Abstract
Environmental pollution and human health are inextricably linked. As the number of environmental pollutants increases, it is increasingly important to develop unique, effective, and intelligent analytical devices to monitor them. Biosensors are devices that capture biological signals and convert them into audible electrical impulses. To detect and observe specific biological analytes, such as the interaction between antibodies and antigens, biological entities such as DNA, RNA, and proteins/enzymes must be integrated with electrochemical transducers. Variety of biosensors has lately gained prominence and is being employed as in situ, real-time, and cost-effective analytical devices for healthy environments. Continuous environmental monitoring necessitates the use of biosensing technologies that are portable, inexpensive, quick, and adaptive. Each sensor, on the other hand, stands apart in terms of selectivity, technique, sensitivity, detection restrictions, sensitizing materials, and speed. Each sensitive element has a distinct selectivity and detection limit based on its sensitivity. This review focuses on the distinguishing characteristics, efficient design, and effectiveness of several types of biosensors, with an emphasis on the detection of environmental contaminants. Accurate devices will also aid in the continuing, parallel investigation of the causes and discharge of environmental toxins from diverse industrial sectors. Furthermore, real-time monitoring has the added benefit of allowing on-site analysis of pollutant components before to discharge into the environment, which can assist reduce the waste of a variety of harsh chemicals and reagents. The goal of this review is to provide an overview of the most recent developments in the field of using biosensors to identify environmental pollutants. Biosensors based on enzymes, entire cells, antibodies, aptamers, DNA, and biomimetic sensors are described. We list their useful qualities as well as their relevance to the detection of various contaminants. Designing biosensors makes use of a number of detection principles, including amperometry, conductometry, luminescence, etc. They differ in terms of design, profitability, sensitivity, and quickness. Further research is necessary to create a powerful biosensor that can identify environmental contaminants in a multifaceted medium, with no prior time-consuming pretreatment or tedious preparation protocols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
On account of excessive industrial expansion, global urbanization, and population growth, numerous dangerous compounds are released into the environment and are built up, becoming a significant environmental threat in the present era. Pollutants come in a wide variety of forms and are widely spread in the soil, air, and waterways. They can be chemical, physical, biological, radioactive, or any combination of these. All biological systems are affected, but the health and way of life of people are severely affected (Xiong et al. 2022). Since environmental safety and security are a major issue on a global scale, monitoring the environment and managing it are two of the top priorities for both the globe and Europe (Justino et al. 2017). Researchers are interested in learning more about long-lasting explanations for the environmental monitoring systems since controlling harmful substances is a vital step in the pollution restoration process. Pollutants are typically detected using the high sensibility and selectivity of classic chromatographic and spectroscopic methods (Deng et al. 2022; Li et al. 2021). However, these processes take a number of stages for sample preparation, are time-consuming, contain dangerous chemicals, and call for expert workers to operate the equipment.
Enhanced biosensing devices were created as a result of the requirement to use some quick, picky, sensitive, and accurate, in-the-moment technologies for identifying and screening contaminants. A transducer, a signal processing system, a display, and a bioreceptor are just a few of the distinctive components that make up a biodetection device (Wu et al. 2022; Dimitrievsaka et al. 2023; Kumar et al. 2023). The complete apparatus produces a measurable detection signal that is correlated with the analyte concentration in the target (Brunnbauer et al. 2021). The biochemical receptor identifies the chemical or biological ingredients from the analysed sample, and the transducing component converts the biochemical result into quantized electrical, thermalor optical signals. Currently, there is a significant curiosity in developing extremely efficient and accurate systems for pinpointing and filtering environmental contaminants. The biosensor consists of the same transducer and signal processing system as a sensor, with the exception of using a biological analyte (Trapananti et al. 2021; Kulkarni et al. 2022). The pollutant is discovered by a bioreceptor, and a transducer converts the sample into a quantifiable signal (Naresh and Lee 2021; Tovar-Lopez 2023; Cimen et al. 2023).
Historical overview on biosensors
Since M. Cremer discovered in 1906 that the electric potential that exists across parts of a liquid on opposite sides of a glass membrane is proportional to the concentration of an acid in a liquid, biosensors have been used to quantify chemical concentrations in biological samples. Despite this, in 1909, Soren Peter Lauritz Sorensen put up the idea of pH (hydrogen ion concentration), and in 1922, W.S. Hughes developed an electrode for measuring pH. The "Father of Biosensors" Leland C. Clark, Jr. invented the first "authentic" biosensor for oxygen detection in 1956. In 1962, he demonstrated an amperometric enzyme electrode for the detection of glucose, which is known as the "Clark electrode" and carries his name. Followed by amperometric enzyme electrode, in the year 1969, Guilbault and Montalvo, Jr. discovered the first potentiometric biosensor for detecting urea and the first commercial biosensor was developed by Yellow Spring Instruments (YSI) in the year 1975. For diagnostics and the development of biopharmaceutical product monitoring, biosensors have made considerable strides recently. In order to achieve desirable pharmacological effects in a label-free environment, biosensors are crucial tools (Bilal and Iqbal 2019; Andryukov et al. 2020; Gaviria-Arroyave et al. 2020). They also help us better understand disease and the interactions between molecules. A typical biosensor comprises (a) bioreceptor, (b) transducer, (c) electronics, (d) display, and (e) an analyte (Fig. 1a, b). The procedure for biorecognition refers to the formation of a signal (in the form of heat, pH, light, plant or animal tissue, charge or mass change, and microbial products) during the interaction of a bioreceptor and an analyte. A transducer is an important key instrument that transforms the state of energy. It turns the biorecognition event into a quantifiable (electrical) signal that corresponds to the quantity or presence of a biological or chemical target. The amount of analyte–bioreceptor interactions is proportional to the number of electrical or optical signals generated by transducers. The electronics unit quantifies the transformed signals from transducer to a digital format. The display unit is made up of a user interpretation system which generates readable and understandable output (a numerical, tabular value, figure, or a pictorial representation).
(a) Schematic representation of Biosensor and (b) Elements of Biosensor (adopted from Grieshaber et al. 2008)
Environmental impurities
Environmental pollution and its consequences, such as acid rain, ozone layer depletion, and climate change, are foremost worldwide concerns that are high on countries’ economic and political agendas. Extensive use of chemicals in agricultural and industrial sectors has led to the discharge of potentially harmful contaminants into the environment. These pollutants represent major hazards to human health and ecological diversity due to their broad dissemination. Traditional chromatographic methods for detecting these environmental pollutants need expensive and specialized equipment, long detection reaction times, and user training. Furthermore, vital environmental variables such as cytotoxicity, bioavailability, mutagenicity, and genotoxicity can only be detected in living cells which is not always possible. As a result, sensitive, rapid, and cost-efficient surveillance of these harmful chemicals is necessary in pollution reduction programmes and management systems (Huang et al. 2023; Fatima et al. 2022). The most precise and sensitive methods for detecting environmental contaminants are biosensors (Patel et al. 2021). These devices create a detectable signal by fusing an electrical element with a biological component—either an enzyme or an antibody. The electronic component detects, records, and transmits information on physiological changes as well as the presence of chemical or biological elements in the surrounding environment. Due to the vast range of applications, including medical care and illness detection, water and food quality monitoring, and environmental monitoring, biosensors have thus assumed an inevitable stage in the last ten years (Willner and Vikesland 2018; Thakur et al. 2022). In general when the pollutants interact with DNA nanosensor to produce signal or to suppress signal. The type of signal may vary from light, electroactivity, pH change, mass change, and heat change upon interaction with pollutant in a concentration-dependent manner. The data are processed using a data processing system, and output is produced in a readable format.
