Abstract
Iron deficiency impairs the formation of hemoglobin, red blood cells, as well the transport of oxygen. The wound healing process involves numerous functions, many of which are dependent on the presence of oxygen. Laser has been shown to improve angiogenesis, increases blood supply, cell proliferation and function. We aimed to study the effect of λ660 nm laser and λ700 nm light-emitting diode (LED) on fibroblastic proliferation on cutaneous wounds on iron-deficient rodents. Induction of iron anemia was carried out by feeding 105 newborn rats with a special iron-free diet. A 1 × 1 cm wound was created on the dorsum of each animal that were randomly distributed into seven groups: I, control anemic; II, anemic no treatment; III, anemic + L; IV, anemic + LED; V, healthy no treatment; VI, healthy + laser; VII, healthy + LED (n = 15 each). Phototherapy was carried out using either a diode laser (λ660 nm, 40 mW, 10 J/cm2) or a prototype LED device (λ700 ± 20 nm, 15 mW, 10 J/cm2). Treatment started immediately after surgery and was repeated at 48-h interval during 7, 14, and 21 days. After animal death, specimens were taken, routinely processed, cut, stained with hematoxylin-eosin, and underwent histological analysis and fibroblast counting. Significant difference between healthy and anemic subjects on regards the number of fibroblast between treatments was seen (p < 0.008, p < 0.001). On healthy animals, significant higher count was seen when laser was used (p < 0.008). Anemic subjects irradiated with LED showed significantly higher count (p < 0.001). It is concluded that the use of LED light caused a significant positive biomodulation of fibroblastic proliferation on anemic animals and laser was more effective on increasing proliferation on non-anemics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of iron deficiencies is very high in developing countries, iron deficiency anemia being the most common of such disorders worldwide. This disease develops itself due to a low ingestion of iron. However, iron deficiency anemia co-exists with other conditions such as vitamin A deficiency, undernourishment, folate deficiency, and infections [1]. This anemia may be considered the last stage of a long period of insufficient iron intake impairing the formation of hemoglobin, which contains iron, by the organism making difficult the formation of red blood cells [1, 2].
Three stages of development of the disease are necessary for its clinical manifestation. On the first stage, there is the depletion of the iron that affects its deposit on the body and represents the period of a more intense vulnerability on relation to the marginal balance of iron, and it may progress to a more severe deficiency. The second stage is known as iron-deficient erythropoiesis that is characterized by biochemical changes that reflects the insufficient level of iron, making the normal and regular production of hemoglobin not possible. This is also seen on other iron composites found on the organism, even when the concentration of hemoglobin is not reduced. The last stage is characterized by the reduction of the level of hemoglobin that causes a severe unbalance of the organism. As much reduced, the level of hemoglobin more severe the anemia is [1, 2].
Wound healing is a complex process that involves the occurrence of many phenomena. Most of these phenomena depend upon the presence of oxygen. The development of epithelial tissue depends on both the level of hydration and oxygenation the injured site. Angiogenesis is very important on the healing process and tissue hypoxia has been point out as a critical trigger on the transcription of angiogenic factors. Both production and maturation of the collagen matrix influence the tensile strength of the healing tissue and it is directly related to the partial pressure of the oxygen on the tissue [3, 4]. The healing deficiency observed on anemic subjects is due to the insufficient delivery of oxygen to the tissue as well as to the reduction of the levels of hemoglobin.
Laser phototherapy (LPT) is a non-invasive treatment modality that has been shown very helpful on the healing wounds being capable of increasing both the speed and quality of the healing process. Increasing use of LPT has been shown efficacious on positively modulating wound healing by stimulating mitotic activity, increased number of fibroblasts, increased deposition of collagen matrix, increased angiogenesis, and others [5, 6].
Low-energy laser irradiation has documented benefits in promoting the healing of hypoxic, ischemic, and infected wounds. In comparison to lasers, light-emitting diode (LED) technology generates negligible amounts of heat. It is clinically proven to be safe and has achieved nonsignificant risk status for human trials by the Food and Drug Administration. LED technology has been shown to be an effective alternative for the treatment of both cutaneous and mucosal wounds [7].
