Abstract
In this study, electro-Fenton (EF) process was used for the decolorization and mineralization of Basic Black 3 (BB3) (a popular textile dye), and the treatment performance of carbon fiber was investigated as a different cathode material to in situ electro-generated H2O2. Total organic carbon (TOC) and color removal were selected as performance indicators of the process. For this purpose, some operational parameters such as pH, Fe2+ concentration, current, oxygen flow rate, and supporting electrolyte concentration were optimized, and the best treatment conditions were found as 3, 100 mg/L, 200 mA, 0.5 L/min, and 50 mM, respectively. At these optimum conditions, complete color removal and 39.1 % TOC removals were achieved in 100 min of electrolysis time. The results obtained from the study indicate that EF process can mineralize and decolorize BB3, therefore, it can be selected as a pre-treatment technology before different conventional treatment methods for reducing toxicity and enhancing the biodegradability of wastewater. According to the results, carbon fiber cathode may offer a wastewater treatment alternative by electrochemical technologies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Textile industries consume a huge amount of water during dyeing, printing, and washing. Their water consumption is between 25 and 250 m3 per ton of product (depending on the type of production process). Studies show that about 40 million tons of textiles are produced every year in the world and the annual effluents produced in these industries are about 4–8 million m3 (Rahimi et al. 2013). From among them, basic dyes (also called cationic dyes) are primarily used for acrylic, nylon, silk, and wool dyeing in textile industries (Dod et al. 2015), and these dyes are included in water-soluble dye class which makes them more stable to degradation and difficult to treat them by conventional treatment methods (Gupta and Suhas 2009).
Textile wastewater originated from various dying processes has a complex structure and its content consists of a large number of pollutants including dyes (Khouni et al. 2012). In general, textile dyes are resistant to heat, light, and oxidizing reactive and mostly non-biodegradable. Most of dyes cause visual problems like transmitting color to the water bodies. Contaminated water environment with dyes affects aquatic life by preventing the transmission of sunlight. The wastewater originated from textile industries includes large amounts of dyes, and some of them are very toxic even at low concentration. Because of potential toxicity of dyes to human health, a lot of them have been called as hazardous pollutants (Hao et al. 2014). Due to the toxicity of the dye substances textile industries have been forced to meet tight environmental legislations (Vasconcelos et al. 2015). In addition, color pollution in aquatic medium causes negative esthetic effect on the water medium (Anirudhan and Ramachandran 2015). The color removal of textile wastewater is hence of great significance before discharging it into the environment.
There are several techniques to remove dyes from aqueous phase efficiently, such as coagulation, biodegradation, photodegradation, adsorption, ozonation, Fenton processes, and biosorption (Shen et al. 2015). Advanced oxidation processes (AOPs) have been widely used to remove toxic and persistent pollutants (Gökkuş et al. 2014). In particular, Fenton oxidation has been applied for textile dye processes and decolorization of wastewater that contains dye (Gökkuş and Çiner 2010). However, Fenton process has a significant disadvantage of producing ferric hydroxide sludge, and its disposal requires additional separation and operations.
In recent years, a large amount of research has been conducted on the application of electrochemical methods to environment protection. In this regard, a novel EF method was developed for the treatment of wastewater containing recalcitrant contaminants (Dimoglo et al. 2004). EF is one of the AOPs and this process is based on the formation of hydroxyl radicals that are highly reactive species. Produced hydroxyl radicals lead to oxidation of recalcitrant organic compounds until their degradation and finally convert them into CO2, water, and inorganic ions (Hammami et al. 2012). The EF oxidation method, which is one of the most known AOPs, appears to be economical, more efficient, and eco-friendly, because H2O2 can be continually produced in situ from reduction of O2 at the cathode surface in acidic solution (Eq. 1), and by this way generates OH* with the iron catalyst in solution by Eq. (2) (Tang et al. 2015).