Pesticides
In order to attain high agricultural productivity, pest management is currently accomplished through the purposeful application of a wide range of harmful compounds, known as pesticides, with significant environmental consequences. Around 3.42 × 106 t/y worth of insecticides were used globally in 2015. The assurance of the quantity and quality of food and feed justifies the use of these insecticides. The majority of pesticides are environmental pollutants with considerable negative impacts since some components are persistent in the environment and have extended half-lives, even when used in compliance with the law; only a tiny part of pesticides meet the stated targets. Pesticides like aldrin, chlordane, DDT, dieldrin, endrin, heptachlor, hexachlorobenzene, mirex, and toxaphene are examples of POPs (persistent organic pollutants) because they take years to decompose. These POPs have the potential to alter the endocrine, reproductive, and respiratory systems of humans as well as non-target creatures in the environment. The automated, precise, and highly specific analytical methods for pesticide identification that integrate chromatographic techniques with various detectors are automated. These systems do have several drawbacks, though, including high costs, time demands, the need for sample pretreatment, and a slow response time. As a result, the study concentrated on creating biosensors, which are quick and accurate pesticide detection tools (Mirres et al. 2022; Samal et al. 2023). The essential idea of these biosensors, which are currently used in a variety of disciplines and businesses, is sample analysis and its recognition, transduction, and amplification.
Electrochemical immunosensors’ high specificity and sensitivity have been used in pesticide detection applications. Mehta et al. (2017) described the invention of an electrochemical immunosensor for detecting the organophosphate insecticide parathion. The immunosensor was created by (i) adding graphene quantum dots to a screen-printed electrode surface, (ii) electrochemically functionalizing with NH2 groups, and (iii) biointerfacing with anti-parathion antibodies. The biosensor has a logarithmic linear range of 0.01 to 106 ng/L and a LOD of 46 pg/L, and it was highly selective for parathion even at high doses (1000 ng/L) of other pesticides such as paraxon, malathion, and chlorpyrifos. Aside from its great sensitivity and selectivity, the immunosensor offered several additional advantages, including a quick response time (15 min), and strong repeatability. Multianalyte immunosensors, in addition to single-analyte detection, have been developed and demonstrated to be successful in the detection of pesticides such as endosulfan and paraoxon at low concentrations (0.05 and 2 ppb, respectively).
Perez-Fernandez et al. (2020a, b) created a direct competitive electrochemical immunosensor for the detection of imidacloprid, a neonicotinoid. Monoclonal antibodies were immobilized on a gold nanoparticle-modified screen-printed carbon electrode, with imidacloprid competing for antibody-binding sites with imidacloprid linked with horseradish peroxidase. The immunosensor had a low LOD (22 pM) and good precision (RDS of 6%), selectivity, and accuracy (relative error of 6%). It also had one-month stability.
Pharmaceuticals impurities
Despite the tight regulatory procedures followed before commercialization, several types of pharmaceutical pollutants gradually have an impact on ecosystems. Aquatic ecosystems tend to accumulate pharmaceutical contaminants more so than terrestrial ones. According to Lan et al. (2018), the sources of pharmaceutical pollutants include the production process, medications used on livestock, streams from animal feeding facilities, and excessive use of norcotic drugs including caffeine and cotinine. Pharmaceuticals accumulate in the environment as a result of improper industry removal and human excretion of unmetabolized medications. Figure 2 elaborates on the overview of how pharmaceutical impurities affect the environment and the ecosystem.
Amperometric biosensors monitor current flows created by an electrochemical reaction at a constant potential, where the intensity of the current is proportional to the concentration of the oxidized and reduced material on the electrode’s surface. These biosensors have been used to quantify aminoglycoside antibiotics, bronchodilators (including theophylline), anti-arrhythmic medicines, and anticancer medications.
Huang et al. (2019) used a multilayer material to modify the glassy carbon electrode to explore the human umami taste receptor (hT1R1) and umami compounds such as monosodium glutamate (MSG). A human umami taste receptor (hT1R1) was linked to the layers generated by the AuNPs during the creation of this electrode. For direct electron transfer to the produced multilayer material, horseradish peroxidase (HRP) is utilized. The researchers believe that hT1R1 is a receptor used by the body to sense nitrogen, which opens up a new avenue of investigation into nutrition and medication adsorption (Mackulak et al. 2020; Wei et al. 2016).
The same researchers constructed another biosensor in 2023 by connecting colon cancer and nearby tissues to GCE in order to visualize the kinetics of responding to C and N nutritional receptors such as glucose and sodium lactate. They did this by combining solutions of starch gum, an aldehyde base, and sodium alginate and spreading them across two microporous polycarbonate membranes into which the colon tissues were put to form a layered assembly aligned with the GCE. Researchers discovered that the cells reacted differently to lactate, implying that this nutrient could be used to treat colon cancer. Lactate has no effect on colon cancer tissue, but it does on neighbouring tissue (Lu et al. 2023).
Heavy metals
It is generally known that mining and related engineering activities increase the build-up of heavy metals in water bodies. Heavy metals do not break down and accumulate in the environment for a very long time. Because reactive oxygen species are produced, the majority of heavy metals seem to have increased hazardous potentials (Yu et al. 2006). However, only a select few of them are needed to exert a variety of biological activations, such as enzyme-mediated reactions, as cofactors, to bring about inhibitory effects (Rebollar-Perez et al. 2016). Consequently, the specific key to designing biosensors for detecting heavy metals is either the induction or repression of enzymes. Alkaline phosphatase and ascorbate oxidase grounded biosensors may detect zinc and copper. A biosensor for detecting the presence of heavy metals such nickel, copper, cobalt, and cadmium was successfully demonstrated when glucose oxidase (GO) was suppressed (Ghicaet al. 2013). By achieving a reporter gene under the control of an inducible promoter for the detection of heavy metals, it was clarified (Rodriguez-Mozaz et al. 2006). According to the contaminants concentration, the reporter signal limits increases during this method. Common reporter genes used in the creation of biosensors include β—galactosidase, luciferase, and green fluorescent protein.
E. coli and the electrochemical redox mediator benzoquinone were co-immobilized within a gelatin/silica hybrid hydrogel on the surface of a glassy carbon electrode, according to Li et al. The toxicity of Hg2+, Cu2+, and Cd2+ ions was tested using this biosensor, with microgram per litre IC50 values reported. Its capacity to identify heavy metal ion combinations in laboratory wastewater has also been established (Hara and Singh 2021).
Types of biosensor
Enzyme biosensor
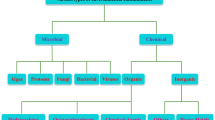
Due to their high selectivity, biological activity, and dependability, enzymes are biocatalysts and can be employed to detect pollutants and contaminants in the environment. By deactivating the pollutant or by catalysing its conversion to less hazardous metabolites, enzymes can detect contaminants. The amount of pollution in a sample can be determined using the signal produced during these procedures. The analyte (pollutant) is passed via the enzyme in the design of the enzyme biosensors, which immobilizes the enzyme on a transducer. The transducer transforms the signal produced by the process into a measurable value. Figure 3 following depicts the classification of enzyme biosensors (Wang et al. 2020).