LEDs present a relatively narrow emission spectrum that may be optimally tuned to correspond to treatment’s requirement and thus eliminate unnecessary wavelengths for the therapy; they allow adjustment of light intensity and produce high light levels with low radiant heat output maintaining useful output for long periods of time. LED-based devices may provide a homogenous light dose in optimal intensity [8].
While lasers provide tissue stimulation, which increase cellular activity during wound healing, they have limitation in wavelength. It is difficult for lasers to produce the efficient wavelength combination optimal for wound healing. The size of wounds that may be treated by the small beam width of laser is also limited. In contrast, LEDs allow the control of spectral composition and may be arranged in flat arrays of all sizes for the treatment of either small or large areas. LEDs offer an effective alternative to the use of conventional light sources [8].
It is known that some wavelengths have the ability to stimulate cell proliferation, including the fibroblasts; and these cells have the capacity to secrete collagen, a main organic component observed during repair [9, 10].
We were not able to find any previous report on the literature on the effects of light sources, such as laser and LEDs, on the healing process of surgical wounds on cases of iron deficiency anemia. The aim of the present investigation was to study the effect of two light sources, λ660 nm laser and λ700 nm LED light, on the fibroblastic proliferation of cutaneous surgical wounds on rodents suffering from iron deficiency anemia.
Materials and methods
Following approval by the Animal research Ethical Committee of the School of Dentistry of the Federal University of Bahia, 105 newborn male Wistar rats (21 days old, ±50 g) were used on the present investigation [11]. The animals were kept under natural conditions of light, humidity, and temperature at the Animal House of Center of Biophotonics of the Federal University of Bahia during the experimental period. Iron deficiency anemia was induced by feeding the animals with a special pelleted iron-free diet (no iron-AIN 93-G, Rhoster, SP, Brazil) during 15 days [12]. Anemia was confirmed by blood testing. The animals were kept in groups of three on individual metallic gages; kept at day/night light cycle and temperature controlled during the experimental period.
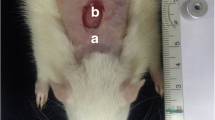
Under intramuscular general anesthesia (0.2 ml/100 mg of quetamine and 0.06 ml/100 g of xylazine), each animal had the dorsum shaven and the skin cleaned with 2 % chlorohexidine solution. A 1 × 1 cm full-thickness excisional wound was created on the dorsum of each animal with a no15 scalpel blade (Fig. 1). The depth of the wound was controlled by the cutting to the depth of the bevel of the scalpel blade that was 1 mm [13].
The animals were randomly distributed into seven groups: group I, control anemic (no wound, no treatment); group II, anemic no treatment (wound, no treatment); group III, anemic + laser; group IV, anemic + LED; group V, healthy no treatment; group VI, healthy + laser; group VII, healthy + LED, (n = 15 each group).
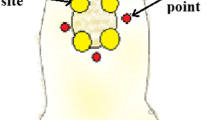
Phototherapy was carried out using either a diode laser (Twin Flex®, MMOptics, São Carlos, SP, Brazil) or a prototype LED device (RED-LED, λ700 ± 20 nm, Kondortech, São Carlos, SP, Brazil). When the laser was used, the energy was delivered to the tissue in four points around the wound (NSEW, 4 × 2.5 J/cm2). Due to the size of the probe of the LED device an adaptor was used to reduce the illuminated area to 1 cm2, and the energy was delivered by contact mode in a single point over the wound. The probes of both devices were covered with a single-use PVC film protection and placed directly in contact with the tissue. As we used two different light sources, we opted to use the energy as a comparative parameter between the two treatments. The parameters of the treatments may be seen on Table 1 . Treatment started immediately after surgery and was repeated at 48-h interval during 7, 14, and 21 days [13].