However, its application is limited by such drawbacks as the difficulty in controlling the dose of ferrous salts for catalytic oxidation, and the high cost and danger in the production, transportation, and storage of H2O2 (Sun et al. 2015). In addition, energy requirement is another important parameter which should be considered in the selection of the process type (Kordkandi and Ashiri 2015). Most AOP methods are energy intensive for radical production to remove organic matter. In this regard, calculation of energy consumption may be beneficial to compare with other AOP’s. Therefore, the EF process has been under intense study due to its continuity in situ electro-generation of H2O2 from carbon-based cathodes (Sun et al. 2015).
When the literature is considered, the studies related to carbon fiber used as cathode in EF reactions appear to be limited. According to the literature, an acceleration of the abatement rate can be achieved in EF process using different carbon-based cathodes which present high electrode surfaces. Oxidation power of the EF system is mainly related to cathode material used in the electrolysis (Özcan et al. 2008). In addition, the electro-generation of hydrogen peroxide, either for EF application or for direct oxidation and disinfection, successfully occurs on carbon-based cathodes due to their high overpotential toward water discharge (Petrucci et al. 2016).
In this study, the main purpose was to evaluate the removal of color and TOC from a BB3 dye solution by the EF process using a carbon fiber cathode material. For this purpose, the effects of the operational parameters such as ferrous ion (catalyst) concentration, pH, supporting electrolyte concentration and applied current on the color, and TOC removal efficiencies were investigated. Moreover, the energy consumption of the EF system at optimum conditions was determined.
Experimental
Materials and reagents
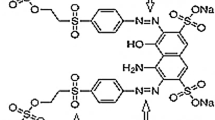
Commercially available BB3 dyestuff was purchased from Sirma Kimya manufacturer in Istanbul/Turkey and used without any pre-treatment. The name, color index (CI) number and chemical structure of the BB3 are shown in Fig. 1 and its main features are shown in Table 1. Deionized water was used to prepare dyestuff solutions. The pH of the sample was adjusted to the desired pH interval (2.0–6.0) using 1 N H2SO4 and 1 N NaOH. Solutions at different concentrations of supporting electrode were prepared from NaCl (Merck, 99.5 %).
Analytical methods
Chemical oxygen demand was determined according to the standard methods (2120 C. spectrophotometric method) (APHA 1998). A UV–vis spectrophotometer (Hach Lange DR2500) was used to measure color absorbance of the dye solutions. The wavelength at maximum absorbance of dye solution was determined as 601 nm by spectrum scanning between 300 and 800 nm. Turbidity of the BB3 solution was measured using a turbidimeter (Hach Lange 2100 AN). The pH and conductivity were measured with a multi parameter electrode (Hach Lange HQ40D). The mineralization of BB3 was monitored by a decrease in TOC value, determined using a Shimadzu TOC-L analyzer. The removal percentage of color was calculated according to Eq. (3).
where A 0 and A t are the absorbance values (nm) at initial and time t at the λmax value, respectively.
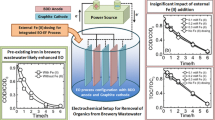
The carbon fiber cathode with the area of 200 cm2 was purchased from Spinteks Textile & Carbon Co. (Denizli, Turkey). After each treatment period the carbon fiber cathode was immersed in sulfuric acid solution for one night, then washed with deionized water and dried in oven. There is a strong relationship between electrochemical H2O2 production and volumetric surface area and the electrode porosity (Coria et al. 2015). In the present study, carbon fiber cathode was preferred because it has a very high specific surface area, and SEM images of carbon fiber are shown in Fig. 2.
Iron (II) sulfate heptahydrate (Merck 99.5 %) was used as catalyst in EF reactions. On the other hand, Pt anode has been successfully used to degrade various toxic and recalcitrant compounds such as herbicides and pesticides because it releases much lower amounts of toxic metallic ions and has no negative effect on environment (Brillas et al. 1998; Jiang and Zhang 2007).
Experimental setup and procedure
The experiments were carried out in a cylindrical and undivided glass cell with volume of 500 ml (105 mm internal diameter and 145 mm height), supplied with a magnetic stirrer (800 rpm). A platinum wire (length = 110 mm) was used as the anode and immersed in the center of the cell, which contained BB3 dye solution. A carbon fiber wounded glass reactor was used as the electrolytic cell and anode was placed vertically in the reactor during the electrolysis (Fig. 3). The potential of working electrodes was controlled by a DC (GWinstek SDP 3606, 30 V, 6A). In case of necessity, Na2SO4 (Merck 99 %) was used as supporting electrolyte. Additionally, the carbon fiber cathode was fed at two different pure O2 gas (96 % purity) flow rates (0.2 and 0.5 L/min) to continuously generate H2O2 from O2 reduction. The length between anode and cathode was 4 cm during all of the experiments.