For several applications, enzyme-based biosensors have been developed using enzymes including horseradish peroxidase, glucose oxidase, and alkaline phosphatase. The enzymatic biofuel sensor was created by Li et al. (2020). It is a self-powered sensor that is encapsulated with laccase enzyme to measure the concentration of bisphenol. The primary raw ingredient in plastics like polyvinyl chloride and polycarbonate is bisphenol, which is also a significant water contaminant. The pollutant could be detected by the biosensor even at a lower concentration of 1.95 × 103 mM due to its great effectiveness. Numerous enzyme biosensors based on enzymes like acetylcholine esterase, butyrylcholinesterase, phosphotriesterase (PTE), and organophosphorus hydrolase (OPH) have been developed as a result of the capacity to detect organophosphate. The idea behind these sensors is to benefit from phosphorous’ ability to reduce acetylcholine esterase activity. The cholinergic substrate cannot be hydrolysed by the enzyme because the serine residue in the active core of the enzyme binds to phosphorus (Wang et al. 2020). Some of the enzyme biosensors for measuring pollution are included in Table 1.
Electrochemical biosensor
Biosensors are a good substitute for conventional chromatography-based approaches because they may be designed with incredibly precise recognition sites. Electrochemical biosensors are among the existing biosensors that have a number of benefits, including real-time monitoring, miniaturization, and improved selectivity and sensitivity. Additionally, electronic signals are produced by electrochemical processes rather than complex signalling components. This makes it easier to create portable devices for on-site environmental monitoring and clinical testing. Biosensors use electrodes to transform biological signals into readable output signals. By altering some biological components, such as DNA, enzymes, or cells, one can increase the selectivity and sensitivity of these signals. Based on the type of transducer, electrochemical biocatalytic sensors have been altered with biological components that may identify a target and cause an electroactive molecule (such as enzymes) to react. Electrochemical affinity sensors, on the other hand, have a binding recognition element that, when attached to the target (such as antibodies), releases a signal. Typical electrochemical biosensor components and their working are illustrated in Fig. 4a, b.
DNA biosensors
Biosensors use nucleic acids as its sensing elements, such as DNA or RNA. Nucleic acids, particularly single-stranded DNA (ss-DNA), are used as recognition components by the majority of DNA-based biosensors. A probe, which is often an artificial oligonucleotide with a specified sequence, and a signal transducer are hybridized to create biosensors in DNA biosensors. Immobilization and probe selection are crucial steps in the creation of DNA biosensors. There are two different action systems, like, (i) hybridization: through single-stranded nucleic acid that has been immobilized on a solid matrix, the analyte that is complementary to the DNA is transferred. The analyte and the nucleic acid combine. The target DNA and its complementary strand stop on the location of the sensing area as a result of an impulsive hydrogen bonding between adenine–thymine (A = T) and cytosine-guanine (C = G) pairs.(Kokkinos, 2019 and Saidur et al. 2017). This hybridization results in a conformational change in nucleic acid from single strand to double strand. A transducer converts the signal produced by this transition into a quantifiable form. (ii) The target analyte’s molecule(s) altering the structure of the ss-DNA (Bacchu et al. 2022). These processes encourage a range of physicochemical changes, which results in the detection of a distinct signal that may be translated into a calculable response by a transducer; typically, optical or electrochemical sensors are used (Wang et al. 2022).
A sensitive, sensitive, affordable, and trustworthy method for quantifying contaminants is using DNA biosensors. The metal ions in pollution are capable of binding the nucleotide bases, especially cytosine and thymine, effectively. In order to detect metal contaminants like copper, cadmium, and zinc, among others, C- and T-rich DNA can be used as a probe. DNA quadraplex, a guanosine-rich tetra helix DNA, can be used to detect lead, zinc, cadmium, salt, and phosphorous. The binding of these metals to the G-rich DNA causes a conformational change that generates a signal that can be detected. Figure 5 illustrates the basic principle of DNA biosensor.
Basic principle of the enzyme assisted DNA amplification reaction (adopted from Wang et al. 2022)
DNAzymes, a kind of nucleic acids having catalytic activity, are frequently used as the biological sensing component in biosensors. DNAzymes have both catalytic and substrate-binding sites. The substrate-binding site’s nucleic acid has a cleavage site. The amount of metal ions present can be determined by analysing the signal produced by the cleavage caused by metal ions such as manganese, magnesium, copper, lead, zinc, and cadmium. According to numerous researches (Bacchu et al. 2022; Sun et al. 2019a, b; He et al. 2020; Sun et al. 2019a, b), DNA-based biosensors can detect trace levels of heavy metals in the environment. The ability of some heavy metal ions to combine with particular DNA bases to form stable duplex structures forms the basis for the functioning of this system. For instance, the mercury ion (Hg2+) binds thymine (T) bases specifically to produce a thermally stable T-Hg2+-T duplex (Muhammad and Huang 2021). Similar to this, C–Ag+-C base pairs are created when two cytosine (C) bases interact preferentially with silver ions (Ag+), aiding in the stabilization of the DNA duplex (Wang et al. 2022; Bacchu et al. 2022). Thus, single-stranded DNA rich in thymine or cytosine can form stable structures that allow metals to be detected using the right transducers in the presence of some metal ions (Bacchu et al. 2022).
The fluorescence quenching of C-rich DNA coated with AgNCs and Cu/AgNCs has also been studied to develop innovative DNA nanosensors (Sun et al. 2019a, b; Muhammad and Huang 2021). Gold nanoparticles were functionalized with thymine-rich DNA templates containing FAM at the long end and undergoing fluorescence reduction in the presence of mercury in order to activate the collapsible DNA template, which brought up FAM in the vicinity of gold nanoparticles (Long et al. 2013). Gold nanoparticles and DNA have been combined to create a nanosensor that can pick up several metal ions at once. The affinities of specific metal ions on FAM-labelled DNA-AuNPs result in fluorescence quenching. Tetra chloroauric acid and hydroxylamine were added to AuNPs, which boosted the structure’s selectivity by causing the external surface to diverge. The change in the external surface area of AuNPs suggested different colour development. Table 2 lists some of these sensors that are used to identify and address Ag+, Hg2+, Pb2+, metals, and foodborne pathogens.
Aptamers biosensors
Like DNA and RNA, nucleic acids, also known as aptamers, are well-known genetic machines that pass on the genetic code to succeeding generations in living things like humans. Nucleic acids may have had a big role in the study of detecting environmental pollution; it has been hypothesized in recent years. The building blocks of aptamers, which preferentially draw inorganic or organic impurities, are deoxyribonucleic acid or ribonucleic acids. It has been discovered that certain genetic base pairs of aptamers can make it possible to build this kind of biosensors specifically. However, the primary technique for creating new aptamers is in vitro selections. Due to their distinct characteristics, aptamers are excellent candidates and high-quality materials for a variety of new sorts, including Systematic Evolution of Ligands by Exponential Enrichment (SELEX). The initial observation and description of this specific type of sensor device were made in 1990 (Zhuo et al. 2017; Ni et al. 2021).
Aptamers have special benefits such stability, improved enzyme resistance, high ionic strength, enhanced temperature or pH tolerance, increased attraction and specificity to target contaminants, and a size range of in vivo and in vitro sensors from nano- to pico-molar. In addition to not requiring synthesis in host animals or a traditional immune response, aptamers have additional benefits over antibodies. Single-stranded nucleic acid aptamers are combined with carbon nanotubes (CNTs) or grapheme sheets through non-covalent or hydrogen contacts to create aptasensors. A fluorescent signal results from the aptamers attaching to the pollutant in the presence of the pollutant and breaking the interaction between the aptamer and the grapheme (Flores-Contreras et al. 2022). A new composite film consisting of carbon black and graphene oxide Fe3O4 has recently been created as an electrochemical aptasensor for the detection of chlorpyrifos in agricultural samples. 94 pM was discovered to be the detection threshold (Perez-Fernandez et al. 2020a, b).