At the end of each experimental time, the animals were killed by an overdose of general anesthetics. The specimens were taken and fixed during 24 h on a 10 % formalin solution, routinely processed to wax, cut and stained with hematoxylin-eosin, and underwent histological analysis by light microcopy (Axiolab®, ZEISS, Germany) at the Laboratory of Surgical Pathology of the School of Dentistry of the Federal University of Bahia. For descriptive analysis, five slides from each group were used [13]. Fibroblast counting was used as assessment parameter. Each slide was divided into six size-standardized fields located just below the healing epithelium and counting was always carried out on the two central fields. The results were statistically analyzed using Minitab 15® software (Minitab, Belo Horizonte, MG, Brazil). Statistical analysis was carried out using Mann–Whitney test.
Results
Histological findings were as follows:
Untreated non-anemic animals showed, on day 7, wounds covered by keratinized squamous epithelium whose dermis showed, sometimes, spindle-shaped fibroblasts, collagen fibers arranged parallel to the surface, as well as congested blood vessels and discrete chronic inflammation. On day 14, the wounds were histologically similar to those seen on day 7. However, inflammatory cells were rarely seen. On day 21, the wounds were covered by keratinized epithelium with flat interface; on dermis, angled, and spindle-shaped fibroblasts and collagen fibers arranged not parallel to the surface were also seen (Fig. 2a).
Histophatological aspects of the healing. a Untreated non-anemic animals: wound covered by keratinized stratified squamous epithelium exhibiting atrophy whose dermis shows an intense interstitial edema, tortuous and congested blood vessels and a relatively fewer collagen fibers. A mild to moderate amount of mononuclear inflammatory cells were also seen. b Untreated anemic animals: wound showing ulceration covered by fibrinous necrosis and crust. The dermis showed a noncompletely organized granulation tissue in which fibroblasts were seen mostly parallel to the surface. Congested blood vessels were also seen. c LED treated non-anemic animals: wound covered by keratinized stratified squamous epithelium, whose dermis shows angular-shaped fibroblasts non-organized and congested and tortuous blood vessels amidst collagen fibers and rare mononuclear inflammatory cells. d Laser treated non-anemic animals: wound covered by keratinized stratified squamous epithelium, whose dermis shows a high number of spindle-shaped fibroblasts organized parallel to the surface. Extravased red blood cells were also seen on superficial dermis. e Anemic animals treated with LED: wound covered partially by keratinized stratified squamous epithelium exhibiting strong atrophy and ulceration recovered by thick crust, whose dermis shows a high number of spindle-shaped fibroblasts organized parallel to the surface amidst organized collagen fibers. Note absence of extravased red blood cells and rare inflammatory cells. f Anemic animals treated with laser: wound covered partially by keratinized stratified squamous epithelium exhibiting intense hyperplasia, whose dermis shows a moderate number of spindle-shaped fibroblasts organized parallel to the surface amidst organized collagen fibers. Note presence of tortuous blood red vessels eventually congested
Untreated anemic animals showed, on day 7, wounds covered by keratinized-stratified squamous epithelium exhibiting atrophy, crust, and fibrinoid necrosis. The dermis showed proliferating angular or oval-shaped fibroblasts, collagen fibers arranged parallel or not to the surface, and congested blood vessels. At both 14 and 21 days, the specimens showed wounds covered by keratinized epithelium exhibiting atrophy. The dermis showed oval- and spindle-shaped fibroblasts amidst collagen fibers arranged parallel to the surface. Blood vessels eventually congested were also seen (Fig. 2b).
LED-treated non-anemic animals showed, on day 7, the majority of surgical wounds covered by keratinized epithelium and, eventually, exhibiting ulceration and extensive epithelium migration. The dermis showed proliferation of oval and spindle fibroblasts and collagen fibers not parallel to the surface. Sometimes, congested blood vessels and mild chronic inflammatory infiltrate were seen scattered within the wound. On day 14, the majority of surgical wounds were covered by keratinized epithelium. The dermis showed fibroblasts and collagen fibers parallel to the surface. Amidst dermis, there were small blood vessels, and a few congested and discrete chronic inflammatory infiltrate. At the end of the experimental period, the specimens covered by epithelium of regular thickness whose dermis had dermal spindle-shaped fibroblasts and collagen fibers parallel to the surface (Fig. 2c).
