Abstract
The objective of this study was to evaluate the effects of chromium:yttrium-scandium-gallium-garnet (Er,Cr:YSGG) laser irradiation on the acid resistance of dental enamel. Forty human enamel samples were divided into four groups. They were manually irradiated with an Er,Cr:YSGG laser device (λ = 2.78 µm, 20 Hz, 20 s), in a scanning mode, with and without water cooling, according to the following parameters: Group 1: 0.25 W, 62.5 J/cm2, no water cooling; group 2: 0.25 W, 62.5 J/cm2, 5.0 ml/min; group 3: 0.5 W, 125 J/cm2, no water cooling; group 4: 0.5 W, 125 J/cm2, 5.0 ml/min. No airflow was used. Afterwards, the samples were submitted to an acid challenge and assessed by cross-sectional Knoop microhardness at different depths (20, 40, 60, 80, and 100 µm) from the outer enamel surface. Average values were obtained for both irradiated and control areas in each sample and they were compared to obtain a percentage of microhardness increase. Data were analyzed by analysis of variance and Fisher’s exact test (α = 5%). The percentage of microhardness increase observed in group 1 (+23.58%) was similar to group 3 (+19.12%), but higher than groups 2 (+3.61%) and 4 (10.9%) (p < 0.05). The comparison of the depths showed that the Er,Cr:YSGG laser acted in the superficial layers of the dental enamel. The findings of the present study suggest that the energy densities of 62.5 and 125 J/cm2 were capable of increasing the acid resistance of human enamel. The presence of water during irradiation makes it difficult to obtain an enamel surface more resistant to acids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental caries is one of the most prevalent worldwide diseases, affecting both children and adults, being the primary cause of oral pain and tooth loss [1]. The mechanism of dental caries involves acidogenic bacteria present in the oral cavity that produce organic acids when they metabolize fermentable carbohydrates. These acids diffuse through the dental biofilm and into the porous subsurface enamel, dissociating to produce hydrogen ions as they infiltrate the hard tissue [2]. These ions easily dissolve the mineral content of the tooth, releasing phosphate and calcium ions, which can diffuse out of the tooth. If this process (called demineralization) is not halted or reversed via the redeposition of mineral via saliva, it becomes a white-spot lesion, the first stage of dental caries [3]. At this stage, dental caries can be arrested and potentially reversed, but it is often not self-limiting and without proper care, caries can progress until the destruction of the teeth occurs [4].
The possibility of increasing the acid resistance of enamel after laser irradiation was first demonstrated in the 1970s using a CO2 laser [5]. Since then, studies have been conducted to verify the effects and interaction of different wavelengths with the dental hard tissues. Currently, it is known that the wavelengths emitted by the erbium and CO2 lasers have higher interaction with dental hard tissues, since they are highly absorbed by water and hydroxyapatite, two of the main components of the human enamel and dentin [6].
Given that enamel is 85% carbonated hydroxyapatite, with 12% water and 3% protein and lipid by volume [7], the use of wavelengths highly absorbed by water and hydroxyapatite together is expected to generate thermal changes in the enamel, which may be able to alter its structure chemically and/or morphologically [8].
The Er,Cr:YSGG (erbium, chromium:yttrium-scandium-gallium-garnet) laser works at a wavelength of 2.78 µm and, for that reason, has been investigated for caries-prevention purposes [9–14], even though they are applied to cavity preparation due to the mechanism of ablation [15]. However, for caries prevention, it is important that lasers do not ablate the treated surface, but change the enamel morphologically or chemically.
So, to avoid ablation of the target tissue, several studies have been performed with low energy densities and without water cooling [9–11]. However, the absence of water cooling can lead to carbonization of the irradiated tissue, which is not desirable [16]. Taking this into consideration, the aim of this study was to evaluate the effect of sub-ablative levels of Er,Cr:YSGG irradiation, with and without water cooling, on the acid resistance of enamel.
