknowledgments
We would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Apoio a Pesquisa do Estado da Bahia (FAPESB) for providing financial support for this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Castro, I.C.V., Rocha, C.A.G., Gomes Henriques, Á.C. et al. Do laser and led phototherapies influence mast cells and myofibroblasts to produce collagen?. Lasers Med Sci 29, 1405–1410 (2014). https://doi.org/10.1007/s10103-014-1537-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-014-1537-0