Abstract
Lithium metal and silicon nanowires, with higher specific capacity than graphite, are the most promising alternative advanced anode materials for use in next-generation batteries. By comparing three batteries designed, respectively, with a lithium metal anode, a silicon nanowire anode, and a graphite anode, the authors strive to analyse the life cycle of different negative electrodes with different specific capacities and compare their cradle-to-gate environmental impacts. This paper finds that a higher specific capacity of the negative material causes lower environmental impact of the same battery. The battery with a lithium metal anode has a lower environmental impact than the battery with a graphite anode. Surprisingly, although the silicon nanowire anode has a higher specific energy than graphite, the production of a battery with silicon nanowires causes a higher environmental impact than the production of a battery with graphite. In fact, the high specific energy of silicon nanowires can decrease the environmental impact of a battery with silicon nanowires, but silicon nanowire preparation causes extremely high emissions. Therefore, batteries with lithium metal anodes are the most environmentally friendly lithium-ion batteries. Batteries with lithium metal anodes could be the next generation of environmentally friendly batteries for electric vehicles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With environmental concerns and the depletion of fossil fuels, an increasing number of studies have been focused on traction batteries and electric vehicles (EVs). As an alternative to internal combustion engine vehicles, EVs are considered the future of road transportation and dramatically reduce the consumption of fossil oil and air pollution during operation. At present, lithium-ion batteries (LIBs), which have a high specific capacity, lightweight, long cycle life compared to conventional battery technologies (Cho et al. 2017), and mature technology (Peters et al. 2017), are widely used in EVs (Manthiram 2017). However, current LIBs are unable to power a vehicle over a long driving distance to meet the demands of practical applications due to the traditional use of graphite (C) materials, which have a small theoretical specific capacity of 372 mAh/g, and the limited specific capacity of the cathode. Therefore, high energy capacity is a key factor to be considered for next-generation traction batteries (Wang et al. 2017). Unfortunately, no significant advancements in the specific capacity of cathode materials have been demonstrated to date. Therefore, seeking replacements for current graphite anodes (C-A) is necessary to improve the specific capacity of LIBs, of which lithium metal (Li) and silicon are the most promising negative materials for their high specific energy, 3860 and 4200 mAh/g, respectively. To increase the specific capacity of batteries, Li and silicon are being pursued as future high-energy-density anode materials in traction batteries (Andre et al. 2017).
Although EVs are complimented for producing zero tailpipe emissions, they still cause damaging impacts on the environment due to serious contamination from the LIB manufacturing process. To solve this problem and provide recommendations for the sustainable development of traction batteries, many studies have used the life cycle assessment (LCA) method to quantify and compare environmental impacts of different batteries or to identify opportunities to improve the environmental performance of the battery manufacturing process (Peters et al. 2017). Matheys et al. (2009) assessed the environmental impacts of various traction battery technologies, such as lead–acid, nickel–cadmium, nickel metal hydride, sodium–nickel chloride, and lithium-ion technologies, and found that the impacts of sodium nickel–chloride and lithium-ion batteries were lower than those of the other batteries. Majeau-Bettez et al. (2011) analysed the LCA results of LIBs and nickel metal hydride batteries (NiMH) and proved that NiMH technology had the highest environmental impact. Yu et al. (2014) performed an LCA on LIB and NiMH batteries and found that batteries with a high energy density and long life expectancy had low environmental impacts. Argonne National Laboratory performed an intensive LCA study on LIBs. The researchers used a process-level approach for LiMn2O4 batteries and found that the cradle-to-gate energy was 75 MJ/kg and greenhouse gas emissions were 5.1 kg CO2-eq/kg (Dunn et al. 2014). Another study on different LIBs focused on their assembly process and indicated that low-throughput facilities consumed higher energy than near-capacity facilities (Dunn et al. 2015).
With advances in enhancing the specific capacity of anode materials, several articles reported LCAs of batteries with different anodes. Authors (Lastoskie and Dai 2015) found that when Li was substituted for C in the anode, the specific capacity of the battery cell increased by 18%, and the environmental impacts were lower. Another group (Kushnir and Sanden 2011) estimated the energy consumption of batteries with different electrodes, and more energy was consumed during the nanomaterial manufacturing, though cathodes or anodes employing nanomaterials had a longer battery life and higher energy efficiency levels than cathodes/anodes using non-nanomaterials. Dunn et al. (2015) showed that the cradle-to-gate energy consumption of a battery with a LNCM (0.5Li2MnO3·0.5LiNi0.44Co0.25Mn0.31O2) cathode and graphite–silicon blend anode was higher than that of a battery with a LNCM cathode and C-A. Li et al. (2014) revealed that the LCA results of batteries with silicon nanowire anodes (SiNW-As) were moderately higher than those of conventional LIBs. SiNW-As contributed a significant share in the total battery global warming potential (15%) and total battery human toxicity potential (10%). For Lastoskie and Dai, the use of higher specific energy anodes can reduce the environmental impact of a battery pack. Several studies by Kushnir and Sanden, Dunn et al. and Li et al. have produced contradictory results showing that batteries with a higher specific energy anode result in a higher impact than batteries with lower specific energy. Furthermore, understanding how an anode’s specific energy affects the cradle-to-gate LCA result is difficult and unclear.
