Abstract
Dentinal hypersensitivity is one of the oldest recorded complaints of discomfort to mankind and yet there appears to be no permanent treatment for this clinical condition. This study was designed to evaluate the clinical efficacy of potassium binoxalate gel and neodymium:yttrium–aluminum–garnet (Nd:YAG) laser on dentin hypersensitivity for a period of 9 months. Eighty teeth (20 subjects, 25–55 years old, M = F) were evaluated in a split-mouth design to receive potassium binoxalate (group A, 40 teeth) and Nd:YAG (group B, 40 teeth: 1 W, 10 Hz, and 60 s, irradiated twice). The diameter of output beam was about 300 μm with a distance of 2 mm between laser fiber or tip and tooth surface. The clinical efficacy was evaluated by air-blast test and cold-water test using visual analog scale. Electron microscopy photomicrographs were taken to confirm the results. Analysis was done at baseline; immediately post-treatment; and at 3, 6, 9 months post-treatment. Student’s paired and unpaired T tests were used to evaluate the statistical analysis. Both treatment modalities were effective in reducing dentine hypersensitivity. However, Nd:YAG laser was better when intragroup comparison was made at 9 months post-treatment. Nd:YAG lasers is better in long-term treatment (up to 9 months) owing to the melting of dentinal tubules. However, due to depth of penetration of microcrystals, gel was better when ease of the procedure is considered. Nevertheless, both treatment modalities resulted in recurrence. Hence, further studies are needed to discover an agent, which can be considered as a “gold standard”.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dentinal hypersensitivity is one of the oldest recorded complaints of discomfort to mankind. It is “an enigma being frequently encountered but poorly understood” and consequently there appears to be no permanent treatment for this clinical condition [1]. It is a common problem with various reports indicating an incidence of 4–74 % of the population, and the teeth most commonly affected are upper premolars [2].
Dentinal hypersensitivity is defined as “sharp pain arising from the exposed dentin typically in response to chemical, thermal, tactile, or osmotic stimuli that cannot be explained as arising from any other form of dentinal defect or pathology” [3]. Recently, this definition has been modified to replace the term “pathology” with the word “disease” to avoid any confusion with other conditions such as atypical odontalgia [4]. It has been described as “common cold of dentistry” by some authors [5] and “toothbrush disease”, when it occurs due to gingival recession [6]. It has been claimed that the condition is dependent on dentin exposure to the oral environment and the patency of dentinal tubules. The exposure can result from loss of enamel by abrasion or erosion, or by a consequence of gingival recession.
Various hypotheses have been proposed to explain the mechanism of dentinal hypersensitivity. The Hydrodynamic theory, given by Gysi in 1900 and later scientifically explained by Branstrom [7] in 1966, is the most commonly accepted theory for dentin hypersensitivity. It is based on the concept that fluid within the dentinal tubule can flow inward or outward, depending on pressure differences in the surrounding tissue. This fluid flow within the tubules serves as a medium to excite intradental nerves, which is perceived as pain by the patient. Hot, cold, sweet, or sour foods and/or beverages, as well as cold air or an explorer touch is common stimuli of dentinal hypersensitivity [7].
It is suggested that the term dentine sensitivity may be considered more appropriate since there is no evidence to indicate that “hypersensitive” dentine differs in any way from normal dentine or that the pulp response is anything but a normal response to stimulation of exposed dentine. However, it should be noted that not all exposed dentine is sensitive, thus both terms could be considered suitable. Nevertheless, the term dentinal hypersensitivity has been used for decades and is appreciated as a distinct entity by clinicians [8].
Numerous agents have been used in the management of dentinal hypersensitivity termed as desensitizing agents. Grossman [9] and Gangarosa [10] suggested a number of requirements for these agents: Therapy for dentinal hypersensitivity should be non-irritant to the pulp, relatively painless on application, easily carried out, rapid in action, effective for long period, and without staining effects. These agents include desensitizing toothpastes and gels containing salts of potassium, strontium, oxalates and fluorides, various varnishes, restorative materials, gels, iontophoresis, lasers, periodontal plastic surgeries, etc. Most of these agents tried and tested have the disadvantage of delayed action and multiple applications. None of them has provided a long-term relief.