Laser-treated non-anemic animals showed, on day 7, wounds covered by keratinized epithelium whose dermis showed oval- and spindle-shaped fibroblasts with collagen fibers arranged parallel to the surface, and discrete chronic inflammatory infiltrate. On day 14, the specimens showed the wounds covered similarly as described on previous period. On day 21, the specimens showed similar aspect to the specimens observed on day 7, but there was no inflammation (Fig. 2d).
Anemic animals treated with LED showed, on both days 7 and 14, wounds covered by keratinized epithelium. The dermis showed fibroblasts and collagen fibers arranged parallel to the surface. In both periods of time, inflammation was rarely seen, but congested blood vessels were present. At the end of the experimental period, the specimens showed the wounds covered as described on previous period. Angular and spindle-shaped fibroblasts with collagen fibers arranged parallel to the surface slightly were seen scattered on the dermis (Fig. 2e).
Anemic animals treated with laser, showed on day 7, that most specimens were covered by keratinized epithelium exhibiting atypical reactive. The dermis showed spindle-shaped fibroblasts among collagen fibers parallel to the surface. There were very congested blood vessels and mild to moderate chronic inflammatory infiltrate. On day 14, the specimens showed the wound covered by keratinized epithelium regularly distributed with dermis similarly distributed as described on the previous period. On day 21, the specimens showed the wounds covered by keratinized epithelium that showed dermis, fibroblasts, and collagen fibers generally arranged parallel to the surface. In addition, there were congested blood vessels (Fig. 2f).
Total mean counts of fibroblast during all the experimental time were as follow: 31 ± 8 (non-anemic controls), 44 ± 7 (laser-irradiated non-anemic), 34 ± 10 (LED-irradiated non-anemic), 23 ± 2 (anemic controls), 48 ± 6 (laser-irradiated anemic), and 63 ± 14 (LED-irradiated anemic). For all non-anemic subjects, the mean count was 37 ± 10 for the anemic 45 ± 19. A summary of the results may be seen on Table 2.
The statistical analysis showed significant difference between healthy and anemic subjects on regards the treatment (p < 0.008 and p < 0.001, respectively). On healthy subjects, significant higher counts of fibroblast were seen when the laser was used (p < 0.008). On the other hand, anemic subjects irradiated with LED showed significant higher results (p < 0.001).
On day 14, the healthy subjects treated with the laser showed significant differences (p < 0.05). On anemic animal, we found significant difference on days 7, 14, and 21 (p < 0.0001, p < 0.001, and p = 0.001, respectively) being the subjects irradiated with the LED the ones showing the highest results.
The Mann–Whitney test did not show significant differences between non-anemic animals. However, on anemic subjects compared to their controls, significant differences were observed on days 7, 14, and 21 treated with laser (p = 0.006, p = 0.007, and p = 0.005, respectively) or LED (p = 0.16, p = 0.03, and p = 0.01).
Discussion
The repair process is a normal physiological tissue response to injury and generally leads to the restoration of its morphology and function. Wound healing begins with an acute inflammation and is followed by vasodilatation and increased vascular permeability [3, 14]. These events are promoted by substances such as histamine that is one of the inflammatory mediators released by mast cells [14]. Cytochrome C oxidase is the target of incident photons inducing signals to cause cytosolic alkalinization. This leads to the Ca+2 increment, resulting in enhanced histamine release by mast cells activated by LPT [15]. LPT influences the inflammatory response within the first days following injury, with increase of the number of polymorfonuclear neutrophyls and mast cells, vasodilatation, and increased numbers of blood vessels, accelerating thus the inflammatory process and resulting in a faster healing of the wound [16].
Anemias are very common disorders worldwide and no previous studies were found evaluating the effect of laser and LED on the fibroblastic proliferation during wound healing in iron deficiency anemia cases. The present model is new and may provide a new option of studying the effects of phototherapies on patients suffering from systemic diseases.
It has been shown that LPT affects most cells involved on the healing wounds. A previous report showed that the use of IR (λ980 nm) laser light improves cell growth [17]. Our results are aligned with this study as healthy animals treated with laser light showed better results on regards the healing.