This paper focuses on the effect of the specific energy of anodes on the LCA result, and the aim is to explain the controversy of existing literature, to propose an eco-friendly and promising future traction battery with an advanced anode material, and to provide suggestions to reduce the emissions of traction battery production. In our work, we present a prospective LCA of three LIBs: new LIBs with a lithium metal anode (Li-A) and a SiNW-A, and a traditional LIB with a C-A. The cradle-to-gate method is conducted. To further understand the effect of total cradle-to-gate anode environmental impacts, we focus on the environmental impacts of anode materials and processing per kg. The contributions of the principal battery components to the overall impacts per kWh are analysed, which can provide a thorough understanding of the significant influence of anode specific energy on the total cradle-to-gate environmental impacts of the battery. We also compare the environmental impacts of three different batteries to provide the most promising and eco-friendly LIB.
Materials and methods
Life cycle assessment (LCA)
LCA is a standardized and objective assessment tool (ISO 14040 2006). Many studies have used LCA to quantify the environmental impacts of products or processes (Peters et al. 2017), and it considers the whole life cycle, from raw material acquisition to the product manufacturing, use, end-of-life treatment, recycling, and disposal phases. LCA can assist in clarifying possible impacts associated with products and can address these impacts or recommend eco-friendlier products to decision-makers. Cradle-to-gate LCA is a variant of LCA that takes material acquisition and product manufacturing as key considerations. Because production is a dominant contributor to environmental impact in the industry, the cradle-to-gate method is used in this study.
First, the environmental impacts of different anode materials on LIBs were measured, including C, Li, and silicon nanowires (SiNWs). Moreover, the total emission potentials of three batteries were compared. Additionally, sensitivity analyses focusing on the specific energy and cycle life were performed. The functional unit (FU) is a basic unit serving both quantification and comparison. There are three functional units used in this study. To compare the LCA results of different anode materials and processing, the first FU used in this cradle-to-gate LCA results is the mass of the anode per kg. The second FU of this study is 1 kWh of storage capacity, which is used to quantify the LCA results of three batteries. Because different battery technologies have different lifetimes, the third FU is based on 1 kWh battery stored energy over the lifetime. The ReCiPe (H) [v1.11] midpoint method, a state-of-the-art method to convert life cycle inventories to life cycle environmental impacts (Huijbregts et al. 2017), is used to calculate the battery LCA results. Initially, 18 impact categories are considered. Finally, in reference to the category of environmental impacts reported by Peters et al. (2016), eight types of impact categories are chosen: fossil depletion potential (FDP), global warming potential (GWP), terrestrial acidification potential (TAP), human toxicity potential (HTP), freshwater eutrophication potential (FEP), particulate matter formation (PMF), metal depletion potential (MDP), and marine eutrophication potential (MEP). OpenLCA (1.6.3), an open-source LCA software developed by GreenDelta, is used for this study. R statistical software, version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria), is used to plot the figures.
Battery modelling
Figure 1 shows the main components of the lithium-ion battery model. The battery pack can be divided into four parts: battery cell, packaging, battery management systems (BMS), and cooling system. The battery cell consists of subcomponents, including an anode, a cathode, a separator, an electrolyte, and a cell container. The capacity of three battery packs is assumed to be the same (100 kWh). Since lithium nickel cobalt manganese oxide in the cathode is widely used in LIBs (Zhang et al. 2009) and has a high specific capacity matching that of Li or silicon, it is used as the cathode in this paper, which agrees with the literature report by Li et al. (2014). For comparison, the positive active materials of the three batteries are all mixed with LiNi1/3Mn1/3Co1/3O2 (NCM), polyvinyl fluoride and carbon black (92:4:4, weight ratio). Positive current collectors are made of aluminium foil. The cathode in this paper is called NCM cathode for short. The negative electrodes are mainly made of graphite (C), lithium metal (Li), and silicon nanowires (SiNWs), respectively. Negative current collectors are made of copper foil in the C-A and SiNW-A. The specific capacity of Li is 3860 mAh/g, and the density is 0.546 g/cm3 (Ye et al. 2017b). The specific capacity of C is 365 mAh/g, and the density is 2.23 g/cm3 (Wu et al. 2016). The specific capacity of SiNWs is 2400 mAh/g, and the density is 2.33 g/cm3 (Li et al. 2014). Lithium hexafluorophosphate is used as the electrolyte. The polypropylene material is used as a separator. The cell case of the battery is made of a multilayer pouch. The cell mass composition data come from our model. Background inventory data are available in Ecoinvent 3.3 with a cut-off system. Battery inventory is based on that in Ellingsen et al. (2014), SiNW-A inventory refers to that from Li et al. (2014), and Li-A inventory refers to that from Zackrisson et al. (2016). The masses of the main components account for 60% (battery cell), 3.7% (BMS), 4.1% (cooling systems), and 32% (packaging), respectively (Ellingsen et al. 2014). European electricity mix data from Ecoinvent 3.3 are used in cell manufacturing. Overall, a LIB pack with a NCM cathode and a Li-A (NCM-Li), a LIB pack with a NCM cathode and a C-A (NCM-C), and a LIB pack with a NCM cathode and a SiNW-A (NCM-SiNWs) are designed in this study. Detailed data about the battery mass composition are available in the supplementary materials (Tables S1–S5).