It has been shown in various studies that lasers can be used in the effective management of DH. Among the lasers, the most commonly used are neodymium:yttrium–aluminum–garnet (Nd:YAG), Er:YAG, GaAlA, diode, and Co2 lasers. The mechanism of action of lasers in treating DH is not very clear. Some authors have shown that Nd:YAG laser application occluded the dentinal tubules [11]. However, White et al. [12] indicated that Nd:YAG lasers damage pulpal tissues when remaining dentin thickness is less than 1 mm. Since one cannot directly measure the remaining dentin thickness in vivo, it is important that the operator choose laser parameters below the safety limits.
Sicilia et al. in 2009 showed that diode lasers were effective in reducing dentinal hypersensitivity immediately following treatment and lasted for only 60 days post-treatment [13]. Clavijio et al. in 2009 concluded that diode lasers were effective till 6 days after the application in the management of dentinal hypersensitivity [14]. Gholami et al. concluded that the 810-nm diode laser sealed tubules to a far lesser degree compared to other lasers, with negligible effects on desensitization [15].
Recently, Al-Tayeb et al. showed that potassium binoxalate gel has the advantage of single application and immediate relief from dentinal hypersensitivity. This potassium binoxalate gel (D/Sens Crystal) contains a patented solution of water, potassium binoxalate, and nitric acid, which reacts with the smear layer to precipitate microcrystals of calcium oxalate and potassium nitrate. These crystals penetrate deeply into the tubules and seal the entire dentinal surface with a continuous, acid-resistant complex. Potassium ions present in this preparation helps in nerve desensitization [4].
Dentinal hypersensitivity literature showed most reported use of Nd:YAG laser in providing immediate relief from dentinal hypersensitivity. The mechanism involved is melting of dentin and thereby closure of exposed dentinal tubule orifices [16]. Even though the immediate relief occurred with the aforementioned treatment modalities, long-term effect was not evaluated. Hence, we compared Nd:YAG laser and potassium binoxalate gel in the present study.
The present study was designed to evaluate the 9-month clinical effectiveness of potassium binoxalate gel and Nd:YAG laser in the management of dentinal hypersensitivity. There are no reports in the periodontal literature, which compares the effect of these agents for 9 months.
Materials and method
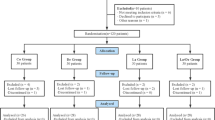
The study was carried out from August 2011 to November 2011. The study population consisted 20 age- and gender-balanced (10 males, 10 females; age range, 25–55 years) systemically healthy subjects, attending the Outpatient Section, Department of Periodontics and Oral Implantology, Dr. DY Patil Dental College and Hospital, Pune, India. Written informed consent was obtained from those who agreed to participate voluntarily. Ethical clearances were obtained from the institution’s ethical committee and review boards.
Subjects with a minimum of 20 natural teeth with at least two hypersensitive teeth, one in either side of the arch or in either of the jaws were included for a split-mouth design (visual analog scale (VAS) score of ≥4). A total of 80 hypersensitive teeth (40 each on contra lateral sides) were included based on the power of the study with confidence interval at α value of 95 %. Teeth having dentinal hypersensitivity on the facial side, to thermal, mechanical, sweet or sour stimuli due to abrasion, erosion, or gingival recession as primary etiology were included for the study. Subjects with chipped teeth, teeth with defective restorations, symptoms of pulpal damage, extensive caries, cracked tooth syndrome, fractured undisplaced cusps, crowns and abutments, and patients on any analgesic, or anti-inflammatory regimens, having pacemaker, undergoing orthodontic therapy, or any treatment for hypersensitivity/periodontal surgery within last 3 months were excluded from the study.
Assessment of dentinal hypersensitivity
The dentinal hypersensitivity was assessed using air-blast test (evaporative stimulus) and cold-water test (thermal stimulus) and the patient response was recorded on VAS.
Air-blast test
A blast of air from a standard dental syringe at 60 lb/in. pressure was directed at the affected area of the tooth for 1 s from a distance of 10 mm while adjacent teeth were isolated using cotton rolls/gloved fingers to prevent false-positive results. The distance was maintained by taping a wire with 10-mm marking to the dental syringe [17].
Cold-water test
A precooled 2 cm3 disposable syringe was filled with freshly melted ice-cold water. After isolating the specific tooth, 0.2 ml of the water was slowly expelled from the syringe into the tooth surface while adjacent teeth were isolated using cotton rolls and gloved fingers to prevent false-positive results [17]. Throughout the study, the stimuli tests were applied in the same order, with a minimum 5-min gap between the applications of the different stimuli.