Some studies have shown that the use of λ630–1,000 nm laser or LED light, both in vivo and in vitro, causes a positive biomodulatory effect on the healing of wounds [10]. Our results indicate that the use of either laser or LED light causes an increased fibroblastic proliferation thus improving the healing process.
The correct dosimetric determination of wavelength, fluence, and irradiance, as well as for each clinical condition and specific cellular modulation, is of great relevance. It is known that, depending on the fluence delivered at the surface tissue, different tissue responses may be observed during the healing process [5, 18].
It seems possible that LED light presents beneficial effects similar to the ones observed when laser light is used as several reports on the benefits of the use of LEDs operating at several wavelengths have been published elsewhere both in vitro and in vivo on both normal and pathologic conditions [19]. It is also possible that the mechanism involved being also similar.
Due to distinct light beams, both LED and laser protocols were adjusted to provide the same energy for both kinds of equipment allowing us the comparison of the therapeutic effect of this different light source based upon a same parameter. The wide absorption window in biological tissues exists and the therapeutic action of both light sources has been observed [20]. On the present investigation, the same energy (10 J/cm2) was used for both treatments. A previous report [8] has indicated that studies involving the use of different light sources might use similar energy densities and power densities. Despite this, many reports are indicative that the use of some types of light results on improvement of healing [21]. Furthermore, LED light is a safe and low-cost option of treatment of wounds on skin and mucous membranes [7].
It is known that light coherence is lost in the first layers of the tissue [22]. In accordance with the positive stimulus to the fibroblastic proliferation found for both light sources in this study, several studies confirm the positive biomodulation by incoherent light [7, 23]. Our results suggest that the fibroblastic proliferation is probably more dependent on wavelength and fluence than on the coherence factor. The LED emission spectrum is broader than that of laser and, despite this feature differentiating the light sources a similar energy was used, but with a distinct spectral distribution. Laser was characterized by a higher energy density concentration in a smaller spectral range of 660 nm. LED energy density was distributed in a broader spectrum of 700 nm, possibly interacting with a higher number of specific photoreceptors [21].
Our results demonstrated that the use of either λ700 ± 20 nm LED or λ660 nm laser light caused stimulation of the fibroblastic proliferation on both anemic and healthy subjects. Many studies have shown that the use of LED light (λ630–1,000 nm) causes positive biomodulatory effects on many biological processes occurring during wound healing, including on the genic expression [23], angiogenesis [21], on the mitochondrial oxidative metabolism [24], and on the prevention and treatment of oral mucosites [25].
On regards, the effects of LED light on the proliferation of fibroblasts, a previous in vitro study showed that the use of λ625–635 nm LED light was capable of keeping the viability of human fibroblasts as well as its synthesis of collagen [26]. A previous study demonstrated also an increased fibroblastic proliferation on mice, in vitro, following these of a combination of different LED’s (λ670, 728, and 880 nm, 4 or 8 J/cm2, 50 mW/cm2) [7]. The study also showed that the stimulation occurred only on the growth phase and not occurred on the stationary phase of the cell cycle, indicating that LED irradiation does not generate an excessive growth or neoplastic transformation. We studied the effect of LED phototherapy of three distinct wavelengths on fibroblast on wound healing, on λ700 nm LED-irradiated subjects there was a marked amount of young fibroblasts that were paralleled located to the surface, on λ530 nm marked amount of fibroblasts was observed on 75 % of the cases and they were parallel to the wound surface and on λ460 nm illuminated subjects, marked amount of fibroblast was seen on 50 % and these were discretely organized on the dermis of the wound [27]. Our results are important as they deal with the effect of two light sources on both anemic and non-anemic animals. We were not able to find any previous reports on using this model in the literature.
Our results showed significant difference between healthy and anemic subjects on regards the treatment used. We also found that on healthy subjects, significant higher results on fibroblast proliferation were seen when the laser was used. On the other hand, anemic subjects irradiated with LED showed significant higher results when compared to non irradiated ones. On anemic animals, we found significant differences at days 7, 14, and 21, the subjects being irradiated with the LED the ones showing the highest counts. It is important to mention that we did not find significant differences between non-anemic animals. However, on anemic subjects compared to their controls, significant differences were observed at days 7, 14, and 21 treated with laser or LED. This is multistep study and further cellular and molecular assessment are planned in order to provide a more conclusive evidence of the effects of light on this model.