Results
This section presents a cradle-to-gate LCA of three anodes and their related processes and three battery packs. Figure 2 explains the cradle-to-gate environmental impacts of three different anodes per kg. To understand the contributions to different anode materials, an LCA of anode-related background processes is conducted (Fig. 3). Figure 4 shows the cradle-to-gate environmental impacts of NCM-C, NCM-Li, and NCM-SiNWs per kWh and provides the most eco-friendly and promising future traction battery. Table 1 describes the influence of cell manufacturing energy consumption on the three battery LCA results. The results of the sensitivity analyses are shown in Figs. 5 and 6.
Contribution of the principal anode components and processing to C-A, Li-A, and SiNW-A, respectively (FU: per kg of anodes). Copper processing = copper processed into copper foil; Lithium chloride processing = lithium chloride processed into Li; Silicon powder processing = silicon powder processed into SiNWs
Environmental impacts in the production of 1 kg anodes
The cradle-to-gate environmental impacts of three anodes are given in Fig. 2. Of the eight impact categories, SiNW-A has the highest impact among all anodes and is attributed to silicon powder processing. Silicon powder processing is the most significant contributor (35–96%) to SiNW-A impacts (see Fig. 3c). C-A has the lowest impact compared to Li-A and SiNW-A for FDP, FEP, GWP, HTP, PMF, and TAP, while for MDP and MEP, Li-A outscores C-A, definitively resulting from copper production, which is the main contributor to C-A impacts for MDP and MEP (96 and 93%, respectively), as shown in Fig. 3a. Li-A in this paper does not include the copper foil because Li as an anode can be used alone as a current collector. Li-A, therefore, has a lower MDP and MEP than C-A. For MDP, the use of lithium is not as metal depletion factor in the ReCiPe method. Figure 3b illustrates the total per impact into three contributors, and lithium chloride and lithium chloride processing are the key contributors. Figure 3c shows that the impact of silicon powder production on the environment is relatively small, and the primary driver is processing silicon powder into SiNWs as mentioned previously. In addition, copper production in SiNW-A is also the principal contributor for MDP (60%) and MEP (42%). In summary, the results indicate that different materials and different production processes significantly affect the total cradle-to-gate anode environmental impacts. For advanced anode materials, the use of Li has greater potential to reduce the emissions of anode production than the use of SiNWs, but Li-A does not exhibit substantial advantages over C-A.
Environmental impacts in the production of 1 kWh battery
As our original assumption, the anode selection is a key factor influencing the environmental impacts of a battery. The higher specific energy of anode indirectly leads to less pollution during battery production. To provide substantial evidence for the assumption, the impact results of NCM-C, NCM-Li, and NCM-SiNWs are compared as follows.
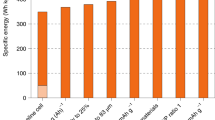
According to specific literature, the C, Li, and SiNWs in this study have specific capacities of 365 mAh/g (Wu et al. 2016), 3860 mAh/g (Ye et al. 2017b), and 2400 mAh/g (Li et al. 2014), respectively. The battery components and electrode materials used in battery pack production are shown in Tables S1–S5. The battery specific capacities of the three batteries designed in this study, the NCM-C, NCM-Li, and NCM-SiNWs, are 127, 212, and 164 Wh/kg, respectively. As expected, using anode materials with high specific capacities, in place of C, could expand the specific capacity of traction batteries.
Figure 4 displays the obvious environmental advantage of NCM-Li. The NCM-Li battery has the highest specific capacity due to the high capacity of Li; therefore, the environmental impact of NCM-Li batteries is all the lowest among the three batteries. Surprisingly, although silicon nanowires have a higher specific capacity than C, the NCM-SiNW battery causes more pollution than NCM-C batteries because the environmental impacts from SiNW-A preparation are much higher than those from C-A preparation, comprising between 31 and 74% of the total impacts of the NCM-SiNWs battery. The use of SiNW-A actually could reduce overall battery emissions. For example, aside from the environmental impacts of the anode, other components of NCM-SiNWs batteries combined contribute a lower impact than components of NCM-C batteries, as we can see from Fig. 4. The results support that a higher specific capacity of anode materials in the batteries produces less pollution. NCM-Li is the most environmentally friendly battery.
