Abstract
The aim of the present study was to determine the effect of low-level laser therapy (LLLT) on the expression of TNF-α and TGF-β in the tibialis anterior muscle of rats following cryoinjury. Muscle regeneration involves cell proliferation, migration and differentiation and is regulated by growth factors and cytokines. A growing body of evidence suggests that LLLT promotes skeletal muscle regeneration by reducing the duration of acute inflammation and accelerating tissue repair. Adult male Wistar rats (n = 35) were randomly divided into three groups: control group (no lesion, untreated, n = 5), cryoinjury without LLLT group (n = 15), and cryoinjury with LLLT group (n = 15). The injured region was irradiated three times a week using an AlGaInP laser (660 nm; beam spot 0.04 cm2, output power 20 mW, power density 500 mW/cm2, energy density 5 J/cm2, exposure time 10 s). Muscle remodeling was evaluated at 1, 7 and 14 days (long-term) following injury. The muscles were removed and total RNA was isolated using TRIzol reagent and cDNA synthesis. Real-time polymerase chain reactions were performed using TNF-α and TGF-β primers; GAPDH was used to normalize the data. LLLT caused a decrease in TNF-α mRNA expression at 1 and 7 days following injury and in TGF-β mRNA expression at 7 days following cryoinjury in comparison to the control group. LLLT modulated cytokine expression during short-term muscle remodeling, inducing a decrease in TNF-α and TGF-β.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal muscle is a dynamic tissue with an extraordinary capacity for repair following injury. Thus, there is considerable interest in skeletal muscle regeneration in situations such as exercise-induced muscle injury, muscle transplantation and muscular dystrophy, as well as the recovery of strength following atrophy due to disuse [1–3]. The enhancement of muscle regeneration and the prevention of muscle fibrosis are the main objectives in improving muscle healing following injury [4].
Muscle repair is a complex process that includes three phases: (1) degeneration and inflammation, (2) muscle regeneration, and (3) fibrosis. A number of molecules and cellular events are essential to the activation of leukocytes and the formation of new blood vessels during the repair process, such as vascular endothelial growth factor, tumor necrosis factor (TNF) and transforming growth factor (TGF) [5, 6]. TNF-α is a potent proinflammatory cytokine that influences various cell types and the chronic inflammatory process by recruiting polymorphonuclear cells and other immune cells, thereby contributing to the amplification and progression of the inflammatory process [7, 8]. TGF-β is known to be the most potent growth factor involved in wound healing throughout the body. Released by degranulated platelets at the site of injury, TGF-β influences the inflammatory response, angiogenesis, re-epithelialization, extracellular matrix deposition and remodeling [9]. In skeletal muscle, members of the TGF-β superfamily have been shown to have potent effects on both muscle development and postnatal skeletal muscle mass by modulating myoblast activity and inhibiting both proliferation and differentiation [10, 11].
Low-level laser therapy (LLLT) has demonstrated favorable effects in modulating the inflammatory response and tissue repair [3, 12–15]. In muscle and tendon injuries, LLLT has been shown to reduce the duration of acute inflammation and accelerate tissue repair [1].
The aim of present study was to determine the effects of LLLT on the expression of TNF-α and TGF-β during muscle repair following cryoinjury in rats.
Materials and methods
Methods
The experimental procedures used in this study were in compliance with the principles of laboratory animal care formulated by the Brazilian College of Animal Experimentation (COBEA) and received approval from the Ethics Committee of the Universidade Nove de Julho São Paulo-SP, Brazil (process number 13/2007).
Adult male Wistar rats (n = 45), weighing 234 ± 37.99 g at the beginning of the procedure were maintained under controlled conditions of room temperature (22°C) and relative humidity (40%), with a 12-h light/dark cycle. The animals were offered solid ration and water ad libitum prior to and throughout the experimental period. The animals were randomly divided into three groups: control group (n = 5, without injury, untreated), cryoinjury without LLLT group (n = 15), and cryoinjury with LLLT group (n = 15). Animals in the control group were killed 1 day after the beginning of the experiment. The cryoinjury groups with and without LLLT were evaluated at 1, 7 and 14 days following injury. Animals evaluated at 1 and 7 days comprised the short-term muscle remodeling groups, and those evaluated at 14 days comprised the long-term muscle remodeling group.