Visual analog scale
A visual analog scale is a scale line of 10 cm in length, the extremes of the line representing the limits of pain a patient might experience from an external stimulus (no pain at one end and severe pain or discomfort at the other end of the line). Patients were asked to place a mark on the 10-cm line, which indicates the intensity of their current level of sensitivity or discomfort following application of test stimuli [18].
Potassium binoxalate gel (group A)
The selected teeth were scaled and roots planed, and were isolated with cotton rolls, cleaned, and dried with cotton pellets. Potassium binoxalateFootnote 1 gel was applied directly on the sensitive surface using a soft needle applicator tip and it was allowed to air dry for 2 min [4].
Nd:YAG laser (group B)
The selected teeth were scaled and the roots were planed and were isolated with cotton rolls, cleaned, and dried with cotton pellets. The sensitive surface was lased with Nd:YAG laser at 1 W, 10 Hz, and 60 s two times according to the study carried out by Birang et al. [19] where no detrimental pulpal effects were seen at these settings. The diameter of the output beam was about 300 μm. The distance between laser fiber or tip and tooth surface was maintained at 2 mm [20]. For this purpose, we used a piece of orthodontic wire joined to the Nd:YAG laser.Footnote 2 All the essential precautions were taken to safeguard both patient as well as the operator [14]. The teeth were evaluated before treatment, i.e., baseline, immediately after treatment, and at 3, 6, and 9 months post-treatment using the two test stimuli.
Environment scanning electron microscopic
Fifteen freshly extracted maxillary or mandibular single-rooted teeth were selected for descriptive analysis and kept in artificial saliva until further use. A cavity was prepared from below the CEJ of buccal surface of each tooth to expose the dentinal tubules. In order to clean off the smear layer from the surfaces, the pieces were embedded in 17 % EDTA for 5 min and then in 5.25 % sodium hypochlorite for 5 min to open up the dentinal tubules. Finally, all the samples were washed with 5 ml of distilled water and kept in artificial saliva. The teeth were randomly divided into three groups. The first group was left as control without any treatment. The second group was irradiated with potassium binoxalate gel and the third group was treated with Nd:YAG laser. After treating, all samples were transferred to the lab for environment scanning electron microscopic (ESEM) analysis at ×2,000 [19].
Ranking criteria for photomicrographs was done using the following grades: [21]
- A:
-
Majority of tubules occluded with some just apparent
- B:
-
Less than or equal to 10 tubules visible with majority occluded
- C:
-
Greater than 10 tubules visible with majority occluded
- D:
-
Less than or equal to 10 tubules visible with majority patent
- E:
-
Greater than 10 tubules visible with majority patent
Statistical tests
As it was a split-mouth design, baseline data for intragroup age, and sex was not required and hence were not analyzed. Student’s paired T test and Student’s unpaired T test were used for statistical analysis in this study. The results were averaged (mean ± standard deviation) for each parameter at different time interval (baseline; immediate post-treatment; 3, 6, and 9 months post-treatment). The difference between each pair of measurement was then calculated. Paired T test was applied to assess the statistical significance between specific time intervals within each group. Student’s unpaired T test was used to assess the difference between the groups. Data collected was entered into MS-Excel worksheet and SPSSFootnote 3 software was used for analysis.
Results
The mean values of VAS for both air-blast and cold-water tests decreased at all the time intervals in both groups A and B. Difference in the mean values of VAS for air-blast test when compared from baseline to post-treatment was highly significant for both the groups A and B with p < 0.01 (Table 1). In addition, difference in the mean values of VAS for cold-water test when compared from baseline to post-treatment was highly significant (p < 0.01) for both groups A and B (Table 2). Intragroup analysis between groups A and B in both tests showed no significance at baseline, immediately post-treatment, at 3 months, and at 6 months. However, values were highly significant (p < 0.01) at 9 months post-treatment (Tables 3 and 4) for both air-blast and cold-water tests.
The following observations were made for descriptive analysis of ESEM photomicrographs:
-
1
All the samples of control group were graded as rank E, i.e., greater than 10 tubules visible with majority patent (Fig. 1).
-
2
Out of the five samples, three samples of potassium binoxalate group were graded as rank C, i.e., greater than 10 tubules visible with majority occluded, while two samples graded as rank A, i.e., majority of tubules occluded with some just apparent (Figs. 2 and 3).