Another important result in Fig. 4 is that anode use significantly affects the total cradle-to-gate battery environmental impacts. Regarding the total NCM-SiNWs battery impact, SiNW-A is the most significant contributor for FDP (50%), GWP (41%), HTP (47%), MEP (74%), and PMF (43%). The SiNW-A also influences three other impact categories of NCM-SiNWs battery production, contributing to 39% of FEP, 31% of MDP, and 38% of TAP, respectively. The driver behind MDP in SiNW-A is the copper production. The use of ethanol from rye causes 43% of SiNW-A’s MEP. The driver behind other impact categories is the hydrogen fluoride production, comprising between 60 and 86% of SiNW-A. Interestingly, for NCM-C, the anode contributes the largest share (74%) for MEP and the second largest share (31%) for MDP, which result from copper production, as discussed previously. Contrary to NCM-SiNWs and NCM-C, Li-A contributes a relatively low percentage (0.3–5%) of the cradle-to-gate NCM-Li battery environmental impacts. Li and SiNWs commonly have higher specific energies than C, which means lower mass of these two anodes is required in the battery to achieve the same performance. Meanwhile, the environmental impacts of Li production are obviously lower than those of SiNW production, as mentioned before. It is these basic differences in the mass of anode need and the production of the anode material supply chain that drive differences in the cradle-to-gate environmental impacts of traction batteries.
Overall, the specific capacity of the negative materials and the anode used has crucial effects on the LCA results of traction batteries. Owing to Li with a high specific capacity and low environmental impact, NCM-Li is the best traction battery.
Total impact of 100 kWh battery production
In Table 1, the results of eight impact capacities are calculated for 100 kWh battery production. To assess the influence of the facility and throughput on the battery LCA results, the energy usage for cell manufacturing is conducted here. Based on previously published data acquired from the factory (Ellingsen et al. 2014), we assume that energy usage from cell manufacturing is approximately 103 MJ/kg when the facility is at full load with the highest energy efficiency. Energy consumption is 168 MJ/kg for near-full-load manufacturing. Energy consumption is 406 MJ/kg when the facility operates at a low capacity. The calculation results in this paper agree with a previously published paper (100–400 MJ/kg) (Ellingsen et al. 2015).
As given in Table 1, the seven impact categories, GWP, FDP, FEP, HTP, MEP, PMF, and TAP, are obviously modified under different energy efficiency values. Notably, the GWP value can decrease from 16.7 to 12.7 tonnes CO2-eq at full load and can increase to 32.2 tonnes CO2-eq at low load. Moreover, for NCM-Li, GWP can be reduced to 8.6 tonnes CO2-eq at full load and can increase to 20.4 tonnes CO2-eq under low-load operation. For NCM-SiNWs, the GWP value can be reduced to a minimum value of 18.9 tonnes CO2-eq at full load and can increase to 34.2 tonnes CO2-eq at low-load conditions. In addition, the window of change in GWP for NCM-Li is slightly smaller than that of NCM-C, which is mainly due to the high specific energy of NCM-Li battery packs and a smaller baseline of the total per impact compared to NCM-C. When emissions reduction reaches a certain level, the emissions reduction rate will show a smooth trend that approaches a boundary value. Moreover, the increase in the impact of NCM-SiNWs is obviously smaller than those of NCM-C and NCM-Li. The main reason is that the anode preparation instead of cell manufacturing is the key contributor to the total NCM-SiNWs battery impact; therefore, the potential of NCM-SiNWs battery production to reduce emissions by improving energy efficiency is smaller than the potential reduction in emission from NCM-C and NCM-Li battery production. Other types of environmental impacts also show the same trend.
Overall, the throughput of the facility clearly has a crucial influence on the environmental impact of battery production. A facility with higher energy efficiency consumes less energy and has a lower impact on cell manufacturing. NCM-Li batteries have a great potential to reduce emissions by improving the energy efficiency of the facility. We suggest that the facilities operated to produce battery cells should be near to or at full load, which agrees with the findings in Ellingsen et al. (2014).
Sensitivity analysis
Influence of specific energy
Since specific energy can be affected by changes to technology, different possible specific energies are considered here. Currently, the specific capacity of C can reach 365 mAh/g (Wu et al. 2016), and the theoretical specific energy is 372 mAh/g. Therefore, two cases of C specific capacity (365 and 372 mAh/g) are used in the analysis. According to the existing literature (Wu et al. 2016), the efficiency of the Li capacity utilization is 33, 50, 80, and 100%, respectively. The four cases of Li (1287/1930/3088/3860 mAh/g) are used to perform a sensitivity analysis. Referencing the peer review article (Li et al. 2014), the specific capacity of SiNWs can reach 2400 mAh/g, and the theoretical specific capacity is 4200 mAh/g. Therefore, the above two cases are considered. Figure 5 shows that the increase in specific energy of the anode material leads to a reduction in the environmental impact of NCM-C, NCM-Li, and NCM-SiNWs, respectively. In addition, even though C and SiNWs reach their maximum specific energy, the total NCM-C battery or NCM-SiNWs battery per impact is still higher than the total NCM-Li battery per impact. Obviously, the result suggests that the NCM-Li battery is the most environment-friendly LIB. New LIBs using Li-A would excel in comparison with traditional LIBs under environmental considerations.