Electrolysis time was kept constant as 100 min for each run. Samples were withdrawn from the reactor at 10 min intervals. Aliquots of 2 mL were withdrawn from EF reactor and finally, centrifuged at 4000 rpm before analysis. All of the experiments were carried out at room temperature.
Results and discussion
The performance of the EF system in the removal of color and TOC from the dye solution depends on the amount of produced hydroxyl radical at the solution. It seems that different electrodes affect significantly on hydrogen peroxide generation rate in the EF reactions (Pignatello et al. 2006). In this sense, cathode materials play an important role on the performance of EF system. Therefore, this study focuses on the efficiency of the carbon fiber cathode in EF treatment. To this end, several experiments were carried out using carbon fiber cathode in order to investigate the effect of operating parameters on EF reaction such as pH, applied current, oxygen flow rate, and supporting electrolyte concentration.
Effect of initial pH
The pH is one of the most important factors for the EF process. It is a well-known fact that Fenton processes are efficient in the acidic range, and the oxidation ability of hydroxyl radical is fairly strong at acidic conditions (Babuponnusami and Muthukumar 2012). In conventional Fenton applications, iron species begin to precipitate as ferric hydroxides at higher pH values. On the other hand, iron species form stable complexes with H2O2 at lower pH values, leading to deactivation of catalysts (Nidheesh and Gandhimathi 2012). In acidic pH values, there is a significant production of OH* directly related to the oxidation of organic matter, evidencing the influence of the pH on the color and COD removal efficiencies (Çiner and Gökkuş 2013). Therefore, the significant production of free radicals at acidic conditions enhances the oxidation of organics (Babuponnusami and Muthukumar 2012). The results of mineralization and color removal at different pH values are shown in Fig. 4.
The effect of pH values on treatment performance was investigated using different pH values within the range of 2–5. According to Fig. 4a, the highest mineralization rate (21.4 %) was found when initial pH was maintained at a value around three. This could be caused by the increase in ionic strength at excessively low pH values. Many researchers report that pH 3 is the most convenient pH value for Fenton reaction. The results obtained in the study showed similarity to those of Rosales (Rosales et al. 2009). On the other hand, the best color removal results (95.5 %) were attained at pH 4 (Fig. 4b), a value different from optimum pH of TOC removal. This result is directly related to that fact H2O2 is electro-generated at the cathode by the two electron reduction of dissolved oxygen in the acidic solution during electrolysis (Eq. 1) and thus, electro-generation of high amount of H2O2 at low pH promotes the production of more OH* radical and provides higher removal results.
Effect of Fe2+ concentration
In the Fenton reactions, hydroxyl radicals could be generated from hydrogen peroxide and ferrous ions (Fenton’s reagent) with proportionate ratios (Eq. 2). There exists an optimal concentration of ferrous ions for the oxidative degradation of TOC (Chen and Liang 2008). Figure 5 shows the effect of Fe2+ concentration on color removal and TOC mineralization by applying 50 mA at pH 3.0 during treatment of BB3 solution of 100 mg/L. In the investigation of optimum Fe2+ optimization experiments, Fe2+ concentration was changed in the range of 25–150 mg/L.
According to Fig. 5, 100 mg/L Fe2+ catalyst concentration provided the best removal results for both TOC (Fig. 5a) and color (Fig. 5b) removals, but further increase on Fe2+ concentration didn’t change the EF treatment results. At these optimum conditions 25.8 % TOC and 83.6 % color removals were obtained. When initial Fe2+ concentration was increased to 150 mg/L, both TOC and color removal efficiencies decreased dramatically. It has been reported by some researchers that an excess of ferrous ions would consume hydroxyl radicals, accordingly, no improvements could have been achieved when Fe2+ concentration increased than that of the optimum value (Fernades Rêgo et al. 2014). Eventually, our findings for the investigation of optimum catalyst concentration are in agreement with the literature.