Materials and methods
Preparation of the samples
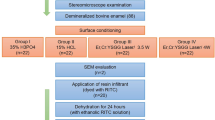
After authorization from the University of São Paulo - Ribeirao Preto School of Dentistry ethical committee, ten freshly extracted, non-erupted, third molars were collected and immediately stored for less than 1 month in 0.1% thymol solution (pH 7.0). Before the experimental procedures, the teeth were observed in a stereoscope. Those who had defects in enamel were rejected. The crowns were separated from the roots at the cement–enamel junction using a section machine (Minitom, Struers Inc., Westlake, OH, USA) with a diamond disk (Isomet; 10.2 cm × 0.3 mm, arbour size 1/2 in., series 15HC diamond; Buehler Ltd., Lake Bluff, IL, USA) at low speed. Each crown was then sectioned in a mesio-distal and in a buccal-lingual direction to obtain four fragments of dental enamel per crown, being one fragment selected for group 1, the other one for group 2, the other one for group 3, and the last one for group 4. This division of the samples was done for all teeth. This allowed the execution of all treatments on samples originated from the same tooth (n = 10). Subsequently, a 4 × 4 mm area in each of the 40 samples of tooth enamel was identified, with the bottom edge located 2.0 mm above of the cementoenamel junction. Around this defined area, two layers of varnish sealer (Colorama Maybelline Ltda, São Paulo, Brazil) were applied.
Experimental groups
In each sample, a 2 × 4 mm area was irradiated and the other 2 × 4 mm area, in the same sample, was used as control (no irradiation and no treatment). At the time of irradiation, the control areas were covered with a silver adhesive tape (Silver Tape 3939, 3M, St. Paul, MN, USA).
The samples of the experimental groups were irradiated immediately after removal from the distilled water in order to prevent drying out of the dental hard substance and any associated corruption of the results. The irradiations were done with an Er,Cr:YSGG laser device (Waterlase Millennium™, Biolase Technologies Inc., San Clemente, CA, USA). This equipment emits photons at a wavelength of 2.78 µm. The repetition rate was fixed at 20 Hz. The pulse duration was fixed on 140 µs. The beam diameter at the focal area for the handpiece was 600 µm. The tip was positioned 1.0 mm from the enamel surface (focused mode). To ensure consistent spot size with the hand irradiation, an endodontic file was fixed at the handpiece, and kept a distance of 1 mm from the surface during irradiation. The energy density per pulse used in each group is shown in Table 1. The handpiece was positioned perpendicularly to the enamel surface, and the samples were irradiated by hand once in each direction in a scanning mode, moving the handpiece slowly horizontally and vertically, in order to promote homogeneous irradiation and to cover the entire sample area. The irradiation was done for 20 s, 10 s vertically and 10 s horizontally, simulating a clinical condition [10]. The output power was measured with a power meter (TM- 744D, Tenmars Electronics Co. Ltd., Taipei, Taiwan). In groups 2 and 4, the water flow was set to 5.0 ml/min, which is the minimum amount this equipment permits. The airflow was always off.
As some past studies used the energy density per pulse, others used the total incident energy density that strikes the experimental area. In this way, we chose to put both of them in Table 1 to facilitate the comparison of the results obtained here with those found in other studies.
pH cycling
For the acid challenge, samples were submitted to a pH-cycling procedure, modified from previously described protocols [9, 15, 17, 18]. The demineralization solution (pH = 4.3) consisted of 2.0 mmol/L of Ca, 2.0 mmol/L of phosphate in buffer solution of acetate 0.075 mol/L, and the remineralization solution (pH = 7.0) consisted of 1.5 mmol/L of Ca, 0.9 mmol/L of phosphate, 150 mmol/L of potassium chloride. Each specimen was cycled in 5.0 ml of both solutions for 6 h in the demineralizing solution and 18 h in the remineralizing solution. This procedure was carried out for 14 days at 37°C. At the end of each 5 consecutive days of cycling, the samples were kept in remineralizing solution for 2 days.