Surgical procedures were performed based on those described by Miyabara et al [16], under anesthesia with 1 ml/kg of 1% ketamine HCl (Dopalen, Vetbrands, Sao Paulo, Brazil) and 2% xylazine (Anasedan, Vetbrands, Sao Paulo, Brazil). The tibialis anterior (TA) muscle was surgically exposed and submitted to the cryoinjury procedure. A round metal probe (3 mm in diameter) that had been cooled with liquid nitrogen was applied to the surface of the exposed TA and maintained in this position for 10 s. After the frozen muscle had thawed, the procedure was repeated on the same area for an additional 10 s.
The injured area was macroscopically identified as a firm, white, disk-shaped region. In the cryoinjury groups, only the left TA muscle was injured and the right side served as the control. The surgical wounds were closed with polyamide sutures and the animals were kept for several hours on a warm plate (37°C) until they had recovered from the effects of the anesthesia in order to prevent hypothermia.
Laser irradiation
The laser device used in this study was an aluminum gallium indium phosphide (AlGaInP) diode laser (MMOptics, São Carlos, SP, Brazil), with a beam spot of 0.04 cm2, an output power of 20 mW, a wavelength of 660 nm, an energy density of 5 J/cm2, a power density of 500 mW/cm2 and an exposure time of 10 s. The laser beam was placed in contact with the skin surface corresponding to the cryoinjured area and radiation was applied to eight points within the area (Fig. 1). The energy per point was 0.2 J, giving a total of 1.6 J per treatment. A LaserCheck power meter (Coherent, Santa Clara, CA) was used to determine the output of the equipment. The experiments were performed with standardized procedures. Laser irradiation was initiated 24 h following the injury and performed three times a week with a 24-h interval between sessions, giving a total of three sessions for the muscles evaluated at 7 days and six sessions for muscles evaluated at 14 days.
Animals were killed with an overdose of ketamine and xylazine. The left and right TA muscles were removed, weighed and immediately frozen in liquid nitrogen.
Total RNA isolation
Frozen TA muscle tissue was homogenized and total RNA was isolated using cold TRIzol reagent (Invitrogen, Carlsbad, CA), following the manufacturer’s instructions. Total RNA was quantified by spectrophotometry and RNA samples were treated with DNAse (Invitrogen) to avoid contamination with genomic DNA. All solutions were prepared with 0.01% diethyl pyrocarbonate-treated water (Sigma, St Louis, MO), while glassware and plasticware were treated against RNase using standard procedures.
cDNA synthesis and real-time PCR
For cDNA synthesis and real-time polymerase chain reaction (PCR) analysis of gene expression, 1 µg of total RNA was used. Contaminating DNA was removed using DNase I (Invitrogen) at a concentration of 1 U/μg RNA in the presence of 20 mM Tris-HCl, pH 8.4, containing 2 mM MgCl2 for 15 min at 37°C, followed by incubation at 95°C for 5 min for enzyme inactivation. Reverse transcription was carried out in a 200-μl reaction mixture in the presence of 50 mM Tris-HCl, pH 8.3, 3 mM MgCl2, 10 mM dithiothreitol, 0.5 mM dNTPs and 50 ng of random primers with 200 U of Moloney murine leukemia virus-reverse transcriptase (Invitrogen). The reactions conditions were 20°C for 10 min, 42°C for 45 min and 95°C for 5 min.
Real-time PCR was accomplished using a SYBRGreen kit (Applied Biosystems, Foster City, CA) on a 7000 sequence detection system (ABI Prism; Applied Biosystems). The thermal cycling conditions were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Experiments were performed in triplicate for each data point. TNF-α and TGF-β mRNA abundance was quantified as a relative value compared with an internal reference (GAPDH), the abundance of which was believed not to change between the different experimental conditions. The primers used for real-time PCR were as follows: GAPDH (GenBank accession number NM 017008) forward primer 5′-TGCACCACCAACTGCTTAGC-3′ and reverse primer 5′-GCCCCACGGCCATCA-3′; rat TNF-α (GenBank accession number X66539) forward primer 5′-AAATGGGCTCCCTCTATCAGTTC-3′ and reverse primer 5′-TCTGCTTGGTGGTTTGCTACGAC-3′; rat TGF-β (GenBank accession number NM 021578.2); sense: 5′-CCCCTGGAAAGGGCTCAACAC-3′; and antisense: 5′-TCCAACCCAGGTCCTTCCTAAAGTC-3′. The volume of the reverse transcription reaction mixture for real-time PCR was 1 µl.
Quantitative values for TNF-α and TGF-β and GAPDH mRNA transcription were obtained from the threshold cycle (Ct) number at which the increase in the signal associated with an exponential growth of PCR products began to be detected. Melting curves were generated at the end of every run to ensure product uniformity. The relative target gene expression level was normalized on the basis of GAPDH expression as an endogenous RNA control. ∆Ct values of the samples were determined by subtracting the average Ct value of integrin-linked kinase mRNA from the average Ct value of the internal control GAPDH. As it is uncommon to use ∆Ct as a relative value due to its logarithmic characteristic, 2−∆Ct was used to express the relative expression data.