Fig. 2 -
3
All the samples of Nd:YAG laser group were graded as rank A, i.e., majority of tubules occluded with some just apparent (Fig. 4).
Discussion
Dentinal hypersensitivity or cervical dentinal sensitivity is a significant clinical problem and it satisfies all the criteria to be classified as a true pain syndrome. Dentinal hypersensitivity identifies itself as a distinct clinical entity and invites the clinician to consider a differential diagnosis, since other conditions may have identical symptoms but require different management strategies [8]. It can significantly affect individual’s quality of life, impede effective oral hygiene, and subsequently affects esthetics.
Dentinal denudation can be due to attrition, abrasion, or erosion. Alternatively, in some individuals (5–10 % cases), the cementum and enamel (normally cover the dentine) do not meet and results in dentine exposure attributed as developmental anomaly [2]. Enamel can also be lost as a result of vigorous or incorrect tooth brushing, overconsumption of acidic food, and tooth grinding caused by stress in parafunctional behaviors. The number of open dentinal tubules per surface area in the exposed dentin surface of teeth with dentinal hypersensitivity can be eight times that of teeth nonresponsive to stimuli [22].
Various agents have been used in the treatment of dentinal hypersensitivity which acts by tubular occlusion or blockage of nerve activity by means of direct ionic diffusion. Most of the agents tried and tested have the disadvantage of delayed action and multiple applications. None of them has reported to provide a long-term relief. So the present study was designed to evaluate the 9-month clinical effectiveness of potassium binoxalate gel and Nd:YAG laser in the management of dentinal hypersensitivity. In addition, ESEM evaluation of microstructural dentinal tubules was done to better understand the mode of action of these treatment modalities.
A mean duration of 8 weeks has been recommended for dentinal hypersensitivity studies, which is an average time taken by the desensitizing agent to reach its peak action [23]. Chesters et al. [24] recommended that a minimum of two stimuli be used to test products or clinical procedures in vivo for dentinal hypersensitivity. Clark and Troullos [25] recommended the least-disturbing stimulus should be used first and the most disturbing to be used at last, so that one stimulus does not interfere with others with a 5-min gap between the test stimuli. Accordingly, we used air-blast and cold-water tests for the assessment of dentinal hypersensitivity in which air-blast test was used first which was followed by cold-water testing with a minimum of 5-min gap between test stimuli. Pashlet [26] showed that prolonged air blasts have an unknown and possibly varying temperature effect, which was avoided by using a short application time, i.e., 1 s [26].
Patient response was recorded on VAS. In comparison to other pain scales, it has been observed that the VAS correlates well with these methods and appears to be more sensitive in discriminating between various treatments and changes in pain intensity [27].
Split-mouth design was chosen to eliminate the effects of individual conception of pain. It is also a highly effective and efficient model for professional application of sensitivity products [28]. The subject acts as his own control which is very powerful tool statistically and the methodology of choice [28].
The mean values of VAS recorded after air-blast and cold-water test decreased significantly for group A at each time interval from baseline up to 9 months. This is in accordance to a study by Al-Tayeb [4] in which there was a statistically significant decrease in the mean scores of VAS at 1 and 6 months post-treatment after the application of potassium binoxalate gel. Similar results were seen in other studies [29–31] conducted for a period of 6 months. The reduction in dentinal hypersensitivity is due to the dual action of potassium binoxalate gel. It reacts with the smear layer to precipitate microcrystals of calcium oxalate and potassium nitrate, which penetrate deeply into the tubules, and seal the entire dentinal surface with a continuous, acid-resistant complex. At the same time, a soluble and active potassium salt penetrate deep into the dentinal tubules to increase extracellular concentration of the active potassium salts, which inhibit the nerve cells repolarization, and the transmission of the pain impulse. Similar observations were made in various other studies [32–36]. Pashley et al. [37] concluded that the formation of calcium oxalate crystals occurs 30 s after the application of oxalate-based solutions. In the present study, potassium binoxalate gel was applied for 2 min as instructed by the manufacturer, a sufficient time for crystal precipitation.
In the present study, three ESEM photomicrographs of potassium binoxalate gel group belonged to rank C while two images belonged to rank A which explains the statistically significant decrease in the mean VAS immediately after treatment. Also, all the photomicrographs in this group showed formation of a homogenous crystalline layer of insoluble salts that precipitate, seal, and occlude the open dentinal tubules. Similar results were obtained by Al-Tayeb [4]. In in vitro study by Greenhill and Pashley [38], potassium oxalate resulted in maximum reduction in dentine permeability, i.e., up to 98 %.