Microbial biosensors
In microbial biosensors, living or dead microbes that have been immobilized on a solid matrix serve as the sensing components. Genetic engineering makes it simple to modify microorganisms to increase their performance and tolerance. Microbial biosensors that have been genetically altered are employed in a variety of applications because they are accurate, cost-effective, portable, and tiny. The main drawbacks of microbial biosensors, however, are their extended recovery and response periods, high sensitivity to temperature and pH, and hysteresis effect.
In comparison with conventional methods, whole cell-based microbial biosensors are shown to be more effective in sensing environmental signals. This is because they can operate under a variety of working conditions. In numerous studies, earthly and aquatic living creatures have been used as microbial biosensors to identify environmental contaminants including pesticides, heavy metals, phenols, and other harmful substances (Vanitha et al. 2017; Gupta et al. 2019; Do et al. 2022; Nigam and Shukla 2015). Regulatory genes and reporter genes are examples of biological recognition components in microbial biosensors. Figure 6 displays the role of regulatory and reporter genes.
Bacterial biosensors are now useful for the detection of heavy metals in environmental tests thanks to the adoption of appropriate genes as bioreceptor that are resistant to detected metals. Many bacterial structures have been recognized as potential biological receptors for heavy metals like zinc, copper, silver, tin, mercury, and cobalt (Webster et al. 2014). The design and operation of the biosensor are portrayed in Fig. 7, and the various types of microbial biosensors used to detect environmental contaminants are shown in Table 3.
Ab-based biosensors (immunosensors)
The majority of glycoproteins that have the capacity to identify and entice antigens (pollutants) for binding are antibodies. These complexes are stable. Based on the transducing mechanism, immunosensors are categorized as electrochemical, which includes amperometric, potentiometric, and impedimetric, colorimetric, optical, and microgravimetric devices. It can also be separated into labelled and unlabelled sensors (Shin et al. 2012; Sagiroglu et al. 2011; Ramasubburayan et al. 2014; Roy et al. 2021; Kharkova et al. 2021; Melnikov et al. 2022; Kolahchi et al. 2018). The labelling contains a sensitive, observable pointing to the target bioreceptor. After analysis, the tag’s activity was scaled. Tags can be made from various enzymes, fluorescent dyes, electroactive compounds, and nanoparticles. The formation of non-labelled immunosensors can be directly predicted by examining the physical changes the antigen–antibody complex results (Choi and Yoon 2023). The idea of unique interactions between antigen and antibody serves as the foundation for designing immunosensors. In electrochemical biosensors, antigen–antibody responses alter the electrical properties of the region between two electrodes (Grieshaber et al. 2008). Immobilizing antibodies on the surface of a solid matrix is one of the most crucial steps in the development of biosensors; yet, immobilization can occasionally cause a loss of function. Non-oriented immobilization is caused by random immobilization and steric hindrance brought on by a high antibody density (Kim et al. 2012; Zhang et al. 2017; Mustafaoglu et al. 2015; Ocsoy et al. 2013; Nathan et al. 2012). Table 4 highlights the list of some of the available antibody-based biosensors.
Nanotechnology-based sensors
Recently, non-metallic nanomaterials including graphite, carbon nanotubes, and graphene as well as metallic nanoparticles like gold, copper, and silver have been used as biosensors. Utilizing nanomaterials broadens the binding surface area and improves surface accessibility. These biosensors’ demonstrated great sensitivity and selectivity enable us to attain very low detection thresholds. The conductive ink-printed trips known as screen-printed electrodes (SPE) are printed on a substrate. This method adjusts a mould, stencil, or other net to serve ink into a substrate via a physical fence. After that, an insulator sheet is used to dry the printed ink and static. Examples of substrate materials include skin, plastics, ceramics, paper, and most recently, paper (Paimard et al. 2023). The redox reaction and its electrochemical significance serve as the foundation for the functioning principle of SPE sensors. The correct chemical alterations must be done in order to produce a superior sensing device, such as strengthening the electrode surface by increasing selectivity and adding new external area (Sailapu and Menon 2022). The best choice for expanding the electrode surface area is nanostructured components. Today, a variety of nanoscale materials, such as metallic nanoparticles, carbon-based nanomaterials like carbon nanotubes and graphene, carbon coatings, membranes, and a few conductive polymers, are used to develop electrode surfaces (Javaid et al. 2021). The fabrication method using screen-printing gives you the freedom to change the electrodes’ morphology. Using SPEs improved by reduced graphene oxide and thionine (Roy et al. 2021) developed a new electrochemical enzyme biosensor to detect 3-hydroxybutyrate.
Conclusion and future perspectives
The goal of this review was to show how biosensors can answer the demand for quick, accurate, and dependable technology for detecting environmental pollutants. However, when applied to complex and irregular environmental samples with changeable compositions, these should be able to meet sensitivity and selectivity requirements. When developing biosensors for environmental pollutants detection, it is critical to consider mobility, cost, automation, and integration into professional devices, regardless of the sensing element or transducer. Continual use would necessitate rapid regeneration of biological activity during detection cycles. Most investigations use standardized laboratory samples to evaluate the biosensor’s performance. Enzymes, aptamers, DNA, antibodies, and microorganisms are a few examples of biological sensing components. Despite difficulties with stability, potential interference, and ideal working circumstances, these components have the advantage of being amenable to enhancements in specificity and selectivity.
Recent study has shown that biomimetic sensors outperform enzyme-based biosensors in terms of kinetic performance. However, specificity and selectivity remain their main shortcomings. Although innovative nanocomposites and improved nanomaterials can be employed to produce environmental biosensors, in situ and real-time monitoring of toxins utilizing various methodologies has lately gained popularity. According to some daily news reports and a few scientific articles in the current literature, environmental monitoring has recently sparked interest in the use of drones, particularly in water and air quality monitoring, agricultural surveillance, and volcano gas measurements.
According to the biosensors evaluated in this review study, electrochemical and enzymatic biosensors are primarily employed for environmental monitoring. Enzymes have predominantly been used as recognition components in biosensors for pesticide detection due to their selectivity. However, enzymes are only helpful under specific circumstances due to their costly and time-consuming purification procedure, low thermal stability, and need for the ideal pH and temperature. Aptamers are a promising alternative for biosensor detection because to their versatility, denaturalization, and rehybridization, identification of targets with diverse functional groups, temperature stability, and in vivo synthesis. The use of immunosensors to identify substances such as endocrine disruptors and toxins has also been studied. Antibodies possessed good antigen specificity, but they also had problems with poor regeneration and difficult immobilization on sensor substrates. This requires research into the optimal settings for antibody formation and immobilization, which may take a long period and be detrimental to sensor development. As antibody activity declines, other antibody properties such as number, orientation, and position on the sensor surface may have an impact on sensor response and optimization.