Influence of cycle life
Cycle life has a vital influence on the environmental impacts of traction battery production. To define the influence of cycle life in three batteries (NCM-C, NCM-Li, and NCM-SiNWs), sensitivity analysis is performed here. NCM-C batteries can be cycled up to 2000 times, according to the results of Ellingsen et al. (2014). Since traction batteries with Li-As or SiNW-As are in an early stage of development, their certain lifetimes are still not well understood in the published paper. Furthermore, Peters et al. summarized the cycle life of NCM-based batteries, and the maximum, minimum, and average cycle life are 3000, 935, and 1006 cycles, respectively (Peters et al. 2017). Therefore, the cycle life of NCM-Li and NCM-SiNWs in this study is assumed in four cases as follows: 935/1006/2000/3000 cycles. The NCM-C cycle life is assumed to be 2000 times. According to the result, the environmental impacts of NCM-Li are all worse when cycle life is assumed to be 935 and 1006 cycles, except for MEP. When the cycle life exceeds 2000 cycles, NCM-Li clearly outperforms NCM-C in all impact categories. For NCM-SiNWs, the cycle life must reach 3000 cycles to achieve similar impacts to those of NCM-C. Consequently, if the cycle life of LIBs with Li-A is the same or even outperforms traditional LIBs, LIBs using Li-A will be very useful in the future. However, the advantage of NCM-SiNWs is still not clear under certain environmental circumstances.
Discussion
To explain how the anode specific energy affects the total battery under environmental aspects, to find significant environmental impact factors, propose some suggestions to reduce emissions of battery production, and provide eco-friendly and promising future LIBs, the midpoint method was used to calculate the life cycle environmental impacts of NCM-Li, NCM-SiNWs, and NCM-C. Since battery production is a key contributor to life cycle impacts, and new LIBs with Li-As and SiNW-As are still in the developing stages and have not yet reached the commercial use stage, this study focused on cradle-to-gate environmental impacts.
For better comparability with each other, we assumed the three battery packs were made of the same materials but contained different anodes. In addition, the anodes (Li-A, SiNW-A, and C-A) were analysed separately first. From Fig. 2, Li-A did not exhibit substantial advantages over C-A in environmental impact or 1 kg anode production. To our surprise, NCM-Li paired with Li-A appeared to be much better than other batteries with a 1 kWh storage capacity battery pack. Increased specific energy could reduce the impacts of battery production, which agrees with Lastoskie et al. Moreover, NCM-Li has a great potential to reduce emissions by further increasing the energy efficiency of a factory. Overall, specific energy is one of the key factors in the total battery impact. We also reveal that the anode used is another key factor for the environmental impact of batteries. In addition, energy efficiency in the factory is a third factor. Moreover, traction batteries with Li-As would be the most promising battery under environmental aspects and 1 kWh of storage capacity.
The NCM-C designed in this study had a specific capacity of approximately 127 Wh/kg, which was in the range 100–155.6 Wh/kg reported in the existing literature (Peters et al. 2017). For LCA results of LIBs with Li–As, some articles were published in recent years. In the analysis of lithium–air battery cells (Li–O2), Zackrisson et al. (2016) concluded that cell manufacturing was the major contributor to battery life cycle environmental impacts. The author reached the same conclusion in other article about LFP-Li (Zackrisson 2016), and both results are similar to our result about NCM-Li. In addition, Zackrisson et al. analysed that the GWP by 1 kg cell of Li–O2 and LFP-Li were 20.91 and 23.05 kg CO2-eq, respectively. In this paper, the GWP result from 1 kg of NCM–Li cells was 21.12 kg CO2-eq, which is consistent with Zackrisson et al. To further prove the environmental advantages of lithium-sulphur batteries (Li–S), LCA results of Li–S and NCM-C were discussed by Deng et al. (2017). The results showed that the GWP of Li–S production was lower than the GWP of NCM-C, which compared well with our study. However, Deng et al. did not explain the effect of the Li specific capacity on the LCA results (2017), which might be limited by the design of the Li–S battery. Because the materials used for the positive electrode and the electrolyte were different from those of the NCM-C battery, the author could not effectively compare the influence of the different negative electrode materials on the LIBs. In contrast, we use the same cathodes, electrolyte and other components in three batteries, and therefore, the use of Li-A was proven to reduce the total battery environmental impacts compared to the environmental impacts from the use of C-A.
Our study has shown that the environmental impact from the production of NCM-SiNWs was significantly higher than those from the production of NCM-C, which agrees with the conclusion of Li et al. (2014). However, compared with the results of Li et al., the disparity between NCM-SiNWs and NCM-C in the LCA results of this study is much smaller. The data of Li et al. are based on GaBi6 professional database, while our LCA result is based on Ecoinvent 3.3 database, which may result in the difference in LCA results. Furthermore, the design data of the cell in this study are different from the data from Li et al. Contrary to Li et al., a cost factor was considered in our analysis. At the same battery capacity, our scheme uses a lower SiNW mass, which is aligned better with the actual situation. This phenomenon can be explained by the capacity ratio of the negative electrode to the positive electrode (N/P ratio), which is an essential factor in cell design. Commonly, the N/P ratio is in the range of 1–1.2 and will affect the battery capacity. For example, Liu et al. (2014) studied this problem and designed an N/P ratio of the two NCM cells to be at a minimum of 1.06 and a maximum of 1.19, respectively. They found that the cell with the higher N/P ratio showed a lower capacity. In this paper, the N/P ratio of the NCM-SiNW and NCM-C battery cells is approximately 1.15, which is similar to that found by Kang et al. (2014), and lies in the range of 1–1.2. However, the N/P ratio in the study by Li et al. was much higher than 1.2, which not only wasted extra anode material, but also occupied a greater volume in EVs, increased the total mass, and reduced the specific capacity of the battery.