With Nd:YAG laser, the mean VAS recorded after air-blast and cold-water tests decreased significantly at each time interval from baseline up to 9 months post-treatment. This was in accordance with other studies [18, 39–41] conducted for a period of 6 months. Nd:YAG laser melts hydroxyapatite structure, which upon cooling, can resolidify forming hydroxyapatite crystals larger than the initial structure. Investigations into this recrystallization of dentin have shown that a glazed, nonporous surface can be produced which may partially or totally obliterate dentinal tubules [11]. Nd:YAG lasers are also thought to work by coagulation of proteins in the dentinal fluid and hence reduce permeability [2].
White et al. [12] indicated that Nd:YAG laser damage pulpal tissues. Accordingly, the laser parameters used in our study were 1 W, 10 Hz, and 60 s two times, which showed no detrimental pulpal effects in a study conducted by Birang et al. [19]. In addition, use of noncontact mode by maintaining a distance of 2 mm between the laser fiber tip and tissue surface prevented excessive heat induction. Similar results were seen in studies conducted by Gutknecht et al. [20]. Nd:YAG laser was found more effective than Er:YAG laser in reduction of patients’ pain [19, 42]. In the present study, all the ESEM photomicrographs of Nd:YAG laser group belonged to the rank A which supports the statistically significant decrease in the mean VAS immediately after treatment.
In the present study, at 9 months, the mean value of VAS increased in both the groups compared to 6 months. This increase in mean value may be due to the removal of the occluding crystals by daily wear and tear (tooth brushing, toothpick, etc.) and citrus food ingestion over the period of time leading to remission of dentinal hypersensitivity. This is in accordance with the study conducted by Kerns et al. [43]. Prati et al. [44] also concluded that use and abuse of acidic drinks may damage dentine and increase the risk of dentinal hypersensitivity.
The comparison between groups A and B showed that there was a decrease in mean VAS score in both the groups but it was not statistically significant except at 9-month interval where the mean VAS score in Nd:YAG laser group was statistically lower compared to potassium binoxalate gel group emphasizing that action of potassium binoxalate gel was effective but short lived compared to Nd:YAG laser which had statistically better long-term clinical effectiveness. The possible reason for this could be that the melting of dentinal tubules and depth of penetration of microcrystals in Nd:YAG laser would be more compared to potassium binoxalate gel group which was 3 μm. Magalhães et al. [45] showed that Nd:YAG laser have the sealing depth of dentinal tubules up to 7 μm. Kerns et al. [43] in a study concluded that the action of oxalates to occlude dentinal tubules is relatively short lived.
Certain limitations of the study need to be addressed, we used a split-mouth design thus subjects acted as his/her own control. In such cases, patient’s expectation of the sided treated with lasers could be higher; also, it may have lead to bias in VAS scores. Further prospective longitudinal studies with larger sample size are warranted to evaluate combinations techniques (lasers + gels) with repeated lasers applications to discover a standard treatment of dentinal hypersensitivity.
Conclusions
It was concluded that both the treatment modalities were effective in the management of dentinal hypersensitivity but comparatively, Nd:YAG laser was better than potassium binoxalate gel in its management for 9 months. As far as short-term relief is concerned (up to a period of 6 months), gel can be considered as a better treatment modality in terms of limited armamentarium, ease of application, and lesser precautions. Nevertheless, both the treatment modalities resulted in the reoccurrence of dentinal hypersensitivity. Hence, it is not a permanent management and further studies are needed to discover an agent, which can be considered as a “gold standard” to permanently treat dentinal hypersensitivity.