Microhardness test
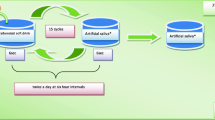
At the end of the pH cycling, the samples were sectioned longitudinally through the exposed area, so that the new fragment contained both the irradiated and the control area. The samples were embedded in epoxy resin, with the cut face exposed, using the methods reported by previous studies [19, 20]. The exposed enamel surfaces were polished using a 1200 silicon carbide grit paper (Buehler, Lake Bluff, IL), followed by a series of fine polishing alumina suspension slurries: 0.05 and 0.03 µm (Arotec, Sao Paulo-SP, Brazil). The specimens were washed separately in distilled water for 10 s before proceeding to the microhardness test, which was done using a Knoop indenter (HMV-2000 Shimadzu Corporation Kyoto, Japan). The indentations were done in enamel starting at 20 µm from the outer surface, with indents at 20 µm intervals between 20 µm and 100 µm from the anatomical surface. In each area, 3 horizontal indentations were done at the same depth, and the distance between measurements was 500 µm, to prevent that the marks overlap each other. Figure 1 outlines this procedure. A static load of 25 g/ 10 s was applied. This procedure was done in both control and experimental areas.
Initially, microhardness values were obtained at each depth for each individual sample. After, an average was obtained for each depth for both the irradiated and the non-irradiated area (control). Subsequently, the percentage of increase or decrease of Knoop microhardness was calculated.
Statistical analysis
The data were analyzed by two-way ANOVA followed by Fisher’s test. The level of significance was set at 5%. Data were analyzed using the software SPSS (SPSS for Windows 16, IBM, Armonk, NY, USA).
Results
Table 2 shows the values of Knoop microhardness (KHN) test for both control and lased area, and the percentage of alterations in microhardness at different depths. Positive percentages indicate that the irradiated enamel presented higher acid resistance than the non-irradiated area.
According to Table 2, group 1, irradiated with a power output of 0.25 W (62.5 J/cm2) and without water cooling, showed a KHN increase of 23.58% for the depth of 20 µm (p < 0.05). The depths represented by 40 and 60 µm in this same group also presented higher KHN (15.33 and 13.60%, respectively). In group 3, the Er,Cr:YSGG laser increased the acid resistance of the enamel located at 20 µm in 19.12% (p < 0.05). The samples irradiated in the presence of water cooling showed no increase in acid resistance when compared to the non-irradiated area.
Figure 2 shows the comparisons of KHN at each depth individually. For the non-irradiated surface (control), an average KHN was calculated based on measurements done in each depth of all groups. At 20 µm, group 1 presented higher KHN (270 ± 12.2) than the control group and groups 2 and 4 (p < 0.05). At the same depth, group 3 also showed higher microhardness than the non-irradiated group (p < 0.05). When the depth of 40 µm was analyzed, it could be observed that group 1 (270 ± 8.5) showed higher KHN than the non-irradiated group (p < 0.05), but it was similar to the other experimental groups. At 60 µm, the KHN of the group 1 was higher than that of the control group (271 ± 9.5; p < 0.05). For depths of 80 and 100 µm, there were no statistically significant differences among the groups (p > 0.05).
Discussion
Erbium lasers (Er:YAG and Er,Cr:YSGG) have been introduced in dentistry primarily to be used for cavity preparation and caries removal. Over time, researchers thought about the possibility of using this wavelength to promote chemical changes in the enamel surface to create a structure resistant to the caries process. In this way, numerous in vitro experiments were able to verify the potential of this wavelength for increasing the acid resistance of dental enamel [9–13]. For caries prevention, dental enamel is not intended to be ablated or melted. So, unlike the high parameters used for cavity preparation to promote chemical modification of the irradiated surface, it is necessary to use sub-ablative parameters.