Silicon has the highest specific capacity (4200 mAh/g) and is of low cost, abundant and environmentally friendly (Martha and Nagaraja 2017). However, the applications of silicon are limited because, during the lithium insertion and extraction process, silicon materials used in the battery suffer a drastic volume change (up to 400%), the silicon materials become pulverized, and the battery capacity fades (Chan et al. 2008). Compared to silicon particles, SiNWs have no significant volume effect during lithium insertion and extraction processes (Chan et al. 2008). Martha and Nagaraja (2017) had the same idea as Chan et al.; however, the preparation technique of SiNWs relies on pollution-prone technologies. The process of creating SiNWs from silicon particles is the leading cause of pollution in the production of NCM-SiNWs. Therefore, reducing the environmental pollution in the SiNWs preparation is particularly important. If the pollution of this process can be reduced, LIBs with SiNW-As would be very competitive candidates for next-generation traction batteries.
Cycle life has an important effect on the LCA results of traction batteries. NCM-Li and NCM-SiNWs are still in the early stages, and the short battery lifetime is also a large problem that urgently needs to be solved. Fortunately, scientists have achieved encouraging results in extending the life span (Ye et al. 2017a). With the development of technology, these problems will all be solved. If the cycle life of NCM-Li can approach or surpass 2000 cycles (80% DOD) and the cycle life of NCM-SiNWs can reach over 3000 cycles (80% DOD), NCM-Li and NCM-SiNWs will have fewer environmental impacts than traditional NCM-C. Li-A and SiNW-A would be expected to replace C-A as next-generation environmentally friendly traction battery anode materials.
Our analysis contains some limitations. Because NCM-Li and NCM-SiNW batteries have not been used in practical EV applications, cycle life and battery efficiency stability data, and the environmental impact of battery use and the recycle phase could not be easily measured. Additionally, the cost of three batteries was not analysed or discussed in this study because the representative data of these costs are hard to obtain. However, the economic conditions are also important factors for practical battery use. In the future, these issues need to be considered in order to provide further insights into the potentials of new LIBs.
Conclusions
This paper presented a prospective cradle-to-gate LCA of three LIBs with a Li-A, a SiNW-A, and a C-A, respectively. The ReCiPe (H) midpoint method was employed to calculate the LCA result. Based on our model, a cell mass composition list of three batteries was provided in this study. This study reveals that NCM-Li is the most environmentally friendly new LIB based on a 1 kWh storage capacity and the cycle life approaching or surpassing that of NCM-C. Since the specific energy of C is too low to meet rapidly growing energy demands, LIBs with Li-As would be eco-friendly and promising future traction batteries. The specific energy of the anode material, the anode production technique, the energy efficiency of the factory and the cycle life are all key factors in the environmental impact of batteries. First, in the same battery, higher specific energy anodes produce less pollution during battery production. Second, the cradle-to-gate environmental impact of SiNW-A production is higher than that of the other two anodes. Third, the NCM-Li battery has a great potential to reduce emissions by improving the energy efficiency of the facility. Finally, with cycle lives of approximately 2000 cycles for NCM-Li and over 3000 cycles for NCM-SiNWs, these batteries would outperform batteries made using NCM-C. Furthermore, battery production facilities should operate at near to or at full load to reduce emissions from cell manufacturing. The preparation technique of SiNWs should rely on technologies that are eco-friendly in order to have environmental advantages, and traction batteries with Li-As should be encouraged.
At present, the main limitations of the Li-A include the problems of safety, low coulombic efficiency, volume expansion (Zuo et al. 2017), and short life span (Zhang et al. 2017). Li-A has not yet been practically used in rechargeable battery (Li et al. 2016). Luckily, in recent years, many scholars have made great progress on the above issues. For example, Ye et al. (2017a) suppressed dendrite formation and achieved a lifespan of 1000 cycles with a lithium surplus of only 5%. Liu et al. summarized modification strategies for Li-A and concluded that Li-A is fascinating for high-energy-density batteries. Scholars have demonstrated the feasibility of future developments of Li-A, which shows its potential. Due to the high specific capacity, lightweight, and great environmental advantages, Li-A will likely be widely used as anode material in future traction batteries.
