Abstract
The aim of this work was to investigate the long-term effects of a single application of a water-cooled pulsed neodymium yttrium aluminium garnet (Nd:YAG) laser, in combination with scaling and root planing (SRP) for the treatment of periodontal inflammation. Twenty-two patients were included in this split-mouth single blind randomized controlled clinical trial. The parameters of the air and water-cooled Nd:YAG laser were: 4 W, 80 mJ/pulse, 50 Hz and a pulse width of 350 μs. The “test side” was treated with a single application of Nd:YAG laser and SRP; while the “control side ” was treated with SRP alone. At baseline, and after a median follow-up time of 20 months (range 12–39), periodontal inflammatory parameters (plaque index [PI], gingival index [GI], probing pocket depth [PPD]), and marginal bone loss (on digital bite-wing radiographs) were measured. Gingival crevicular fluid (GCF) was collected from the teeth 35, 36, 45, and 46 at baseline and at follow-up. Pl (p < 0.01), GI (p < 0.01), and PPD (p < 0.001) were significantly lower on the test side compared to the control side at follow-up. Radiological results showed significantly less bone loss on the test side compared to the control side (p < 0.05). GCF volume was lower on the test side compared to the control side (p < 0.01). In conclusion, a single application of Nd:YAG laser in combination with SRP had a positive long-term effect on periodontal health compared to treatment by SRP alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lasers are used for periodontal treatments including removal of calculus, epithelial lining of periodontal pockets, and granulomatous tissue [1–5]. The neodymium-yttrium-aluminium-garnet (Nd:YAG) laser, approved for periodontal treatment by the US Food and Drug Administration, has been in use for periodontal curettage for nearly three decades [6–8].
Theoretically, the Nd:YAG laser has a potential application in periodontal therapy because the wavelength is not readily absorbed by hard tissues such as cementum or dentin. Within the dose ranges recommended for clinical application, the Nd:YAG laser (even without water cooling) affects only the soft tissues such as the pocket epithelial lining [3]. The Chanthaboury and Irinakis study [8] has reported that the Nd:YAG is comparable to scaling and root planing (SRP) in reducing periodontal inflammation. However, there is limited evidence to support the efficacy of laser treatment as an adjunct to non-surgical periodontal treatment in adults with periodontal inflammation [9–11]. A debatable issue is that the Nd:YAG laser may cause overheating of the irradiated tissues and hence expose the soft and hard oral tissues to damage [12]. It should be noted that most previous studies used laser instruments (without water cooling) with an optical fiber of 300-μm diameter [13, 14]. However, the risk of thermal damage to periodontal tissue and the root surface may be evaded by using water-cooled laser instruments with a probe diameter of 600 μm. A larger diameter of the laser tip helps reduce the energy density at the laser tip. Water-irrigation also reduces clogging of the probe with debris, thereby preventing a build-up of areas of excessive heat.
In this context, the aim of the present study was to assess the long-term efficacy of a water-cooled pulsed Nd:YAG laser (1,064-nm) in supplement to SRP in the treatment of periodontal inflammation.
Materials and methods
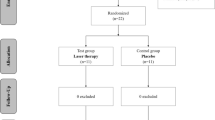
The trial was approved by the regional ethics review board in Stockholm, Sweden. The study participants (aged between 26 and 70 years) were recruited for a study of the short-term effects of a combined treatment with scaling and root planning and irradiation with Nd:YAG laser [15]. Consenting individuals underwent a preliminary clinical dental examination and their mandibular probing pocket depths were measured by the main author (TQ). In order to be included, the participants had to have at least six periodontal pockets of 4–8 mm (periodontal inflammation) on each side of the mandible. Subjects were asked about their systemic health, medications, as well as tobacco habits. The exclusion criteria were based on the following: intake of medications for systemic illnesses, use of antibiotics over the previous 3 months, tooth mobility (class II or III), mandibular third molars, and/or patients who had previously undergone laser treatment for periodontal inflammation.
In an attempt to investigate if our previous findings [15] were valid over a longer time, we invited these patients (n = 30) for a follow-up analysis. Twenty-two individuals volunteered to participate in the present study and the duration of follow-up ranged from 12–39 months.
Laser parameters and irradiation
The parameters of the air- and water-cooled pulsed Nd:YAG laser were: 4 W, 80 mJ/pulse, 50 Hz, and a pulse width of 350 μs.
Water and air settings were “9” on the machine. Angulation of the tip was between 20 and 30°. The fiber tip was cleaned after each pocket debridement. Power was automatically controlled by the device. The time spent on each tooth varied between 60 and 120 s, depending on accessibility. The fiber was held in constant motion in contact with the pocket epithelial lining. The power density and peak power density reported above are calculated by a hypothetical 100% emission through the small fiber tip. However, the energy is not emitted solely from the tip of the fiber; there is also considerable lateral emission. Thus, due to the high uncertainty about the actual light-emitting surface and the total area of tissue irradiated, the energy density (J/cm2) was not calculated.