Statistical analysis
TNF-α and TGF-β mRNA data are presented as mean values ± standard deviation (SD). The groups were compared using one-way analysis of variance (ANOVA), and the Tukey test was used to determine the significance of differences among all experimental groups. Values of p <0.05 were considered statistically significant. Data were analyzed using GraphPad Prism 4.0 statistical software (GraphPad Software, San Diego, CA).
Results
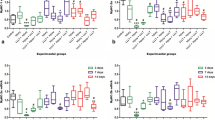
The results of the TNF-α mRNA analysis in the control group and the injured groups with and without LLLT are shown in Fig. 2. There was a significant decrease in TNF-α mRNA expression in the injured group with LLLT in comparison with the injured group without LLLT at 1 and 7 days following injury, and in comparison with the control group. In addition, at 7 days there was a significant increase in TNF-α mRNA expression in the injured group without LLLT in comparison with the other groups. At 14 days following injury there were no significant differences in TNF-α mRNA expression between the injured groups with and without LLLT or between these groups and the control group.
The results of the TGF-β mRNA analysis in the control group and the injured groups with and without LLLT are shown in Fig. 3. There was a significant decrease in TGF-β mRNA expression in the injured group with LLLT at 7 days following injury in comparison with the injured group without LLLT, in comparison with the injured with LLLT at 1 day following injury, and in comparison with the control group. In addition, there was an increase in TGF-β mRNA expression at 7 days in the injured group without LLLT in comparison with the other groups except the control group. At 14 days following injury, there were no significant differences in TGF-β mRNA expression between the injured groups with and without LLLT but there was a decrease in TGF-β mRNA expression in the injured group with LLLT in comparison with the control group.
Discussion
LLLT is used in many biomedical sciences in an attempt to modulate inflammatory process and promote tissue regeneration. In this study LLLT promoted a decrease in TNF-α mRNA expression at 1 and 7 days and a decrease in TGF-β mRNA expression at 7 days following muscle injury. These findings strongly suggest the positive influence of this kind of therapy on the muscle repair process.
The muscle regeneration process is divided into three distinct phases. In the inflammation phase, necrosis of damaged muscle fiber segments and phagocytosis of the necrotic debris are the main features. The goals of treatment in this phase are to limit the size of the hematoma and to avoid an excessive inflammatory reaction [17, 18]. In the repair or regeneration phase, the main feature is the proliferation of reserve satellite cells and endomysial fibroblasts, which is followed by active protein synthesis. Quiescent mononucleated muscle precursor cells (myoblasts, satellite cells) become activated, proliferate, differentiate and fuse to form young multinucleated muscle cells, known as myotubes. These myotubes then undergo further differentiation and mature to form fully functional muscle fibers [4]. These processes are controlled by various components of the disintegrated extracellular matrix and plasmalemma and by the availability of growth factors. The muscle healing, maturation or remodeling phase is characterized by a gradual recovery of the functional properties of the muscle, including the recovery of the tensile strength of its connective tissue component. The remodeling of the extracellular matrix provides structural support and protection and is important in maintaining the functional integrity of the fibers [19, 20].
This study indicated a short-term decrease in TNF-α and TGF-β mRNA expression during muscle healing . After a muscle is injured, tissue necrosis occurs followed by several stages of cell infiltration, generally characterized by early neutrophil invasion and a sequential increase in macrophages. Macrophages play a decisive role in the removal of necrotic tissue and, together with fibroblasts, produce complementary chemotactic signals (cytokines, growth factors and chemokines) to attract circulating inflammatory cells and proinflammatory cytokines, such as TNF-α, IL-1β, IL-6 and IL-8, which are important in the modulation of chemotaxis to the injured muscle [6]. Langen et al. [21] found that the administration of TNF-α induces muscle atrophy, demonstrating a causal role for this cytokine in inflammation-associated muscle wasting and demonstrated that myoblast differentiation and fusion are inhibited in response to inflammatory stimuli, including TNF-α [22].