Abbreviations
- 1,4-DB:
-
1,4-Dichlorobenzene
- BMS:
-
Battery management systems
- C:
-
Graphite
- C-A:
-
Graphite anode
- CO2 :
-
Carbon dioxide
- DoD:
-
Depth of discharge
- EVs:
-
Electric vehicles
- FDP:
-
Fossil depletion potential
- Fe:
-
Iron
- FEP:
-
Freshwater and marine eutrophication
- FU:
-
Functional unit
- GWP:
-
Global warming potential
- HTP:
-
Human toxicity potential
- kg eq:
-
Kilograms equivalents
- LCA:
-
Life cycle assessment
- LFP:
-
LiFePO4
- LFP-Li:
-
Battery with LiFePO4 cathode and lithium metal anode
- Li:
-
Lithium metal
- Li-A:
-
Lithium metal anode
- LIBs:
-
Lithium-ion batteries
- Li–O2 :
-
Lithium–air battery cells
- Li–S:
-
Lithium–sulphur battery
- LNCM:
-
0.5Li2MnO3·0.5LiNi0.44Co0.25Mn0.31O2
- MDP:
-
Metal depletion potential
- MEP:
-
Marine eutrophication potential
- N:
-
Nitrogen
- N/P ratio:
-
Capacity ratio of the negative electrode to the positive electrode
- NCM:
-
Lithium nickel cobalt manganese oxide, LiNi1/3Mn1/3Co1/3O2
- NCM-C:
-
Lithium-ion battery pack with NCM cathode and graphite anode
- NCM-Li:
-
Lithium-ion battery pack with NCM cathode and lithium metal anode
- NCM-SiNWs:
-
Lithium-ion battery pack with NCM cathode and silicon nanowire anode
- P:
-
Phosphor
- PM10:
-
Particulate matter less than 10 μm in diameter
- PMF:
-
Particulate matter formation
- SiNWs:
-
Silicon nanowires
- SiNW-A:
-
Silicon nanowire anode
- SO2 :
-
Sulphur dioxide
- TAP:
-
Terrestrial acidification potential
References
Andre D, Hain H, Lamp P, Maglia F, Stiaszny B (2017) Future high-energy density anode materials from an automotive application perspective. J Mater Chem A 5:17174–17198. https://doi.org/10.1039/c7ta03108d
Chan CK, Peng H, Liu G, McIlwrath K, Zhang XF, Huggins RA, Cui Y (2008) High-performance lithium battery anodes using silicon nanowires. Nat Nanotechnol 3:31–35. https://doi.org/10.1038/nnano.2007.411
Cho S, Jang HY, Jung I, Liu LC, Park S (2017) Synthesis of embossing Si nanomesh and its application as an anode for lithium ion batteries. J Power Sources 362:270–277. https://doi.org/10.1016/j.jpowsour.2017.07.048
Deng YL, Li JY, Li TH, Gao XF, Yuan C (2017) Life cycle assessment of lithium sulphur battery for electric vehicles. J Power Sources 343:284–295. https://doi.org/10.1016/j.jpowsour.2017.01.036
Dunn JB, Gaines L, M. B, Sullivan J, Wang M (2014) Material and energy flows in the materials production, assembly, and end-of-life stages of the automotive lithium-ion battery life cycle. Argonne National Laboratory. https://greet.es.anl.gov/publication-lib-lca. Accessed on 5 Oct 2017
Dunn JB, Gaines L, Kelly JC, James C, Gallagher KG (2015) The significance of Li-ion batteries in electric vehicle life-cycle energy and emissions and recycling’s role in its reduction. Energy Environ Sci 8:158–168. https://doi.org/10.1039/c4ee03029j
Ecoinvent 3.3. http://www.ecoinvent.org/database. Accessed on 10 Sept 2017
Ellingsen LAW, Majeau-Bettez G, Singh B, Srivastava AK, Valoen LO, Stromman AH (2014) Life cycle assessment of a lithium-ion battery vehicle pack. J Ind Ecol 18:113–124. https://doi.org/10.1111/jiec.12072
Ellingsen LAW, Majeau-Bettez G, Stromman AH (2015) The significance of Li-ion batteries in electric vehicle life-cycle energy and emissions and recycling’s role in its reduction in energy and environmental science. J Ind Ecol 19:518–519. https://doi.org/10.1111/jiec.12309
Huijbregts MAJ et al (2017) ReCiPe2016: a harmonised life cycle impact assessment method at midpoint and endpoint level. Int J Life Cycle Assess 22:138–147. https://doi.org/10.1007/s11367-016-1246-y
ISO 14040 (2006) Environmental management—life cycle assessment—principles and framework. International Organization of Standardization, Geneva
Kang KS et al (2014) Effect of additives on electrochemical performance of lithium nickel cobalt manganese oxide at high temperature. J Power Sources 253:48–54. https://doi.org/10.1016/j.jpowsour.2013.12.024
Kushnir D, Sanden BA (2011) Multi-level energy analysis of emerging technologies: a case study in new materials for lithium ion batteries. J Clean Prod 19:1405–1416. https://doi.org/10.1016/j.jclepro.2011.05.006
Lastoskie CM, Dai Q (2015) Comparative life cycle assessment of laminated and vacuum vapour-deposited thin film solid-state batteries. J Clean Prod 91:158–169. https://doi.org/10.1016/j.jclepro.2014.12.003
Li BB, Gao XF, Li JY, Yuan C (2014) Life cycle environmental impact of high-capacity lithium ion battery with silicon nanowires anode for electric vehicles. Environ Sci Technol 48:3047–3055. https://doi.org/10.1021/es4037786