Notes
D/Sense® Crystal™, Shelton, CT 06484, USA
Fidelis Plus Laser System, Fotona, Slovenia
Statistical Package for Social Sciences, Version 15
References
Pashley DH (1986) Dentin permeability, dentin sensitivity, and treatment through tubule occlusion. J Endodontol 12:465–73
Bartold PM (2006) Dentinal hypersensitivity: a review. Australian Dent J 51(3):212–218
Von Troil B, Needleman I, Sanz M (2002) A systematic review of the prevalence of root sensitivity following periodontal therapy. J Clin Periodontol 29:173–77
Al-Tayeb D (2008) Management of root-dentine hypersensitivity following non-surgical periodontal therapy: clinical and scanning electron microscopic study. Egyptian Dent J 54:1–15
Strassler HE, Drisko CL, Alexander DC (2008) Dentin hypersensivity: its inter-relationship to gingival recession and acid erosion. Compend Contin Educ Dent 29(5):1–9
Bamise CT, Olusile AO, Oginni AO (2008) An analysis of the etiological and predisposing factors related to dentin hypersensitivity. J Contemp Dent Pract 9(5):52–59
Brannstrom M, Astrom A (1972) The hydrodynamics of the dentine, its possible relationship to dentinal pain. Int Dent J 22:219–227
Dababneh RH, Dababneh RH, Khouri AT, Addy M (1999) Dentine hypersensitivity—an enigma? a review of terminology, epidemiology, mechanisms, aetiology and management. Brit Dent J 187(11):606–611
Gangarosa LP Sr (1994) Current strategies for dentist applied treatment in the management of hypersensitive dentine. Arch Oral Biol 39(suppl):101S–106S
Grossman LI (1935) A systematic method for the treatment of hypersensitive dentine. J Am Dent Asso 22:592–602
Schwarz F, Arweiler N, Georg T, Reich E (2002) Desensitizing effects of an Er:YAG laser on hypersensitive dentine. A controlled, prospective clinical study. J Clin Periodontol 29:211–215
White JM, Fagan MC, Goodis HE (1994) Intrapulpal temperatures during pulsed Nd:YAG laser treatment of dentin, in vitro. J Periodontol 65:255–259
Gholami GA, Fekrazad R, Esmaiel-Nejad A, Kalhori KA (2011) An evaluation of the occluding effects of Er; Cr:YSGG, Nd:YAG, CO2 and diode lasers on dentinal tubules: a scanning electron microscope in vitro study. Photomed Laser Surg 29(2):115–21
Sicilia A, Cuesta-Frechoso S, Suáre A, Suáre A, Angulo J, Pordomingo A, De Juan P (2009) Immediate efficacy of diode laser application in the treatment of dentine hypersensitivity in periodontal maintenance patients: a randomized clinical trial. J Clin Periodontol 36(8):650–660
Clavijo EMA, Clavijo VRG, Clavijo VRG, Bandéca MC, Bandéca MC, Nadalin MR, Nadalin MR, Andrade MF, Andrade MF, Saad JRC, Pelegrine AA (2009) Clinical efficiency of low-level diode laser in reducing dentin hypersensitivity. Laser Physics 19(10):2041–2044
Lan W, Liu H (1996) Treatment of dentin hypersensitivity by Nd:YAG laser. J Clin Laser Med Surg 14(2):89–92
Singhal P, Gupta R, Pandit N (2005) 2 % Sodium fluoride-iontophoresis compared to a commercially available desensitizing agent. J Periodontol 76:351–357
GauthamKumar N, Mehta DS (2005) Short term assessment of the Nd:YAG laser with and without sodium fluoride varnish in the treatment of dentine hypersensitivity—a clinical and scanning electron microscope study. J Periodontol 76:1140–1147
Birang R, Yaghini J (2006) Comparative evaluation of dentine surface changes following Nd:YAG laser irradiation by SEM. Dent Res J 3(2):1–7
Gutknecht N, Moritz A, Dercks HW, Lampert F (1997) Treatment of hypersensitive teeth using neodymium:yttrium–aluminum–garnet lasers: a comparison of the use of various settings in an in vivo study. J Clin Laser Med Surg 15(4):171–174
West N, West N, Addy M, Hughes J, Hughes J (1998) Dentin hypersensitivity: the effects of brushing desensitizing toothpastes, their solid and liquid phases, and detergents on dentin and acrylic: studies in vitro. J Oral Rehab 25:885–895
Absi EG, Addy M, Adams D (1987) Dentine hypersensitivity: a study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J Clin Periodontol 14:280–4
Ricarte JM, Matoses VF, Fernandez AJF, Moreno BM (2008) Dentinal sensitivity: concept and methodology for its objective evaluation. Med Oral Patol Oral Cir Bucal 13(3):E201–6