Effect of applied current
The mineralization of organic pollutants in the EF system is closely associated with the amount of OH* radicals produced by the Fenton’s reaction (Eq. 2), where H2O2 is generated in situ by O2 reduction on the cathode (Eq. 1). As reported earlier by many authors the production rate of H2O2 on the cathode is affected by the applied current (Bañuelos et al. 2014; Fernades Rêgo et al. 2014; Nidheesh and Gandhimathi 2013). In order to investigate the effect of the applied current on pollutant removal efficiencies of EF process several experiments were carried out in the range of 50–200 mA current values at operation time 0–100 min and at initial pH of 3.0. The results of experiments are depicted in Fig. 6. Removal of color and TOC was directly proportional to the applied current values, and increased with electrolysis time.
As shown in Fig. 6a, the best TOC removal efficiency of 27.5 % was achieved at 200 mA current value in 100 min. As seen in Fig. 6a there are significant differences between 200 mA and other applied current values which may be explained by the greater production of H2O2 from Eq (1) leading to generation of higher amount of hydroxyl radicals from Fenton’s reaction Eq (2). Likewise, color removal results have shown similar trends to the TOC removal, and 200 mA current values have provided the best removal results for both TOC and color removals. At this optimum current, 98.7 % color removal was obtained in 100 min (Fig. 6b). The optimum current for this study was selected as 200 mA. Another remarkable result derived from Fig. 6 showed that the time required for the treatment decreased with the increase in applied current density. The time required was drastically shortened in particular when the applied current density was raised from 50 to 200 mA.
Effect of oxygen flow rate
The solution pH, electrolyte type, and cathode material have important roles on EF process regarding electrochemical H2O2 production (Zhang et al. 2008). In the EF reactions, oxygen is passed through the liquid medium at the beginning of the reaction. Then, the diffused oxygen is adsorbed onto active cathodic sites, and as a result, it is converted into hydrogen peroxide by cathodic reduction. Eventually, the removal efficiency would increase when the flow rate of oxygen is enhanced (Daneshvar et al. 2008). In the current study, the effect of O2 flow rate on color and TOC removals at optimum conditions was investigated using two different O2 flow rates (0.2 and 0.5 L/min). Figure 7a, b shows the TOC and color removal changes at different O2 flow rates.
In this part of the study, two different oxygen flow rates were tested to compare the effect of oxygen flow rate on TOC and color removal performance. The results show that the removal efficiencies increased just slightly, with the increase in the oxygen flow rate, and after 100 min of electrolysis, the removal efficiencies at 0.5 L/min oxygen flow rate were 28.4 and 100 % for TOC and color, respectively while TOC and color removal efficiencies were 27.5 and 98.7 % at 0.2 L/min oxygen flow rate, respectively. These results are in accordance with the report from Wang et al. (2008). The difference between the TOC and color removals is probably attributable to the higher dissolved oxygen concentration around the carbon fiber cathode and enhancement of mass transfer by the flow of oxygen bubbles with increasing flow rate from 0.2 to 0.5 L/min. Accordingly, the amount of H2O2 production is thought to be higher at high O2 flow rates. This result can be easily observed in the 80th min of electrolysis. Later than the 80th min of the electrolysis, 0.5 L/min flow rate provided better results than 0.2 L/min oxygen flow rate. Hence, 0.5 L/min was selected as optimum oxygen flow rate and it was used for the next step in this study.
Effect of supporting electrolyte concentration
Supporting electrolyte improves the solution conductivity, and accelerates the electron transfer, thus benefiting the EF reaction. When the solution conductivity is not sufficient, supporting electrolyte is needed (Nidheesh and Gandhimathi 2012). In this study, Na2SO4, one of the most commonly used supporting electrolytes was added both to increase the electrical conductivity of dye solution and to test its effect on the removal efficiency. Five different Na2SO4 concentrations (20, 30, 40, 50, and 60 mM) were tested for this purpose, and results are depicted in Fig. 8a, b.