Data availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- DNA:
-
Deoxyribonucleic acid
- RNA:
-
Ribonucleic acid
- YSI:
-
Yellow spring instruments
- DDT:
-
Dichlorodiphenyltrichloroethane
- POPs:
-
Persistent organic pollutants
- GO:
-
Glucose oxidase
- OPH:
-
Organophosphorus hydrolase
- PTE:
-
Phosphotriesterase
- AMP:
-
Amperometric
- Vol:
-
Voltammetric
- Col:
-
Calorimetric
- Imp:
-
Impedimetric
- AChE:
-
Acetylcholine esterase
- SELEX:
-
Systematic evolution of ligands by exponential enrichment
- CNTs:
-
Carbon nanotubes
- SPE:
-
Screen-printed electrodes
References
Andryukov BG, Besednova NN, Romashko RV, Zaporozhets TS, Efimov TA (2020) Label-free biosensors for laboratory-based diagnostics of infections: current achievements and new trends. Biosens Basel 10(2):11. https://doi.org/10.3390/bios10020011
Asal M, Ozen O, Sahinler M, Polatoglu I (2018) Recent Developments in Enzyme. DNA Immuno-Based Biosens Sens 18:1924. https://doi.org/10.3390/s18061924
Aynalem B, Muleta D (2021) Microbial biosensors as pesticide detector: an overview. Hindawi J Sens 12:5538857. https://doi.org/10.1155/2021/5538857
Bacchu M, Ali M, Das S, Akter S, SakamotoH SSI, Rahman M, Campbell K, KhanM, (2022) A DNA functionalized advanced electrochemical biosensor for identification of the food borne pathogen Salmonella enterica serovar Typhi in real samples. Anal Chim Acta 1192:339332. https://doi.org/10.1016/j.aca.2021.339332
Bhatt K, Maheshwari DK (2020) Zinc solubilizing bacteria (Bacillus megaterium) with multifarious plant growth promoting activities alleviates growth in Capsicum annuum L. Biotech 10(2):36. https://doi.org/10.1007/s13205-019-2033-9
Bilal M, Iqbal HM (2019) Microbial-derived biosensors for monitoring environmental contaminants: recent advances and future outlook. Process SafEnviron Protect 124:8–17
Borah H, Dutta RR, Gogoi S, Medhi T, Puzari P (2017) Glutathione-S-transferase-catalyzed reaction of glutathione for electrochemical biosensing of temephos, fenobucarb and dimethoate. Anal Methods 9:4044–4051. https://doi.org/10.1039/C7AY01258F
Brunnbauer L, Gonzalez J, Lohninger H, Bode J, Vogt C, Nelhiebel M, Larisegger S, Limbeck A (2021) Strategies for trace metal quantification in polymer samples with an unknown matrix using Laser-Induced Breakdown Spectroscopy. Spectrochim Acta BAt Spectrosc 183:106272. https://doi.org/10.1016/j.sab.2021.106272
Cai B, Han Y, Liu B, Ren Y, Jiang S (2003) Isolation and characterization of an atrazine-degrading bacterium from industrial wastewater in China. Lett Appl Microbiol 36(5):272–6. https://doi.org/10.1046/j.1472-765x.2003.01307.x
Cao C, Kemper AF, Agapito L, Zhang JW, He Y, Rinzler A, Cheng HP, Zhang XG, Rocha AR, Sanvito S (2009) Nonequilibrium green’s function study of Pd4-cluster-functionalized carbon nanotubes as hydrogen sensors. Phys Rev B 79(7):075127. https://doi.org/10.1103/PhysRevB.79.075127
Chen Y, Luo Z, Lu X (2019) Construction of novel enzyme-graphene oxide catalytic interface with improved enzymatic performance and its assembly mechanism. ACS Appl Mater Interfaces 11(12):11349–11359. https://doi.org/10.1021/acsami.8b20744
Choi HK, Yoon J (2023) Enzymatic electrochemical/fluorescent nanobiosensor for detection of small chemicals. Biosensors 13(4):492. https://doi.org/10.3390/bios13040492
Chung TH, Meshref MNA, Dhar BR (2021) A review and roadmap for developing microbial electrochemical cell-based biosensors for recalcitrant environmental contaminants, emphasis on aromatic compounds. Chem Eng J 424:130245. https://doi.org/10.1016/j.cej.2021.130245
Çimen D, Bereli N, Denizli A (2023) Advanced plasmonic nanosensors for monitoring of environmental pollutants. Curr Anal Chem 19(1):2–17. https://doi.org/10.2174/1573411018666220618155324
Cunha I, Biltes R, Sales MGF, Vasconcelos V (2018) Aptamer-based biosensors to detect aquatic phycotoxins and cyanotoxins. Sens Basel 18(7):2367. https://doi.org/10.3390/s18072367
Deng F, Zhang D, Yang L, LiL LuY, Wang J, Fan Y, Zhu Y, Li X, Zhang Y (2022) Effects of antibiotics and heavy metals on denitrification in shallow eutrophic lakes. Chemosphere 291:132948. https://doi.org/10.1016/j.chemosphere.2021.132948
Dimitrievska I, Paunovic P, Grozdanov A (2023) Recent advancements in nano sensors for air and water pollution control. Mater Sci Eng 7(2):113–128
Do MH, Ngo HH, Guo W, Chang SW, Nguyen DD, Pandey A, Sharma P, Varjani S, Nguyen TAH, Hoang NB (2022) A dual chamber microbial fuel cell based biosensor for monitoring copper and arsenic in municipal wastewater. Sci Total Environ 811:152261. https://doi.org/10.1016/j.scitotenv.2022.152261
Dwivedi HP, Smiley RD, Jaykus L-A (2010) Selection and characterization of DNA aptamers with binding selectivity to Campylobacter jejuni using whole-cell SELEX. Appl Microbiol Biotechnol 87:2323–2334. https://doi.org/10.1007/s00253-010-2728-7
Fatima A, Younas I, Ali MW (2022) An overview on recent advances in biosensor technology and its future application. Arch Pharm Pract 13(1):5–10. https://doi.org/10.51847/LToGI43jil
Flores-Contreras EA, González-González RB, González-González E, Melchor-Martínez EM, Parra-Saldívar R, Iqbal HMN (2022) Detection of emerging pollutants using aptamer-based biosensors: recent advances, challenges, and outlook. Biosens Basel 12(12):1078. https://doi.org/10.3390/bios12121078
Gaviria-Arroyave MI, Cano JB, Peñuela GA (2020) Nanomaterial-based fluorescent biosensors for monitoring environmental pollutants: a critical review. Talanta Open 2:100006. https://doi.org/10.1016/J.TALO.2020.100006
Gavrila S, Ursachi CS, Pera-Crian S, Munteanu FD (2022) Recent trends in biosensors for environmental quality monitoring. Sensors Basel 22(4):1513. https://doi.org/10.3390/s22041513
Ghica ME, Carvalho RC, Amine A, Brett CMA (2013) Glucose oxidase enzyme inhibition sensors for heavy metals at carbon film electrodes modified with cobalt or copper hexacyanoferrate. Sens Actuat B Chem 178:270–278. https://doi.org/10.1016/j.snb.2012.12.113
Grieshaber D, MacKenzie R, Vörös J, Reimhult E (2008) Electrochemical biosensors-sensor principles and architectures. Sensors 8(3):1400–1458. https://doi.org/10.3390/s80314000
Gupta N, Renugopalakrishnan V, Liepmann D, Paulmurugan R, Malhotra BD (2019) Cell-based biosensors: recent trends, challenges and future perspectives. Biosens Bioelectron 141:111435. https://doi.org/10.1016/j.bios.2019.111435