The results obtained in the present study showed that the Er,Cr:YSGG laser was able to increase the enamel acid resistance in 23% compared to the control surface. This result was obtained when the lowest possible output power (0.25 W) and the lowest energy density (62.5 J/cm2) were used without air/water cooling. The samples of the groups 1 and 3 showed higher KHN than the control group (p < 0.05) for the depth of 20 µm. Group 1 also presented higher KHN than the non-irradiated area for the depths of 40 and 60 µm. It can be noted in Table 2 that the microhardness was increased in about 10% for some depths. The percentage of increase of acid resistance shown in Table 2 was calculated based on the average KHN of each depth. Thus, despite the high increase of hardness in some depths, the standard deviation was also high in those cases. So, these results were not statistically significant.
Previous studies showed that the Er,Cr:YSGG laser promotes an increase in enamel acid resistance by heating the surface during irradiation, which leads to structural and chemical changes in that dental hard tissue [11, 14, 21]. It is currently not known whether other interactions between light and matter occur, such as photochemical effects or nonlinear interactions, thus leading to changes within the enamel. Thermal changes in dental enamel and the associated effects on acid solubility have already been investigated and also serve to explain the effect of laser radiation. According to Perhavec and Diaco [22], even at low energy densities, the temperature at the superficial layers of the dental enamel reaches values above 400°C during irradiation. This temperature decreases to 250°C 2.5 ms after Er,Cr:YSGG laser irradiation and the enamel surface reaches the ambient temperature 82.5 ms after laser application [22]. To use the Er,Cr:YSGG laser to prevent acid demineralization, the temperature at the enamel surface should not exceed 600°C during irradiation. If this situation occurs, the dental tissue will be ablated, which does not agree with the principles already mentioned here [22].
The increase in the enamel’s acid resistance after laser irradiation is attributed to photothermal effects that occur when the temperature in the enamel surface rises from 100 to 650°C and promote chemical alterations in that dental hard tissue. In this situation, the main chemical changes that occur in the tooth are that the major CO3 component in the phosphate position decreases and the acid phosphate ions condense to form pyrophosphate ions. At 650–1,100°C, the main changes are thermal re-crystallization and crystal size growth, and pyrophosphate reacts with apatite to form PO4 along with the formation of β-TCP (tri-calcium-phosphate); at temperatures above 1,100°C, the main change is that the β-TCP is converted to α-TCP, and when the temperature reaches 1,430°C, this compound changes into a high-temperature polymorph. TCP α and β are potentially soluble in an acid environment [23, 24].
Another theory could explain the increase in acid resistance of enamel irradiated with erbium lasers. This hypothesis proposed that the partial decomposition of the organic matrix of the enamel during irradiation leads to a blockage of the inter- and intraprismatic spaces. Consequently, ion diffusion in enamel is compromised, which results in the retardation of enamel demineralization [25]. This effect occurs when the enamel surface reaches temperatures near 400°C. Similar results obtained by other authors support this theory [26].
In this way, it is important not only to increase the acid resistance of the superficial layers of the enamel but also inhibit the diffusion of the acids into the tissue. Probably even a little change in the enamel surface can block the inter- and intraprismatic spaces, preventing the penetration of the acids trough deeper layers of the enamel. The results of the present study suggest that the action of the demineralizing solution was higher in the superficial layers of enamel. Thus, the deeper layers do not have much contact with the acid solution and therefore they are not significantly demineralized. Similarly, the laser action occurs in the superficial layers of the enamel. As the deeper layers of enamel located more than 60 µm from the outer surface of the tissue were not significantly affected by the demineralizing solution, they presented similar KHN values with the layers located under the irradiated surface at the same depth. The percentage of KHN increase found in 60 µm (group 1) probably occurred because the demineralization solution reached that depth in the non-irradiated area, reducing the KHN. In the same group, it is possible that the laser has decreased the porosity of the enamel enough to inhibit a deeper diffusion of the acid solution in the irradiated area, since group 1 showed the most important increase in acid resistance. In the present study, the acid challenge was performed for 14 days. Thus, it is unclear whether these results would be different if the cariogenic challenge were performed for a longer period. Thus, it is important to conduct studies about this issue and about the effects of Er,Cr:YSGG in the enamel permeability.