The most important factor in any electrochemical method is the energy consumption. The ohmic potential drop in the solution and the anode and cathode overpotentials cause higher electrical energy consumption in electrochemical systems (Tezcan et al. 2013). According to Fig. 8a, TOC removals increased with increasing supporting electrolyte concentration up to 50 mM (39.3 %). Then, a drastic decrease in TOC removal was observed when the supporting electrolyte concentration was increased to 60 mM (21.3 %). The possible explanation for this is the consumption of the generated hydroxyl radical by high SO4 2− concentration (Zhou et al. 2007) by the following equation:
Optimal supporting electrolyte concentration, therefore, would be 50 mM for this system. In addition, color of the dye solution at all investigated supporting electrolyte concentrations was completely removed (Fig. 8b). However, according to Fig. 8b, 50 mM supporting electrolyte concentration shows best removal trend in the first 60 min with similar results to the TOC removal. Using EF process, the most effective treatment results were achieved in short treatment times. In addition, Fig. 9 shows the color differences of the dye solutions during electrolysis period.
Specific energy consumption (SEC)
The SEC of the EF process at a given time “t” was obtained from the following equation:
where V (voltage) and I (ampere) are the average cell voltage until time t (hour) and applied current, respectively. By using Eq. (5), energy consumption of EF process for each supporting electrolyte concentrations were determined and results were given in Fig. 10.
As seen in Fig. 10, energy consumption of the process decreases with an increase in supporting electrolyte concentration, and the minimum energy consumption belongs to 60 mM Na2SO4 concentration. According to Fig. 10, while the maximum energy consumption with 20 mM Na2SO4 concentration is 4.96 kWh m−3, energy consumption is 3.67 kWh m−3 when supporting electrolyte concentration is increased to 60 mM. What could be the possible reason for this is the conductivity of solution increased by the addition of a supporting electrolyte decreasing ohmic resistance between the anode and the cathode to minimal levels. Hence, the energy consumption decreases because of the reduction of the applied potential (Tezcan et al. 2013). Although energy consumption decreases with increasing supporting electrolyte concentration, TOC removal trend is not similar to energy consumption in this study as stated before (Sect. 3.5). On the other hand, when the cathode potential is above the optimal value, the competitive electrode reactions (parasitic reactions), such as the discharge of O2 and H2, may take place and as a result, it may decrease the amount of generated H2O2 in the electrolytic cell (Lei et al. 2010). Consequently, this situation may affect the EF results in a negative way. Accordingly, in order to provide cost-effectiveness, EF system should be carried out at pre-determined optimal operational conditions and by this way, higher removal efficiencies associated with leads to some extend to an increase lower energy consumption can be provided. However, future experiments should be carried out in order to better understand the impact of operational parameters for carbon fiber cathode on different wastewater samples.
Conclusions
In this study, treatment of BB3 dye solution was studied, and the effects of operational parameters on the EF processes using carbon-based carbon fiber cathode and Pt wire anode were evaluated based on removal efficiencies (TOC and color). Batch experiments in EF experiments show that the best operational conditions for the treatment of BB3 are pH 3, I = 200 mA, Fe2+ = 100 mg/L, pure oxygen flow rate = 0.5 L/min in terms of color removal and TOC mineralization. At these optimum operational conditions, color and TOC removals in 100 min electrolysis time are 100 and 39.3 %, respectively. According to the results while pH, current, Fe2+ concentration have an significant influence on the EF processes, oxygen flow rate does not have so much effect as others. Accordingly, the best removal percentages were achieved with 0.5 L/min oxygen flow rate; yet, it would be advisable to use 0.2 L/min for the treatment of BB3 as it provides similar results to those of 0.5 L/min. Fe(II) as a catalyst, leads to some extend to an increase in TOC removal of basic dye BB3, but a further increase in Fe2+ concentration does not change the EF treatment results because redundancy of iron (II) catalyst consumes hydroxyl radicals and causes a decrease in TOC removal performance. To decrease energy consumption, Na2SO4 was used as the supporting electrolyte in the experiments. Although the energy consumption decreases with increasing supporting electrolyte concentration, a higher supporting electrolyte concentration than 50 mM causes negative effect on the performance of the process in terms of the TOC mineralization efficiency. The experimental results of color and TOC removal (%) indicate that the use of carbon fiber cathode in EF process may be a suitable treatment alternative for textile wastewater containing various basic dyes.