Hara TO, Singh B (2021) Electrochemical biosensors for detection of pesticides and heavy metal toxicants in water: recent trends and progress. ACS EST Water 1(3):462–47. https://doi.org/10.1021/acsestwater.0c00125
Hashem A, Hossain MAM, Marlinda AR, Mamun MA, Simarani K, Johan MR (2021) Nanomaterials based electrochemical nucleic acid biosensors for environmental monitoring: a review. Appl Surf Sci Adv 4:100064. https://doi.org/10.1016/j.apsadv.2021.100064
He Z, Yin H, Chang CC, Wang G, Liang X (2020) Interfacing DNA with gold nanoparticles for heavy metal detection. Biosensors 10:167. https://doi.org/10.3390/bios10110167
Hernandez-Vargas G, Sosa-Hernández JE, Saldarriaga-Hernandez S, Villalba-Rodríguez AM, Parra-Saldivar R, Iqbal HM (2018) Electrochemical biosensors: a solution to pollution detection with reference to environmental contaminants. Biosensors 8(2):29
Huang S, Wang W, Cheng F, Yao H, Zhu JJ (2017) Highly sensitive detection of mercury ion based on T-rich DNA machine using portable glucose meter. Sens Actuat B Chem 242:347–354. https://doi.org/10.1016/j.snb.2016.10.123
Huang Y, Lu D, Liu H, Liu S, Jiang S, Pang GC, Liu Y (2019) Preliminary research on the receptor–ligand recognition mechanism of umami by an hT1R1 biosensor. Food Func 10(3):1280–1287. https://doi.org/10.1039/C8FO02522C
Huang CW, Lin C, Nguyen MK, Hussain A, Bui XT, Ngo HH (2023) A review of biosensor for environmental monitoring: principle, application, and corresponding achievement of sustainable development goals. Bioengineered 14(1):58–80. https://doi.org/10.1080/21655979.2022.2095089
Iravani S, Varma RS (2022) Genetically engineered organisms: possibilities and challenges of heavy metal removal and nanoparticle synthesis. Clean Technol 4(2):502–511. https://doi.org/10.3390/cleantechnol4020030
Jain U, Saxena K, Hooda V, Balayan S, Singh AP, Tikadar M, Chauhan N (2022) Emerging vistas on pesticides detection based on electrochemical biosensors—an update. Food Chem 371:131126. https://doi.org/10.1016/j.foodchem.2021.131126
Javaid PM, Haleem A, Rab S, Singh RP, Suman R (2021) Sensors for daily life: a review. Sens Int 2:100121. https://doi.org/10.1016/j.sintl.2021.100121
Jeon Y, Lee Y, Jang G, Kim BG, Yoon Y (2022) Design of Pb(II)-specific E. coli-based biosensors. Front Microbiol 13:881050. https://doi.org/10.3389/fmicb.2022.881050
Justino CI, Duarte AC, Rocha-Santos TA (2017) Recent progress in biosensors for environmental monitoring: a review. Sens 17:2918. https://doi.org/10.3390/s17122918
Khan S, Burciu B, Filipe CDM, Li Y, Dellinger K, Didar TF (2021) DNAzyme-based biosensors: immobilization strategies, applications, and future prospective. ACS Nano 15(9):13943–13969. https://doi.org/10.1021/acsnano.1c04327
Kharkova AS, Arlyapov VA, Ilyukhina AS, Ponamoreva ON, Alferov VA, Reshetilov AN (2021) A kinetic approach to the formation of two-mediator systems for developing microbial biosensors as exemplified by a rapid biochemical oxygen demand assay. 3 Biotech 11:1–13
Kim YS, Jung HS, Matsuura T, Lee HY, Kawai T, Gu MB (2007) Electrochemical detection of 17β-estradiol using DNA aptamer immobilized gold electrode chip. Biosens Bioelectron 22:2525–2531. https://doi.org/10.1016/j.bios.2006.10.004
Kim HJ, Lim JW, Jeong H, Lee SJ, Lee DW, Kim T, Lee SJ (2016) Development of a highly specific and sensitive cadmium and lead microbial biosensor using synthetic CadC-T7 genetic circuitry. BiosensBioelectron 79:701–708. https://doi.org/10.1016/j.bios.2015.12.101
Kim HJ, Park D, Park Y, Kim DH, Kim J (2022) Electric-field-mediated in-sensor alignment of antibody’s orientation to enhance the antibody-antigen binding for ultrahigh sensitivity sensors. Nano Lett 22(16):6537–6544. https://doi.org/10.1021/acs.nanolett.2c01584
Kolahchi N, Braiek M, Ebrahimipour G, Ranaei-Siadat SO, Lagarde F, Jaffrezic-Renault N (2018) Direct detection of phenol using a new bacterial strain-based conductometric biosensor. J Environ Chem Eng 6:478–484. https://doi.org/10.1016/j.jece.2017.12.023
Kulkarni MB, Ayachit NH, Aminabhavi TM (2022) Recent advancements in nanobiosensors: current trends, challenges, applications, and future scope. Biosensors 12(10):892. https://doi.org/10.3390/bios12100892
Kumar S, Deep A, Wangoo N, Bhardwaj N (2023) Recent advancements in nanomaterials based optical detection of food additives: A review. Analyst 148:5322–5339. https://doi.org/10.1039/D3AN01317K
Kumar J, Melo JS (2017) Overview on biosensors for detection of organophosphate pesticides. Curr Trends Biomed Eng Biosci 5:555–663. https://doi.org/10.19080/CTBEB.2017.05.555663
Lan Y, Coetsier C, Causserand C, Serrano KG (2018) An experimental and modelling study of the electrochemical oxidation of pharmaceuticals using a boron-doped diamond anode. Chem Eng J 333:486–494. https://doi.org/10.1016/j.cej.2017.09.164
Li X, Li D, Zhang Y, Lv P, Feng Q, Wei Q (2020) Encapsulation of enzyme by metal-organic framework for single-enzymatic biofuel cell-based self-powered biosensor. Nano Energy 68:104308. https://doi.org/10.1016/j.nanoen.2019.104308
Li L, He J, Gan Z, Yang P (2021) Occurrence and fate of antibiotics and heavy metals in sewage treatment plants and risk assessment of reclaimed water in Chengdu. China Chemosphere 272:129730. https://doi.org/10.1016/j.chemosphere.2021.129730
Liu J, Lu Y (2003) A colorimetric lead biosensor using DNAzyme-directed assembly of gold nanoparticles. J Am Chem Soc 125:6642–6643. https://doi.org/10.1021/ja034775u
Long F, Zhu A, Shi H, Wang H, Liu J (2013) Rapid on-site/in-situ detection of heavy metal ions in environmental water using a structure-switching DNA optical biosensor. Sci Rep 3:2308. https://doi.org/10.1038/srep02308
Lu D, Liu D, Liu Y, Wang X, Liu Y, Yuan S, Ren R, Pang G (2023) Comparative study on the sensing kinetics of carbon and nitrogen nutrients in cancer tissues and normal tissues based electrochemical biosensors. Molecules 28(14):1453. https://doi.org/10.3390/molecules28031453
Mackulak T, Medvecka E, StanovaAV BP, Grabic R, Golovko O, Marton M, Bodik I, Medvedova A, Gal M, Plany M, Kromka A, Spalkova V, Skulcova A, Horakova I, Vojs M (2020) Boron doped diamond electrode—The elimination of psychoactive drugs and resistant bacteria from wastewater. Vacuum 171:108957. https://doi.org/10.1016/j.vacuum.2019.108957
Mehne FMP, Gunka K, Eilers H, Herzberg C, Kaever V, Stülke J (2013) Cyclic Di-AMP Homeostasis in Bacillus subtilis: Both lack and high level accumulation of the nucleotide are detrimental for cell growth. J Biol Chem 288(3):2004–2017. https://doi.org/10.1074/jbc.M112.395491