An in vitro study demonstrated that when enamel surfaces are irradiated with both Er:YAG and Er,Cr:YSGG lasers with a fluence of 8 J/cm2, the temperature at the tissue surface increases enough to change the chemical structure of the enamel, turning it into a less-soluble structure [13]. That energy density is also capable of promoting melting areas and small spots of carbonization on the enamel surface [14]. According to de Freitas et al. [11], the Er,Cr:YSGG laser irradiation with 8.5 J/cm2 led to an increase in enamel acid resistance, comparable to the cariostatic effect obtained with the NaF dentifrice-treated samples, showing 64% caries inhibition [27].
Although there were no statistically significant differences from the other parameters used, we observed that the energy density of 62.5 J/cm2 appears to provide more favorable results. This suggests that the fluence of 125 J/cm2 leads to a decrease in enamel acid resistance, and this situation is possible because higher parameters increase the chance of tissue ablation or even promote cracks in the enamel surface [10]. The development of cracks in the tooth surface would make erbium laser irradiation unsuitable for use in caries prevention, since the aim of prophylactic treatment is to preserve the tissue integrity, and not to destroy it.
For this reason, the possibility of using water during irradiation was considered. The researchers were aware that the presence of high amounts of water hindered the preventive effect promoted by the Er,Cr:YSGG laser [27]. Previous studies showed that the presence of water in great amounts during irradiation increases the chance of ablation as well as the porosity of the dental surface [28, 29]. This situation facilitates the diffusion of acids into the enamel structure, increasing the depth of demineralization. Hossain et al. [30] investigated the influence of water cooling on the caries-preventive effect of laser radiation and found that the samples irradiated without water mist showed a higher Ca2+ content when compared to the samples irradiated with water cooling. These authors did not mention the amount of water used, making it difficult to compare theier data to the results obtained in the present study [30]. However, we thought about the possibility of using a minimum quantity of water (5.0 ml/min) in order to decrease the chance of occurrence of thermal damage and enamel ablation as well. Thus, it was noted that none of the samples had visible carbonization spots but, on the other hand, the presence of water, even in minimal amounts, was not so favorable for the preventive effect of the laser irradiation, as observed in groups 2 and 4.
According to previous studies, the absorption coefficients for the Er:YAG laser are approximately 150 mm−1 in enamel, and 200 mm−1 in dentin. The absorption coefficients for the Er,Cr:YSGG laser are approximately three times lower. In this way, the Er,Cr:YSGG laser wavelength penetrates 21 µm in enamel [31, 32]. This situation can be noted in the present study, since groups 1 and 3 showed that percentages of alterations in enamel microhardness are higher in the superficial than in deeper layers, suggesting that the wavelength emitted by the Er,Cr:YSGG laser is absorbed mainly in the superficial layers of enamel, which reduces the risk of thermal damage to the pulp tissue.
However, the heating is not restricted to a depth of 21 µm, since heat-diffusion penetration depth is larger than the optical penetration depth. Heat distribution within the tissue is also created by conductive spreading of heat, i.e., heat diffusion into the surrounding tissue [22]. This could explain why the deeper layers of enamel (40 µm in the present study) irradiated with the conditions used in group 1 showed higher KHN than the control group.
In addition, the Er,Cr:YSGG laser was applied manually (scanning mode) in order to simulate a clinical condition. So even though the operator is properly trained to irradiate the dental tissue, due to the small spot size of the laser beam, it is possible that some microscopic areas of the enamel have not been irradiated. Maybe if the entire enamel surface had been irradiated in the same manner, the increase in acid resistance of the tissue could be more significant.