Abbreviations
- Ao :
-
Absorbance value (nm) at initial
- AOPs:
-
Advanced oxidation processes
- APHA:
-
American Public Health Association
- At :
-
Absorbance value (nm) at time “t”
- BB3:
-
Basic Black 3
- CI:
-
Color index
- COD:
-
Chemical oxygen demand (mg/L)
- DC:
-
Direct current
- EF:
-
Electro-Fenton
- I :
-
Applied current (mA)
- OH*:
-
Hydroxyl radical
- SEC:
-
Specific energy consumption (kWh m−3)
- SEM:
-
Scanning electron microscopy
- t:
-
Time (min)
- TOC:
-
Total organic carbon (mg/L)
- V :
-
Potential (V)
- λmax :
-
Wavelength at maximum absorbance (nm)
- η:
-
Efficiency of removal (%)
References
Anirudhan TS, Ramachandran M (2015) Adsorptive removal of basic dyes from aqueous solutions by surfactant modified bentonite clay (organoclay): kinetic and competitive adsorption isotherm. Process Safe Environ Prot 95:215–225. doi:10.1016/j.psep.2015.03.003
Apha A (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington DC
Babuponnusami A, Muthukumar K (2012) Advanced oxidation of phenol: a comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem Eng J 183:1–9
Bañuelos JA et al (2014) Study of an air diffusion activated carbon packed electrode for an electro-Fenton wastewater treatment. Electrochim Acta 140:412–418. doi:10.1016/j.electacta.2014.05.078
Brillas E, Mur E, Sauleda R, Sanchez L, Peral J, Domènech X, Casado J (1998) Aniline mineralization by AOP’s: anodic oxidation, photocatalysis, electro-Fenton and photoelectro-Fenton processes. Appl Catal B 16:31–42
Chen W-S, Liang J-S (2008) Decomposition of nitrotoluenes from trinitrotoluene manufacturing process by electro-Fenton oxidation. Chemosphere 72:601–607. doi:10.1016/j.chemosphere.2008.03.005
Çiner F, Gökkuş Ö (2013) Treatability of dye solutions containing disperse dyes by fenton and fenton-solar light oxidation processes. CLEAN Soil Air Water 41:80–85. doi:10.1002/clen.201000500
Coria G, Pérez T, Sirés I, Nava JL (2015) Mass transport studies during dissolved oxygen reduction to hydrogen peroxide in a filter-press electrolyzer using graphite felt, reticulated vitreous carbon and boron-doped diamond as cathodes. J Electroanal Chem. doi:10.1016/j.jelechem.2015.09.031
Daneshvar N, Aber S, Vatanpour V, Rasoulifard MH (2008) Electro-Fenton treatment of dye solution containing orange II: influence of operational parameters. J Electroanal Chem 615:165–174. doi:10.1016/j.jelechem.2007.12.005
Dimoglo A, Akbulut HY, Cihan F, Karpuzcu M (2004) Petrochemical wastewater treatment by means of clean electrochemical technologies. Clean Techn Environ Policy 6:288–295. doi:10.1007/s10098-004-0248-9
Dod R, Banerjee G, Saini D (2015) Removal of methylene blue (MB) dye from water environment by processed jowar stalk [Sorghum bicolor (L.) Moench] adsorbent. Clean Technol Environ Policy 17:2349–2359. doi:10.1007/s10098-015-0977-y
Fernades Rêgo FE, Sales Solano AM, da Costa Soares IC, da Silva DR, Martinez Huitle CA, Panizza M (2014) Application of electro-Fenton process as alternative for degradation of Novacron Blue dye. J Environ Chem Eng 2:875–880. doi:10.1016/j.jece.2014.02.017
Gökkuş Ö, Çiner F (2010) Investigation of color and COD removal from wastewater containing disperse yellow 119 and disperse red 167 using Fenton oxidation process. J Facult Eng Arch Gazi Univ 25:49–55