Clinical periodontal investigations
The patients underwent two different treatment modalities. The teeth on the test side of the mandible received SRP and laser treatment; whereas the control side was treated with SRP alone. Assignment of left or right side for the respective treatments was randomly determined (by tossing a coin) before any treatment. Under local anesthesia, the mandibular teeth from 35, 36, 45, and 46 underwent SRP using hand instruments (American Eagle Curette, Missoula, USA) and ultrasonic scalers (Sonosoft Lux, Kavo Dental, Germany).
SRP and laser treatments were performed by one operator (TQ), while the baseline and follow-up periodontal examinations (plaque index [PI] [16], gingival index [GI] [17], and probing pocket depth [PPD] (Perio Wise, Premier, Canada), were conducted by two calibrated examiners (FJ and PP) who were blinded to the test and control groups.
Measurement of gingival crevicular fluid (GCF) volume and immunological investigations
Trained investigators (FJ and PP) collected the baseline and post-operative GCF samples from the teeth 36, 35, 46, and 45. Supragingival plaque was removed from the sites of GCF collection (mesial pocket of the second premolar and the first molar on the test and control sites) with cotton rolls. The GCF was collected with prefabricated paper strips (Periopaper, Oraflow Inc., Plainview, NY, USA), which were inserted into the pockets until resistance was felt and kept in place for 30 s. Blood-contaminated samples were discarded. The collected volume was measured with a calibrated Periotron 8000 (Oraflow Inc. Plainview, NY, USA).
Radiological investigations
Digital bite-wing radiographs (Siemens, Bensheim/Germany) were taken with the vertical long axis of the hemi-mandible using a software program (Schick, Technologies, Inc., NY, USA). All radiographs were taken by the main author (TQ). Baseline and post-operative mandibular alveolar bone loss were gauged (in millimeters) from the mesial surface of second molars to the distal surface of canine teeth by a trained investigator (FJ and AG). Alveolar bone loss was measured from the cementoenaemel junction (CEJ) to the most apical portion of the alveolar bone. Teeth with indistinct or carious lesions at the CEJ were excluded.
Statistical analyses
Statistical analyses were performed using a software program (Statistica v. 6.0, Statsoft, Tulsa, OK, USA). The paired t test was performed to assess the changes in the clinical parameters from baseline to follow-up, and between the treatment modalities. p-values less than 0.05 were considered as statistically significant. Normality was tested with Kolmogorov–Smirnov test.
Results
In total, 22 patients (nine males and 13 females) with periodontal inflammation with a mean age 50 years were included in the study. Four patients were smokers and one subject used smokeless tobacco. The median follow-up time was 20 months (range 12–39 months).
Clinical and radiological results
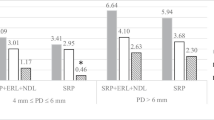
At the follow-up examination, PI (p < 0.01), GI (p < 0.01), and PPD (p < 0.001) were significantly lower on the test side compared to the control side. Radiological results showed a significant increase in marginal bone height on the test side compared to the control side (p < 0.05). These results are summarized in Table 1.
Gingival crevicular fluid volume
GCF volume was significantly lower on the test side (mean change: –0.57 μl, range: –0.4 μl to 1.68 μl) compared to the control side (mean change: 0.15 μl, range: –0.12–1.11 μl) (p < 0.01). These results are summarized in Table 1.
Discussion
In the current study, sites irradiated with a single application of Nd:YAG laser as an adjunct to SRP showed a reduction in periodontal inflammation and bone loss compared to the control side. The clinical reduction of inflammation measured as gingival index was corroborated by the simultaneous reduction of the GCF volume on the test side compared to the control side [18]. Our present study showed a minor bone loss on the SRP alone side while the side treated with laser and SRP showed some bone gain. This is in line with results from a recent experimental study in rats demonstrating an increase in marginal bone height following laser therapy [19].
Besides reducing the periodontal inflammatory conditions, Nd:YAG laser treatment also supports new connective tissue formation. The Yukna study [20] investigated the effect of Nd:YAG laser therapy in patients with periodontal inflammation. The results showed a significant reduction in PPD with increased clinical attachment levels [20]. An interesting finding of this study was that Nd:YAG laser therapy showed new cementum and connective-tissue formation [21]. It has been shown that the Nd:YAG laser when used at low energy does not cause damage to the cementum and dental pulp. The Radvar study [22] also showed that the Nd:YAG laser does not have a negative influence on cementum; thereby suggesting the formation of new connective tissues around the periodontium.
The present study has obvious weaknesses such as the small number of participants, the relatively long unsupervised and varying observation time, and a lack of positioning devices to standardize the radiographs. Since the patients know which side of the lower jaw that was irradiated with the laser, it is possible that they brushed this side more carefully, but considering the long follow-up time, it is not probable that this had a measurable effect.
A difference in bone level of 0.18 mm is not clinically relevant but it is statistically significant and shows that one treatment with a Nd:YAG laser can have a long-term effect on the alveolar bone.
In conclusion, a single application of a water-cooled pulsed Nd:YAG laser in combination with SRP significantly reduced the severity of periodontal inflammation compared to treatment by SRP alone. However, further human and experimental studies are required to assess the influence of combining Nd:YAG laser with SRP for the treatment of periodontal inflammation.