Thus, analyzing the results obtained in this study, it was possible to verify, after the Knoop microhardness test, that the Er,Cr:YSGG laser can increase the acid resistance of the human dental enamel. Moreover, the data showed that the presence of water, even in small amounts, should not be used during irradiation. Further studies should be performed in order to evaluate the permeability of irradiated enamel with sub-ablative parameters, as well as the morphological characteristics and chemical composition of that dental tissue. These data will help to define what would be the most correct theory to explain increasing the acid resistance of enamel irradiated with erbium lasers.
Conclusions
Taking into consideration the experimental conditions of the present research, it can be concluded that the irradiation with the Er,Cr:YSGG laser was able to increase acid resistance of the superficial layers of enamel, while the power output of 0.25 W (62.5 J/cm2) without air/water cooling showed better results.
References
Kidd EA, Fejerskov OE (2004) What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms. J Dent Res 83:C35–C38
Featherstone JD, Rodgers BE (1981) Effect of acetic, lactic and other organic acids on the formation of artificial carious lesions. Caries Res 15:377–385
Featherstone JD (2000) The science and practice of caries prevention. J Am Dent Assoc 131:887–899
Selwitz RH, Ismail AI, Pitts NB (2007) Dental caries. Lancet 369:51–59
Stern RH, Vahl J, Sognnaes RF (1972) Lased enamel: ultrastructural observations of pulsed carbon dioxide laser effects. J Dent Res 51:455–460
Bader C, Krejci I (2006) Indications and limitations of Er:YAG laser applications in dentistry. Am J Dent 19:178–186
Theuns HM, Arends J, Groeneveld A (1980) Polarizing microscopy and microradiography of sound enamel. J Biol Buccale 8:229–238
Bevilacqua FM, Zezell DM, Magnani R, Ana PA, Eduardo CP (2008) Fluoride uptake and acid resistance of enamel irradiated with Er:YAG laser. Lasers Med Sci 23:141–147
Apel C, Birker L, Meister J, Weiss C, Gutknecht N (2004) The caries-preventive potential of sub-ablative Er:YAG and Er:YSGG laser radiation in an intraoral model: a pilot study. Photomed Laser Surg 22:312–317
Apel C, Meister J, Götz H, Duschner H, Gutknecht N (2005) Structural changes in human dental enamel after sub-ablative erbium laser irradiation and its potential use for caries prevention. Caries Res 39:65–70
de Freitas PM, Rapozo-Hilo M, Eduardo CP, Featherstone JD (2010) In vitro evaluation of erbium, chromium:yttrium-scandium-gallium-garnet laser-treated enamel demineralization. Lasers Med Sci 25:165–170
Apel C, Meister J, Schmitt N, Gräber HG, Gutknecht N (2002) Calcium solubility of dental enamel following sub-ablative Er:YAG and Er,Cr:YSGG laser irradiation in vitro. Lasers Surg Med 30:337–341
Fried D, Featherstone JDB, Visuri SR, Seka WTJ (1996) The caries inhibition potential of Er:YAG and Er:YSGG laser radiation. Proc SPIE 2672:73–78
Ana PA, Zezzel DM, Blay CC, Blay A, Eduardo CP, Miyakawa W (2004) Thermal analysis of dental enamel following Er,Cr:YSGG laser irradiation at low fluencies. Lasers Surg Med 34(16):53–53
Geraldo-Martins VR, Lepri CP, Palma-Dibb RG (2012) Effect of different root caries treatments on the sealing ability of conventional glass ionomer cement restorations. Lasers Med Sci 27:39–45