There are still controversies on regards the effects of some characteristics of the laser light such as the coherence. It has been shown that the biological effects of the light depend mostly on the specific relationship between the wavelength of the light and the photoreceptor molecule. Monochromaticity has been considered the main factor responsible for the positive stimulation of the tissues. Consequently, it has been proposed that the LED light, even with it spectral band, possesses similar effects to the laser light [7, 26, 28]. Karu [29] has suggested that high monochromaticity is not essential and that it is important that the bandwidth to be within the absorption band of the photoreceptor. We found that the fibroblastic proliferation on anemic animals treated with the red LED light showed better results than when the laser was used. This may have been caused by the fact that hemoglobin, photoreceptor molecule that is deficient on anemic subjects, absorbs better the LED light probably due to greater bandwidth when compared to the laser [22, 26, 28]. New ATP production occurs rapidly after LED photomodulation, triggering subsequent metabolic activity of fibroblasts [28].
This study showed positive biomodulatory effects of both laser and LED phototherapies when comparing irradiated and non-irradiated animals. LED (λ700 ± 20 nm) irradiation showed better results on anemic animals when compared to the use of laser light. On healthy animals, our results are aligned with previous reports when either laser [22, 30] or LED light were used [21, 31] on regards increased vasodilation and microcirculation. These phenomena are important for the nutritional support of the healing tissues. A previous report [31] demonstrated the stimulation of the angiogenesis by the upregulation of both vascular endothelial growth factor and nitric oxide synthase following the use of laser light (λ804 nm). Another research [21], by a comparative study using laser or LED, found no significant difference between the two protocols and suggested that the effect on angiogenesis could be attributed to the wavelength. This aspect may also be indicative that the same occurs on regards fibroblastic activity as found on the present investigation.
Based upon our results, it is concluded that the use ofλ700 ± 20 nm LED Light caused a significant positive biomodulation of the fibroblastic proliferation on anemic subjects and laser (λ660) irradiation was more effective on increasing proliferation on non-anemic animals.
References
Olivares M, Qalter T, Hertrampf E, Pizarro F (1999) Anaemia and iron deficiency disease in children. Br Med Bull 55:534–543
Almeida AP, Zandonade E, Abrantes MM, Lamounier JA (2004) Iron deficiency and anemia among children in Vitória, ES [Deficiência de ferro e anemia em crianças de Vitória, ES]. Pediatrics 26:140–150
Jonshon K, Jensen A, Goodson WH, Scheuenstuhl H, West J, Hopf HW, Hunt TK (1991) Tissue oxygenation, anemia, and perfusion in relation to wound healing in surgical patients. Ann Surg 214:605–613
Shweiki D, Neeman M, Itin A, Keshet E (1992) Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843–845
Medrado AP, Pugliese LS, Reis SRA, Andrade ZA (2003) Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Med Sci 32:239–244
Demir H, Menku P, Kirnap M, Calis M, Ikizceli I (2004) Comparison of the effects of laser, ultrasound, and combined laser + ultrasound treatments in experimental tendon healing. Lasers Surg Med 35:84–89
Whelan HT, Smits RL, Buchman EV, Whelan NT, Tuner SG, Margolis DA et al (2001) Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg 19:305–314
Barolet D (2008) Light-emitting diodes (LEDs) in dermatology. Semin Cutan Med Surg 27:227–238
Corazza AV, Jorge J, Kurachi C, Bagnato VS (2007) Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources. Photomed Laser Surg 25:102–106
Smith KC (2005) Laser (and LED) therapy is phototherapy. Photomed Laser Surg 23:78–80