References
Radvar M, MacFarlane TW, MacKenzie D, Whitters CJ, Payne AJ, Kinane DF (1996) An evaluation of the Nd:YAG laser in periodontal pocket therapy. Br Dent J 80:57–62
Ishikawa I, Sculean A (2007) Laser dentistry in periodontics. In: Gutknecht N (ed) Proceedings of the 1st International Workshop of Evidence-Based Dentistry on Lasers in Dentistry. Quintessence Publishing Co., pp 115–129
Gómez C, Costela A, García-Moreno I, García JA (2006) In vitro evaluation of Nd:YAG laser radiation at three different wavelengths (1064, 532 and 355 nm) on calculus removal in comparison with ultrasonic scaling. Photomed Laser Surg 24:366–376
Gold SI, Vilardi MA (1994) Pulsed laser beam effects on gingiva. J Clin Periodontol 21:391–396
Grassi RF, Pappalardo S, Frateiacci A, Scortechini A, De Benedittis M, Petruzzi M, Frasca M (2004) [Antibacterial effect of Nd:YAG laser in periodontal pockets decontamination: a in vivo study] (article in Italian). Minerva Stomatol 53:355–359
Romanos GE (1994) Clinical applications of the Nd:YAG laser in oral soft tissue surgery and periodontology. J Clin Laser Med Surg 12:103–108
Wang QQ, Zhang CF, Yin XZ (2007) Evaluation of the bactericidal effect of Er, Cr:YSGG, and Nd:YAG lasers in experimentally infected root canals. J Endod 33:830–832
Chanthaboury R, Irinakis T (2005) The use of lasers for periodontal debridement: marketing tool or proven therapy? J Can Dent Assoc 71:653–658
Karlsson MR, Diogo Löfgren CI, Jansson HM (2008) The effect of laser therapy as an adjunct to non-surgical periodontal treatment in subjects with chronic periodontitis: a systematic review. J Periodontol 79:2021–2028
Schwarz F, Aoki A, Becker J, Sculean A (2008) Laser application in non-surgical periodontal therapy: a systematic review. J Clin Periodontol 35(8 Suppl):29–44
Slot DE, Kranendonk AA, Paraskevas S, Van der Weijden F (2009) The effect of a pulsed Nd:YAG laser in non-surgical periodontal therapy. J Periodontol 80:1041–1056
Miserendino LJ, Levy GC, Abt E, Rizoiu IM (1994) Histologic effects of a thermally cooled Nd:YAG laser on the dental pulp and supporting structures of rabbit teeth. Oral Surg Oral Med Oral Pathol 78:93–100
Ben Hatit Y, Blum R, Severin C, Maquin M, Jabro MH (1996) The effects of a pulsed Nd:YAG laser on subgingival bacterial flora and on cementum: an in vivo study. J Clin Laser Med Surg 14:137–143
Andrade AK, Feist IS, Pannuti CM, Cai S, Zezell DM, De Micheli G (2008) Nd:YAG laser clinical assisted in class II furcation treatment. Lasers Med Sci 23:341–347
Qadri T, Poddani P, Javed F, Tunér J, Gustafsson (2010) A short-term clinical evaluation of Nd:YAG laser as an adjunct to scaling and root planing in treatment of periodontal inflammation. J Periodontol Accepted April 16 [Epub ahead of print]
Löe H (1967) The gingival index, the plaque index and the retention index system. J Periodontol 38:610–616
Silness J, Löe H (1964) Periodontal disease in pregnancy. II Correlation between oral hygiene and periodontal conditions. Acta Odontol Scand 22:121–131
Wakao T, Yoshinaga E, Numabe Y, Kamoi K (1989) Examination of periodontal disease with gingival crevicular fluid. Correlation between capacitance and clinical finding. Nippon Shishubyo Gakkai Kaishi 31:573–582
de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Oshiiwa M, Garcia VG (2008) In vivo effect of photodynamic therapy on periodontal bone loss in dental furcations. J Periodontol 79:1081–1088
Yukna RA, Carr RL, Evans GH (2007) Histologic evaluation of an Nd:YAG laser-assisted new attachment procedure in humans. Int J Periodontics Restor Dent 27:577–587
Romeo U, Palaia G, Botti R, Leone V, Rocca JP, Polimeni A (2009) Non-surgical periodontal therapy assisted by potassium-titanyl-phosphate laser: a pilot study. Lasers Med Sci Nov 21 [Epub ahead of print]
Radvar M, Creanor SL, Gilmour WH, Payne AP, McGadey J, Foye RH, Whitters CJ, Kinane DF (1995) An evaluation of the effects of an Nd:YAG laser on subgingival calculus, dentine and cementum. An in vitro study. J Clin Periodontol 22:71–77
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qadri, T., Javed, F., Poddani, P. et al. Long-term effects of a single application of a water-cooled pulsed Nd:YAG laser in supplement to scaling and root planing in patients with periodontal inflammation. Lasers Med Sci 26, 763–766 (2011). https://doi.org/10.1007/s10103-010-0807-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-010-0807-8