Furthermore, muscle regeneration following muscle damage or during recovery from atrophy requires the activation of quiescent satellite cells, which enter the cell cycle and proliferate. This proliferative phase is followed by terminal differentiation and fusion of myoblasts to damaged muscle fibers leading to the repair of the fibers or to each other leading to the formation of new muscle fibers [11, 21, 23, 24]. Studies indicate that TGF-β and TNF-α prevent myogenic differentiation and could cause failure of skeletal muscle regeneration [11, 21]. TNF-α has been associated with decreased MyoD protein stability and abundance [22]. Thus, a decrease in the level of these factors could enhance the muscle repair process by facilitating muscle cell differentiation.
In agreement with the findings of the present study, other authors employing LLLT have reported differences in the inflammatory response of animals treated with laser radiation. Silveira et al. [13] found an elevated number of polymorphonuclear cells, especially mast cells, within the first hours of the experiment, followed by decreased levels on day 3 after LLLT and, consequently, in cytokine levels. In inflamed bronchial smooth muscles in rats, LLLT significantly decreased the expression of TNF-α mRNA in comparison with bronchial smooth muscle segments incubated with lipopolysaccharide without any laser treatment [25]. In another study, laser irradiation at both 660 nm and 684 nm wavelengths of inflamed rat paw tissue at a dose of 7.5 J/cm2 reduced the expression of TNF-α, IL-1β and IL-6 mRNA within 3 h in the irradiated tissue [26]. The decrease in TNF-α mRNA expression observed in injured group with LLLT at 1 and 7 days (early phase of inflammation) induced a decrease in the recruitment of polymorphonuclear cells and, consequently, in the progression of the inflammatory process. Thus, LLLT could contribute to a faster resolution of muscle repair.
We used the same muscle injury model in a previous study (in press). The results of the morphological analysis revealed that the nontreated and cryoinjury LLLT groups exhibited similar morphological aspects throughout skeletal muscle repair process. At 1 day following injury, the skeletal muscle exhibited neutrophil infiltration with edema and necrotic fibers. At 7 days, the tissue exhibited a reduction in inflammatory infiltration, with the appearance of immature skeletal muscle cells. At 14 days, there was a decrease in inflammatory infiltration and the fibers exhibited an increase in diameter, but the majority of fibers had a centrally located nucleus. At 21 days, most cells had a mature appearance. These results are in agreement with the present findings regarding laser-induced changes in the expression of TGF-β and TNF-α, as there was a decrease in both cytokines at 7 days in injured group with LLLT, with the beginning of a decrease in inflammatory cell infiltration, along with more evident muscle cell proliferation.
TGF-β and TNF-α are involved in the fibrosis and stiffness observed in dystrophic muscle and TGF-β inactivation has been found to reduce fibrosis and improve contractile properties following muscle laceration [27, 28]. Moreover, overproduction of TGF-β has been associated with tissue fibrosis in injured skeletal muscle [29]. Considering these findings, the present results indicate that LLLT enhances the muscle repair process by decreasing both TGF-β and TNF-α, thereby preventing fibrosis and deficits in contractile properties.
In conclusion, the present study demonstrated that LLLT was able to modulate cytokine expression, with a reduction in TNF-α and TGF-β during the short-term period of muscle healing following cryoinjury to the TA muscle in rats. This was a preliminary study and further studies are needed in order to clarify how LLLT induces changes in cytokine expression during skeletal muscle repair using different parameters and injury models.
References
Lopes-Martins RA, Marcos RL, Leonardo PS et al (2006) Effect of low-level laser (Ga-Al-As 655 nm) on skeletal muscle fatigue induced by electrical stimulation in rats. J Appl Physiol 101:283–288
Grounds MD, White JD, Rosenthal N, Bogoyevitch MA (2002) The role of stem cells in skeletal and cardiac muscle repair. J Histochem Cytochem 50:589–610
Fisher BD, Rathgaber M (2006) Denervation does not change the ratio of collagen I and collagen III mRNA in the extracellular matrix of muscle. J Phys Ther Sci 18(1):57–66
Huard J, Li Y, Fu FH (2002) Muscle injuries and repair: current trends in research. J Bone Joint Surg Am 84:822–832
Araújo FA, Rocha MA, Mendes JB, Andrade SP (2010) Atorvastatin inhibits inflammatory angiogenesis in mice through down regulation of VEGF, TNF-a and TGF-b1. Biomed Pharmacother 64:29–34