Chesters R, Kaufman HW, Wolff MS, Huntington E, Kleinberg I (1992) Use of multiple sensitivity measurements and logit statistical analysis to assess the effectiveness of potassium citrate containing dentrifice in reducing dentinal hypersensitivity. J Clin Periodontol 19:256–261
Clark GE, Troullos ES (1990) Designing hypersensitivity clinical studies. Dent Clin North Am 34:531–544
Pashley DH (1990) Mechanisms of dentin sensitivity. Dent Clin North Am 34:449–473
Gillam DG, Newman HN (1993) Assessment of pain in cervical dentinal sensitivity studies: a review. J Clin Periodontol 20:383–394
Hoang-Dao BT, H-Hoang T, Tranthi NN, Koubi G, Camps J (2009) Clinical efficiency of a natural resin fluoride varnish (Shellac F) in reducing dentin hypersensitivity. J Oral Rehab 36:124–131
Muzzin KB, Johnson R (1989) Effects of potassium oxalate on dentin hypersensitivity in vivo. J Periodontol 60(3):151–8
Pereira JC, Martinella A, Santiago SL (2001) Treating hypersensitive dentin with three different potassium oxalate based gel formulations: a clinical study. Rev FOB 9(3/4):123–130
Pillon FL, Romani ID, Schmidt ER (2004) Effect of a 3 % potassium oxalate topical application on dentinal hypersensitivity after subgingival scaling and root planning. J Periodontol 75:1461–1464
Gillam DG, Bullman JS, Jackson RJ, Newman HM (1996) Efficancy of a potassium nitrate mouthwash in alleviating cervical dentin sensitivity. J Clin Periodontol 23:993–997
Tarbet WJ, Silverman G, Stolman JM, Fratarcangelo PA (1980) Clinical evaluation of a new treatment for dentinal hypersensitivity. J Periodontol 51:535–540
Tarbet WJ, Silverman G, Fratarcangelo PA, Kanapka JA (1982) Home treatment for dentinal hypersensitivity: a comparative study. J Am Dent Assoc 105:227–230
Tzanova S, Ivanova Z, Velinova S (2005) Clinical evaluation of dentinal hypersensitivity treatment with 5 % potassium nitrate dentifrice. Folia Med (Plovdiv) 47(2):65–9
Bedi G, Pratibha PK, Bhat KM, Bhat GS (2011) Clinical and scanning electron microscopic evaluation of various concentrations of potassium nitrate as a desensitizing agent. Smile Dent J 6(1):28–35
Pashley DH, O’Meara JA, Kepler EE, Galloway SE (1984) Dentine permeability—effects of desensitizing dentrifices in vitro. J Periodontol 55:522–525
Greenhill JD, Pashley DH (1981) The effects of desensitizing agents on the hydraulic conductance of human dentin in vitro. J Dent Res 60(3):686–698
Wan-Hong L, Hsin-Cheng L (2009) Treatment of dentin hypersensitivity by Nd:YAG laser. J Clin Laser Med Surg 14(2):89–92
Ciaramicoli MT, Carvalho RCR, Eduardo CP (2003) Treatment of cervical dentin hypersensitivity using neodymium: yttrium–aluminum–garnet laser. Clin Eval Lasers Surg Med 33:358–362
H Deiab, K EL-Soudany (2007) Comparative study of the clinical effectiveness between Nd:YAG laser and gluma desensitizer in the treatment of dentin hypersensitivity. Cairo Dent J (23) Part (II): 193–200
Dilsiz A, Aydin T, Canakci V, Gungormus M (2010) Clinical evaluation of Er:YAG, Nd:YAG, and diode laser therapy for desensitization of teeth with gingival recession. Photomed Laser Surg 28(S2):11–17
Kerns DG, Scheidmt MJ, Pashley DH, Hoenes JA, Strong SL, Van Dyke TE (1991) Dentinal tubule occlusion and root hypersensitivity. J Periodontol 62:421–428
Prati C, Montebugnoli L, Suppa P, Valdre G, Mongiorgi R (2003) Permeability and morphology of dentine after erosion induced by acidic drinks. J Periodontol 74:428–436
Figueiredo M, Magalhães D et al (2004) A morphological in vitro study of the effects of Nd:YAG laser on irradiated cervical dentin. Photomed Laser Surg 22(6):527–532
Acknowledgments
We are thankful to Centrix Dental, Shelton, USA, for providing free samples of D/Sense® Crystal™, for the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talesara, K., Kulloli, A., Shetty, S. et al. Evaluation of potassium binoxalate gel and Nd:YAG laser in the management of dentinal hypersensitivity: a split-mouth clinical and ESEM study. Lasers Med Sci 29, 61–68 (2014). https://doi.org/10.1007/s10103-012-1239-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1239-4