Geraldo-Martins VR, Tanji EY, Wetter NU, Nogueira RD, Eduardo CP (2005) Intrapulpal temperature during preparation with the Er:YAG laser: an in vitro study. Photomed Laser Surg 23:182–186
Featherstone JD (1996) Modeling the caries-inhibitory effects of dental materials. Dent Mater 12:194–197
Rehder Neto FC, Maeda FA, Turssi CP, Serra MC (2009) Potential agents to control enamel caries-like lesions. J Dent 37:786–790
Featherstone JD, ten Cate JM, Shariati M, Arends J (1983) Comparison of artificial caries-like lesions by quantitative microradiography and microhardness profiles. Caries Res 17:385–391
ten Cate JM, Shariati M, Featherstone JDB (1985) Enhancement of salivary remineralization by “dipping” solutions. Caries Res 19:335–341
Moslemi M, Fekrazad R, Tadayon N, Ghorbani M, Torabzadeh H, Shadkar MM (2009) Effects of Er,Cr:YSGG laser irradiation and fluoride treatment on acid resistance of the enamel. Pediatr Dent 31:409–413
Perhavec T, Diaci J (2009) Comparison of heat deposition of Er:YAG and Er,Cr:YSGG lasers in hard dental tissues. J Laser Health Acad 2:1–6
Fowler BO, Kuroda S (1986) Changes in heated and in laser irradiated human tooth enamel and their probable effects on solubility. Calcif Tissue Int 38:197–208
Featherstone JDB, Fried D (2001) Fundamental interactions of lasers with dental hard tissues. Med Laser Appl 16:181–194
Hsu CY, Jordan TH, Dederich DN, Wefel JS (2000) Effects of low-energy CO2 laser irradiation and organic matrix on inhibition of enamel demineralization. J Dent Res 79:1725–1730
Ying D, Chuah GK, Hsu CY (2004) Effect of Er:YAG laser and organic matrix on porosity changes in human enamel. J Dent 32:4l–46l
Hossain M, Kimura Y, Yamada Y, Nakamura Y, Yamada Y, Kinoshita JI, Matsumoto K (2001) A study on acquired acid resistance of enamel and dentin irradiated by Er,Cr:YSGG laser. J Clin Laser Med Surg 19:159–163
Visuri SR, Walsh JT Jr, Wigdor HA (1996) Erbium laser ablation of dental hard tissue: effect of water cooling. Lasers Surg Med 18:294–300
Olivi G, Angiero F, Benedicenti S, Iaria G, Signore A, Kaitsas V (2010) Use of the erbium, chromium:yttrium-scandium-gallium-garnet laser on human enamel tissues. Influence of the air–water spray on the laser-tissue interaction: scanning electron microscope evaluations. Lasers Med Sci 25:793–797
Hossain M, Nakamura Y, Kimura Y, Yamada Y, Ito M, Matsumoto K (2000) Caries-preventive effect of Er:YAG laser irradiation with or without water mist. J Clin Laser Med Surg 18:61–65
Majaron B, Sustersic D, Lukac M, Skaleric U, Funduk N (1998) Heat diffusion and debris screening I Er:YAG laser ablation of hard biological tissues. Appl Phys B 66:1–9
Ivanov B, Hakimian AM, Peavy GM, Haglund RF (2003) Mid-infrared laser ablation of hard biocomposite material: mechanistic studies of pulse duration and Interface effects. Appl Surf Sci 208–209:77–84
Acknowledgements
The authors are grateful to the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil, for providing financial support (grant # 2007/08140-5 and 2008/07403-5). The authors would like to thank The Special Laboratory on Lasers in Dentistry, School of Dentistry, São Paulo University, Brazil (LELO-FOUSP), for allowing the use of the Er,Cr:YSGG laser device.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geraldo-Martins, V.R., Lepri, C.P. & Palma-Dibb, R.G. Influence of Er,Cr:YSGG laser irradiation on enamel caries prevention. Lasers Med Sci 28, 33–39 (2013). https://doi.org/10.1007/s10103-012-1056-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1056-9