Filippin LI, Moreira AJ, Marroni NP, Xavier RM (2009) Nitric oxide and repair of skeletal muscle injury. Nitric Oxide 21:157–163
Pereira MC, Pinho CB, Medrado ARP, Andrade ZA, Reis SRA (2010) Influence of 670 nm low-level laser therapy on mast cells and vascular response of cutaneous injuries. J Photochem Photobiol B Biol 98:188–192
Herbein G, O'Brien WA (2000) Tumor necrosis factor (TNF)-alpha and TNF receptors in viral pathogenesis. Proc Soc Exp Biol Med 223(3):241–257
Riedel K, Riedel F, Goessler UR, Germann G, Sauerbier M (2007) TGF-beta antisense therapy increases angiogenic potential in human keratinocytes in vitro. Arch Med Res 38(1):45–51
Chargé SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84(1):209–238
Kollias HD, McDermott JC (2008) Transforming growth factor-β and myostatin signaling in skeletal muscle. J Appl Physiol 104:579–587
Moreira MS, Velasco IT, Ferreira LS, Ariga SKK, Barbeiro DF, Meneguzzo DT, Abatepaulo F, Marques MM (2009) Effect of phototherapy with low intensity laser on local and systemic immunomodulation following focal brain damage in rat. J Photochem Photobiol B 97:145–151
Silveira PCL, Silva LA, Fraga DB, Freitas TP, Streck EL, Pinho R (2009) Evaluation of mitochondrial respiratory chain activity in muscle healing. by low-level laser therapy. J Photochem Photobiol B 95:89–92
Medrado ARAP, Pugliese LS, Reis SRA, Andrade ZA (2003) Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med 32:239–244
Shefer G, Barash I, Oron U, Halevy O (2003) Low-energy laser irradiation enhances de novo protein synthesis via its effects on translation-regulatory proteins in skeletal muscle myoblasts. Biochim Biophys Acta 1593:131–139
Miyabara EH, Aoki MS, Soares AG, Moriscot AS (2005) Expression of tropism-related genes in regenerating skeletal muscle of rats treated with cyclosporin-A. Cell Tissue Res 319(3):479–489
Rantanen J, Thorsson O, Wollmer P, Hurme T, Kalimo H (1999) Effects of therapeutic ultrasound on the regeneration of skeletal myofibers after experimental muscle injury. Am J Sports Med 27(1):54–59
Freitas LS, Freitas TP, Silveira PC, Rocha LG, Pinho RA, Streck EL (2007) Effect of therapeutic pulsed ultrasound on parameters of oxidative stress in skeletal muscle after injury. Cell Biol Int 31:482–488
Kjaer M (2004) Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84:649–698
Carmeli E, Moas M, Reznick AZ, Coleman R (2004) Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve 29(2):191–197
Langen RCJ, Schols AMWJ, Kelders MCJM, Van Der Velden JL, Wouters EFM, Janssen-Heininger YMW (2006) Muscle wasting and impaired muscle regeneration in a murine model of chronic pulmonary inflammation. Am J Respir Cell Mol Biol 35:689–696
Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM (2004) Tumor necrosis factor-alpha inhibits myogenic differentiation through MyoD protein destabilization. FASEB J 18:227–237
Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM (2001) Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J 15:1169–1180
Shi X, Garry DJ (2006) Muscle stem cells in development, regeneration, and disease. Genes Dev 20:1692–1708
Lima FM, Costa MS, Albertini R, Silva JA Jr, Aimbire F (2009) Low level laser therapy (LLLT): attenuation of cholinergic hyperreactivity, b2-adrenergic hyporesponsiveness and TNF-a mRNA expression in rat bronchi segments in E. coli lipopolysaccharide-induced airway inflammation by a NF-kB dependent mechanism. Lasers Surg Med 41:68–74
Albertini R, Villaverde AB, Aimbire F, Bjordal J, Brugnera A, Mittmann J, Silva JA, Costa M (2008) Cytokine mRNA expression is decreased in the subplantar muscle of rat paw subjected to carrageenan-induced inflammation after low-level laser therapy. Photomed Laser Surg 26(1):19–24
Bernasconi P, Di Blasi C, Mora M, Morandi L, Galbiati S, Confalonieri P et al (1999) Transforming growth factor-beta1 and fibrosis in congenital muscular dystrophies. Neuromuscul Disord 9:28–33
Fukushima K, Badlani N, Usas A, Riano F, Fu FH, Huard J (2001) The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med 29:394–402
Li Y, Foster W, Deasy BM et al (2004) Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol 164:1007–1019
Acknowledgements
The authors would like to thank UNINOVE for financial support.
Conflicts of interest
The authors declare that there were no conflicting financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mesquita-Ferrari, R.A., Martins, M.D., Silva, J.A. et al. Effects of low-level laser therapy on expression of TNF-α and TGF-β in skeletal muscle during the repair process. Lasers Med Sci 26, 335–340 (2011). https://doi.org/10.1007/s10103-010-0850-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-010-0850-5