Murray-Kolb LE, Takaiwa F, Goto F, Yoshihara T, Theil EC, Beard JL (2002) Transgenic rice is a source of iron for iron-depleted rats. J Nutr 132(5):957–960
Lobo AR, Gaievski EHS, Colli C (2011) Hemoglobin regeneration efficiency in anemic rats: effects on bone mineral composition and biomechanical properties. Biol Trace Elem Res 143:403–411
Pinheiro ALB Advances and perspectives on tissue repair and healing. Photomed Laser Surg. doi:10.1089=pho.2009.2716
Sawasaki I, Geraldo-Martins VR, Ribeiro MS, Marques MM (2009) Effect of low-intensity laser therapy on mast cell degranulation in human oral mucosa. Lasers Med Sci 24:113–116
Wu Z, Zhou Y, Chen J, Zhou L (2010) Mitochondrial signaling for histamine releases in laser-irradiated RBL-2 H3 mast cells. Lasers Med Sci 42:503–509
Pereira MCMC, Pinho CB, Medrado ARP, Andrade ZA, Reis SRA (2010) Influence of 670 nm low-level laser therapy on mast cells and vascular response of cutaneous injuries. J Photochem Photobiol 98:188–192
Skopin MD, Molitor SC (2009) Effects of near-infrared laser exposure in a cellular model of wound healing. Photodermatol Photoimmunol Photomed 25:75–80
Al-Watban FA, Andres BL (2003) Polychromatic LED therapy in burn healing of non-diabetic and diabetic rats. J Clin Laser Med Surg 21:249–258
Vinck EM, Cagnie BJ, Cornelissen MJ, Declercq HA, Cambier DC (2003) Increased fibroblast proliferation induced by light emitting diode and low power laser irradiation. Lasers Med Sci 18:95–99
Da Costa RS, Andersson H, Wilson BC (2003) Molecular fluorescence excitation-emission matrices relevant to tissue spectroscopy. Photochem Photobiol 78:384–392
Tachiara R, Farinelli WA, Anderson R (2002) Low intensity light-induced vasodilation in vivo. Lasers Surg Med 30:11
Whelan HT, Buchmann EV, Dhokalia A, Kane MP, Whelan NT, Wong-Riley MTT et al (2003) Effect of NASA light emitting diode irradiation on molecular changes for wound healing in diabetic mice. J Clin Laser Med Surg 21:67–74
Desmet KD, Paz DA, Corry JJ (2006) Clinical and experimental applications of NIR-LED photobiomodulation. Photomed Laser Surg 24:121–128
Corti L, Chiarion-Sileni V, Aversa S, Ponzoni A, D’Arcais R, Pagnuttis S (2006) Treatment of chemotherapy-induced oral mucositis with light emitting diode. Photomed Laser Surg 24:207–213
Huang P, Huang Y, Su M, Yang TY, Huang JR, Jiang CP (2007) In vitro observations on the influence of copper peptide aids for the LED photoirradiation of fibroblast collagen synthesis. Photomed Laser Surg 25:183–190
De Souza AP, Santos JN, Dos Reis JA Jr, de Souza J, Cangussú MC, Pinheiro ALB (2010) Effect of LED phototherapy of three distinct wavelengths on fibroblasts on wound healing: a histological study in a rodent model. Photomed Laser Surg 28:547–552
Weiss RA, McDaniel DH, Geronemus RG, Weiss MA (2005) Clinical experience with light emitting diode (LED) photomodulation. Dermatol Surg 31:1199–1205
Karu TI (1989) Photobiology of low-power laser effects. Health Phys 56:691–704
Ihsan FRM (2005) Low-level laser therapy accelerates collateral circulation and enhances microcirculation. Photomed Laser Surg 23:289–294
Lanzafame RJ, Stadler I, Whelan HT (2002) NASA LED photoradiation influences nitric oxide and collagen production in wounded rats. Lasers Surg Med 30(Suppl):14
Tuby H, Maltz L, Oron U (2006) Modulations of VEGF and INOS in the rat heart by low level laser therapy are associated with cardio protection and enhanced angiogenesis. Lasers Surg Med 38:682–688
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oliveira Sampaio, S.C.P., de C. Monteiro, J.S., Cangussú, M.C.T. et al. Effect of laser and LED phototherapies on the healing of cutaneous wound on healthy and iron-deficient Wistar rats and their impact on fibroblastic activity during wound healing. Lasers Med Sci 28, 799–806 (2013). https://doi.org/10.1007/s10103-012-1161-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1161-9