Mehta J, Bhardwaj N, Bhardwaj SK, Tuteja SK, Vinayak P, Paul AK, Kim KH, Deep A (2017) Graphene quantum dot modified screen printed immunosensor for the determination of parathion. Anal Biochem 523:1–9. https://doi.org/10.1016/j.ab.2017.01.026
Melnikov P, Bobrov A, Marfin Y (2022) On the use of polymer-based composites for the creation of optical sensors: a review. Polymers. https://doi.org/10.3390/polym14204448
Mirres ACDM, Silva BEPDMD, Tessaro L, Galvan D, Andrade JCD, Aquino A, Joshi N, Conte-Junior CA (2022) Recent advances in nanomaterial-based biosensors for pesticide detection in foods. Biosensors 12(8):572. https://doi.org/10.3390/bios12080572
Muhammad M, Huang Q (2021) A review of aptamer-based SERS biosensors: design strategies and applications. Talanta 227:122188. https://doi.org/10.1016/j.talanta.2021.122188
Mustafaoglu N, Alves NJ, Bilgicer B (2015) Oriented immobilization of fab fragments by site-specific biotinylation at the conserved nucleotide binding site for enhanced antigen detection. Langmuir 31(35):9728–9736. https://doi.org/10.1021/acs.langmuir.5b01734
Naresh V, Lee N (2021) A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 21(4):1109. https://doi.org/10.3390/s21041109
Ni S, Zhuo Z, Pan Y, Yu Y, Li F, Liu J, Wang L, Wu X, Li D, Wan Y, Zhang L, Yang Z, Zhang BT, Lu A, Zhang G (2021) Recent progress in aptamer discoveries and modifications for therapeutic applications. ACS Appl Mater Interfaces 13(8):57057. https://doi.org/10.1021/acsami.0c05750
Nigam VK, Shukla P (2015) Enzyme based biosensors for detection of environmental pollutants—a review. J Microbiol Biotechnol 25(11):1773–1781. https://doi.org/10.4014/jmb.1504.04010
Nikbakt S, Kamarian S, Shakeri M (2018) A review on optimization of composite structures Part I: laminated composites. Compos Struct 195:158–185. https://doi.org/10.1016/j.compstruct.2018.03.063
Ocsoy I, Gulbakan B, Shukoor MI, Xiong X, Chen T, Powell DH, Tan W (2013) Aptamer-conjugated multifunctional nanoflowers as a platform for targeting, capture, and detection in laser desorption ionization mass spectrometry. ACS Nano 7(1):417–427. https://doi.org/10.1021/nn304458m
Paimard G, Ghasali E, Baeza M (2023) Screen-printed electrodes: fabrication, modification, and biosensing applications. Chemosensors 11(2):113. https://doi.org/10.3390/chemosensors11020113
Patel V, Pramod R, Khanna N, Pawar P, Mathuriya AS, Pandit S (2021) Fundamentals of biosensor application in environmental pollutant monitoring. In: Shah MP (eds) Removal of emerging contaminants through microbial processes. Springer, Singapore. https://doi.org/10.1007/978-981-15-5901-3_15
Perez-Fernandez B, Costa-Garcia A, de la Escosura-Muniz A (2020a) Electrochemical (Bio)sensors for pesticides detection using screen-printed electrodes. Biosens 10(4):32. https://doi.org/10.3390/bios10040032
Perez-Fernandez B, Mercader JV, Abad-Fuentes A, Checa-Orrego BI, Costa-García A, de la Escosura-Muniz A (2020b) Direct competitive immunosensor for imidacloprid pesticide detection on gold nanoparticle-modified electrodes. Talanta 209:120465. https://doi.org/10.1016/j.talanta.2019.120465
Porras A, Maranon A (2012) Development and characterization of a laminate composite material from polylactic acid (PLA) and woven bamboo fabric. Compos B Eng 43:2782–2788. https://doi.org/10.1016/j.compositesb.2012.04.039
Prathap MUA, Chaurasia AK, Sawant SN, Apte S (2012) Polyaniline-based highly sensitive microbial biosensor for selective detection of lindane. Anal Chem 84:6672–6678. https://doi.org/10.1021/ac301077d
Ramanathan S, Shi W, Rosen BP, Daunert S (1997) Sensing antimonite and arsenite at the subattomole level with genetically engineered bioluminescent bacteria. Anal Chem 69(16):3380–3384. https://doi.org/10.1021/ac970111p
Ramasubburayan R, Susan T, Pradeep Kumar V, Immanuel G, Palavesam A (2014) Isolation, screening and optimization of culture conditions for enhanced antibacterial activity by a marine epibiotic bacterium Bacillus flexus APGI against fouling bacterial strains. J Pure Appl Microbiol 8:2909–2920
Rathnayake I, Megharaj M, Naidu R (2021) Green fluorescent protein based whole cell bacterial biosensor for the detection of bioavailable heavy metals in soil environment. Environ Technol Innv 23:101785. https://doi.org/10.1016/j.eti.2021.101785
Rebollar-Perez G, Campos-Teran J, Ornelas-Soto N, Mendez-Albores A, Torres E (2016) Biosensors based on oxidative enzymes for detection of environmental pollutants. Biocatalysis 1:118–129. https://doi.org/10.1515/boca-2015-0010
Rodriguez-Mozaz S, Lopez de Alda MJ, Barcelo D (2006) Biosensors as useful tools for environmental analysis and monitoring. Anal Chem 386:1025–1041. https://doi.org/10.1007/s00216-006-0574-3
Roy R, Ray S, Chowdhury A, Anand R (2021) Tunable multiplexed whole-cell biosensors as environmental diagnostics for ppb-level detection of aromatic pollutants. CS Sens 6(5):1933–1939. https://doi.org/10.1021/acssensors.1c00329
Sagiroglu A, Paluzar H, Ozcan HM, Okten S, Sen B (2011) A novel biosensor based on Lactobacillus acidophilus for determination of phenolic compounds in milk products and wastewater. Prep Biochem Biotechnol 41:321–336. https://doi.org/10.1080/10826068.2010.540607
Saidur M, Aziz AA, Basirun W (2017) Recent advances in DNA based electrochemical biosensors for heavy metal ion detection. Biosens Bioelectron 90:125–139. https://doi.org/10.1016/j.bios.2016.11.039
Sailapu SK, Menon C (2022) Engineering self-powered electrochemical sensors using analyzed liquid sample as the sole energy source. Adv Sci Weinh 9(29):2203690
Samal S, Mohanty RP, Mohanty PS, Giri MK, Pati S, Das B (2023) Implications of biosensors and nanobiosensors for the eco-friendly detection of public health and agro-based insecticides: A comprehensive review. Heliyon 9:e15848. https://doi.org/10.1016/j.heliyon.2023.e15848
Shin HJ (2012) Agarose-gel-immobilized recombinant bacterial biosensors for simple and disposable on-site detection of phenolic compounds. Appl Microbiol Biotechnol 93:1895–1904. https://doi.org/10.1007/s00253-011-3700-x
Sun C, Liu M, Sun H, Lu H, Zhao G (2019a) Immobilization-free photoelectrochemical aptasensor for environmental pollutants: design, fabrication and mechanism. Biosens Bioelectron 140:111352. https://doi.org/10.1016/j.bios.2019.111352
Sun C, Ou X, Cheng Y, Zhai T, Liu B, Lou X, Xia F (2019b) Coordination-induced structural changes of DNA-based optical and electrochemical sensors for metal ions detection. Dalton Trans 48:5879–5891. https://doi.org/10.1039/C8DT04733B