Li NW, Yin YX, Yang CP, Guo YG (2016) An artificial solid electrolyte interphase layer for stable lithium metal anodes. Adv Mater 28:1853–1858. https://doi.org/10.1002/adma.201504526
Liu S, Xiong L, He C (2014) Long cycle life lithium ion battery with lithium nickel cobalt manganese oxide (NCM) cathode. J Power Sources 261:285–291. https://doi.org/10.1016/j.jpowsour.2014.03.083
Majeau-Bettez G, Hawkins TR, Stromman AH (2011) Life cycle environmental assessment of lithium-ion and nickel metal hydride batteries for plug-in hybrid and battery electric vehicles. Environ Sci Technol 45:5454. https://doi.org/10.1021/es2015082
Manthiram A (2017) An outlook on lithium ion battery technology. ACS Central Sci 3:1063–1069. https://doi.org/10.1021/acscentsci.7b00288
Martha R, Nagaraja HS (2017) Effect of current density and electrochemical cycling on physical properties of silicon nanowires as anode for lithium ion battery. Mater Charact 129:24–30. https://doi.org/10.1016/j.matchar.2017.04.001
Matheys J, Timmermans JM, Van Mierlo J, Meyer S, Van den Bossche P (2009) Comparison of the environmental impact of five electric vehicle battery technologies using LCA. Int J Sust Manuf 1:318–329
OpenLCA. http://www.openlca.org/. Accessed on 11 Sept 2017
Peters J, Buchholz D, Passerini S, Weil M (2016) Life cycle assessment of sodium-ion batteries. Energy Environ Sci 9:1744–1751. https://doi.org/10.1039/c6ee00640j
Peters JF, Baumann M, Zimmermann B, Braun J, Weil M (2017) The environmental impact of Li-Ion batteries and the role of key parameters—a review. Renew Sustain Energy Rev 67:491–506. https://doi.org/10.1016/j.rser.2016.08.039
Wang D, Zhang W, Zheng W, Cui X, Rojo T, Zhang Q (2017) Towards high-safe lithium metal anodes: suppressing lithium dendrites via tuning surface energy. Adv Sci (Weinh) 4:1600168. https://doi.org/10.1002/advs.201600168
Wu JY, Liu P, Hu YS, Li H (2016) Calculation on energy densities of lithium ion batteries and metallic lithium ion batteries. Energy Storage Sci Technol 5:443–453
Ye H, Xin S, Yin YX, Li JY, Guo YG, Wan LJ (2017a) Stable Li plating/stripping electrochemistry realized by a hybrid Li reservoir in spherical carbon granules with 3D conducting skeletons. J Am Chem Soc 139:5916–5922. https://doi.org/10.1021/jacs.7b01763
Ye H et al (2017b) Synergism of Al-containing solid electrolyte interphase layer and Al-based colloidal particles for stable lithium anode. Nano Energy 36:411–417. https://doi.org/10.1016/j.nanoen.2017.04.056
Yu YJ, Chen B, Huang K, Wang X, Wang D (2014) Environmental impact assessment and end-of-life treatment policy analysis for Li-ion batteries and Ni–MH batteries. Int J Environ Res Pub He 11:3185–3198. https://doi.org/10.3390/ijerph110303185
Zackrisson M (2016) Life cycle assessment of long life lithium electrode for electric vehicle batteries http://ri.diva-portal.org/smash/get/diva2:1131667/FULLTEXT01.pdf. Accessed on 17 Oct 2017
Zackrisson M, Fransson K, Hildenbrand J, Lampic G, O’Dwyer C (2016) Life cycle assessment of lithium-air battery cells. J Clean Prod 135:299–311. https://doi.org/10.1016/j.jclepro.2016.06.104
Zhang CF, Yang P, Dai X, Xiong X, Zhan J, Zhang YL (2009) Synthesis of LiNi1/3Co1/3Mn1/3O2 cathode material via oxalate precursor. T Nonferrous Met Soc 19:635–641. https://doi.org/10.1016/S1003-6326(08)60325-8
Zhang R, Li NW, Cheng XB, Yin YX, Zhang Q, Guo YG (2017) Advanced micro/nanostructures for lithium metal anodes. Adv Sci (Weinh) 4:1600445. https://doi.org/10.1002/advs.201600445
Zuo TT, Wu XW, Yang CP, Yin YX, Ye H, Li NW, Guo YG (2017) Graphitized carbon fibers as multifunctional 3D current collectors for high areal capacity Li anodes. Adv Mater. https://doi.org/10.1002/adma.201700389
Acknowledgements
We are very grateful to Professor Xiaoming Ma for helpful discussions, to the editor and reviewers for their valuable comments, and to Qinhong Luo for his valuable help with plotting the data. We would like to thank James Ding and Lianyi Quan for helping the researchers to check grammar errors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, Z., Kong, D. Comparative life cycle assessment of lithium-ion batteries with lithium metal, silicon nanowire, and graphite anodes. Clean Techn Environ Policy 20, 1233–1244 (2018). https://doi.org/10.1007/s10098-018-1548-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-018-1548-9