Taguchi R, Terai T, Ueno T, Komatsu T, Hanaoka K, Urano Y (2018) A protein-coupled fluorescent probe for organelle-specific imaging of Na. Sens Actuat B Chem 265:575–581. https://doi.org/10.1016/j.snb.2018.03.090
Tang YS, Dong WC, Tang L, Zhang YK, Kong J, Gu JW (2018) Fabrication and investigations on the polydopamine/KH-560 functionalized PBO fibers/cyanate ester wave-transparent composites. Compos Commun 8:36–41. https://doi.org/10.1016/j.coco.2018.03.006
Thakur M, Wang B, Verma ML (2022) Development and applications of nanobiosensors for sustainable agricultural and food industries: Recent developments, challenges and perspectives. Environ Technol Innov 26:102371. https://doi.org/10.1016/j.eti.2022.102371
Tibazarwa C, Corbisier P, Mench M, Bossus A, Solda P, Mergeay M, Wyns L, Van der Lelie D (2001) A microbial biosensor to predict bioavailable nickel in soil and its transfer to plants. Environ Pollut 113:19–26. https://doi.org/10.1016/s0269-7491(00)00177-9
Tovar-Lopez FJ (2023) Recent progress in micro-and nanotechnology-enabled sensors for biomedical and environmental challenges. Sensors 23(12):5406. https://doi.org/10.3390/s23125406
Trapananti A, Eisenmann T, Giuli G, Mueller F, Moretti A, Passerini S, Bresser D (2021) Isovalent versus aliovalent transition metal doping of zinc oxide lithium-ion battery anodes. Mater Today Chem 20:100478. https://doi.org/10.1016/j.mtchem.2021.100478
Tschmelak J, Proll G, Riedt J, Kaiser J, Kraemmer P, Bárzaga L, Wilkinson JS, Hua P, Hole JP, Nudd R (2005) Biosensors for unattended, cost-effective and continuous monitoring of environmental pollution: AWACSS and RIANA. Int J Environ Anal Chem 85:837–852. https://doi.org/10.1080/03067310500149619
Tsopela A, Laborde A, Salvagnac L, Ventalon V, Bedel-Pereira E, Séguy I, Temple-Boyer P, Juneau P, Launay IR (2016) Development of a lab-on-chip electrochemical biosensor for water quality analysis based on microalgal photosynthesis. Biosens Bioelectron 79:568–573. https://doi.org/10.1016/j.bios.2015.12.050
Tucci M, Grattieri M, Schievano A, Cristiani P, Minteer SD (2019) Microbial amperometric biosensor for online herbicide detection: Photocurrent inhibition of Anabaena variabilis. Electrochim Acta 302:102–108. https://doi.org/10.1016/j.electacta.2019.02.007
Vanitha UM, Natarajan M, Sridhar H, Umamaheswari S (2017) Microbial fuel cell characterisation and evaluation of Lysinibacillus macroides MFC02 electrigenic capability. World J Microbiol Biotechnol 33(5):91. https://doi.org/10.1007/s11274-017-2252-3
Vask A, Green T, Polyak B, Mor A, Kahru A, Virta M, Marks R (2007) Fibre-optic bacterial biosensors and their application for the analysis of bioavailable Hg and As in soils and sediments from Aznalcollar mining area in Spain. Biosens Bioelectron 22:1396–1402. https://doi.org/10.1016/j.bios.2006.06.019
Vopalenska I, Vachova L, Palkova Z (2015) New biosensor for detection of copper ions in water based on immobilized genetically modified yeast cells. Biosens Bioelectron 72:160–167. https://doi.org/10.1016/j.bios.2015.05.006
Wang Q, Wang J, Huang Y, Du Y, Zhang Y, Cui Y, Kong DM (2022) Development of the DNA-based biosensors for high performance in detection of molecular biomarkers: more rapid, sensitive, and universal. Biosens Bioelectron 197:113739. https://doi.org/10.1016/j.bios.2021.113739
Wang B, Li Y, Hu H, Shu W, Yang L, Zhang J (2020) Acetylcholinesterase electrochemical biosensors with graphenetransition metal carbides nanocomposites modified for detection of organophosphate pesticides. PLoS ONE 15(4):e0231981. https://doi.org/10.1371/journal.pone.0231981
Webster DP, TerAvest MA, Doud DF, Chakravorty A, Holmes EC, Radens S, Sureka CM, Gralnick JA, Angenent LT (2014) An arsenic-specific biosensor with genetically engineered Shewanella oneidensis in a bioelectrochemical system. Biosens Bioelectron 62:320–324. https://doi.org/10.1016/j.bios.2014.07.003
Wei L, Wang X, Lu D, Li Y, Pang G, Xie J (2016) A novel staphylococcal enterotoxin q immunosensor based on horseradish peroxidase and double-layer gold nanoparticles. Food Anal Methods 10:892–899. https://doi.org/10.1007/s12161-016-0632-1
Willner MR, Vikesland PJ (2018) Nanomaterial enabled sensors for environmental contaminants. J Nanobiotechnol 16(1):1–16. https://doi.org/10.1186/s12951-018-0419-1
Wu W, Qu S, Nel W, Ji J (2022) Tracing and quantifying the sources of heavy metals in the Pearl River Basin. Chemosphere 288:132630. https://doi.org/10.1016/j.chemosphere.2021.132630
Xiong J, Sun Z, Yu JH, Liu H, Wang X (2022) DThermal self-regulatory smart biosensor based on horseradish peroxidase-immobilized phase-change microcapsules. Chem Eng J 430:132982. https://doi.org/10.1016/j.cej.2021.132982
Yang N, Chen X, Ren T, Zhang P, Yang D (2015) Carbon nanotube based biosensors. Sens Actuat B Chem 207:690–715. https://doi.org/10.1016/j.snb.2014.10.040
Yu J, Qian Y, Wang J, Zhang Y, Zhi J (2006) Novel biosensor based on prussian blue-modified screen-printed electrode and its application. Sensors Actuators B Chem 114:774–780. https://doi.org/10.1016/j.snb.2005.07.041
Zhang S, Geryak R, Geldmeier J, Kim S, Tsukruk VV (2017) Synthesis, assembly, and applications of hybrid nanostructures for biosensing. Chem Rev 117(20):12942–13038. https://doi.org/10.1021/acs.chemrev.7b00088
Zhao S, Zhou T, Khan A, Chen Z, Liu P, Li XA (2022) A novel electrochemical biosensor for bisphenol A detection based on Engineered Escherichia coli cells with a surface-display of Tyrosinase. Sens Actuators B Chem 353:131063. https://doi.org/10.1016/j.snb.2021.131063
Zhuo Z, Yu Y, Wang M, Li J, Zhang Z, Liu J, Wu X, Lu A, Zhang G, Zhang B (2017) Recent advances in SELEX technology and aptamer applications in biomedicine. Int J Mol Sci 18(10):2142
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
J.M., M.R., H.S, and S.S.M. contributed to writing—original draft. R.S. and B.P. were involved in reviewing. S.N contributed to figure edition. L.G. was involved in validation. N.T. contributed to supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manjunathan, J., Revathi, M., Sowmya, H. et al. Recent advancements in nanotechnological approaches for pollution monitoring and environmental sustainability. Clean Techn Environ Policy (2023). https://doi.org/10.1007/s10098-023-02676-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10098-023-02676-z