Gökkuş Ö, Çoşkun F, Kocaoğlu M, Yıldız YŞ (2014) Determination of optimum conditions for color and COD removal of reactive blue 19 by Fenton oxidation process. Desalin Water Treat 52:6156–6165. doi:10.1080/19443994.2013.812523
Gupta VK, Suhas (2009) Application of low-cost adsorbents for dye removal—a review. J Environ Manag 90:2313–2342. doi:10.1016/j.jenvman.2008.11.017
Hammami S, Oturan MA, Oturan N, Bellakhal N, Dachraoui M (2012) Comparative mineralization of textile dye indigo by photo-Fenton process and anodic oxidation using boron-doped diamond anode. Desalin Water Treat 45:297–304. doi:10.1080/19443994.2012.692059
Hao Y-f, Yan L-g, Yang K, Yu S-j, Shan R-r, Du B (2014) Comparative study on adsorption of basic and acid dyes by hydroxy-aluminum pillared bentonite. J Mol Liquid 199:202–207. doi:10.1016/j.molliq.2014.09.005
Jiang C-C, Zhang J-F (2007) Progress and prospect in electro-Fenton process for wastewater treatment. J Zhejiang Univ Sci A 8:1118–1125
Khouni I, Marrot B, Amar RB (2012) Treatment of reconstituted textile wastewater containing a reactive dye in an aerobic sequencing batch reactor using a novel bacterial consortium. Sep Purif Technol 87:110–119. doi:10.1016/j.seppur.2011.11.030
Kordkandi SA, Ashiri R (2015) Modeling and kinetics study of acid anthraquinone oxidation using ozone: energy consumption analysis. Clean Technol Environ Policy 17:2431–2439. doi:10.1007/s10098-015-0967-0
Lei H, Li H, Li Z, Li Z, Chen K, Zhang X, Wang H (2010) Electro-Fenton degradation of cationic red X-GRL using an activated carbon fiber cathode. Process Safe Environ Prot 88:431–438. doi:10.1016/j.psep.2010.06.005
Nidheesh P, Gandhimathi R (2012) Trends in electro-Fenton process for water and wastewater treatment: an overview. Desalination 299:1–15
Nidheesh PV, Gandhimathi R (2013) Removal of Rhodamine B from aqueous solution using graphite–graphite electro-Fenton system. Desalin Water Treat 52:1872–1877. doi:10.1080/19443994.2013.790321
Özcan A, Şahin Y, Koparal SA, Oturan MA (2008) Carbon sponge as a new cathode material for the electro-Fenton process: comparison with carbon felt cathode and application to degradation of synthetic dye basic blue 3 in aqueous medium. J Electroanal Chem 616:71–78. doi:10.1016/j.jelechem.2008.01.002
Petrucci E, Da Pozzo A, Di Palma L (2016) On the ability to electrogenerate hydrogen peroxide and to regenerate ferrous ions of three selected carbon-based cathodes for electro-Fenton processes. Chem Eng J 283:750–758. doi:10.1016/j.cej.2015.08.030
Pignatello JJ, Oliveros E, MacKay A (2006) Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol 36:1–84
Rahimi S et al (2013) Application of TiO2/UV-C photocatalytic process in removal of reactive red 198 dye from synthetic textile wastewater. Asian J Chem 25:7427
Rosales E, Pazos M, Longo MA, Sanromán MA (2009) Electro-Fenton decoloration of dyes in a continuous reactor: a promising technology in colored wastewater treatment. Chem Eng J 155:62–67. doi:10.1016/j.cej.2009.06.028
Shen J, Wu Y-n, Zhang B, Li F (2015) Adsorption of rhodamine B dye by biomimetic mesoporous SiO2 nanosheets. Clean Technol Environ Policy 17:2289–2298. doi:10.1007/s10098-015-0970-5
Sun M, Zhang G, Liu Y, Liu H, Qu J, Li J (2015) Highly efficient AuPd/carbon nanotube nanocatalysts for the electro-Fenton process. Chem A Eur J 21:7611–7620. doi:10.1002/chem.201406676
Tang Q, Wang D, Yao D, Yang C, Sun Y (2015) Heterogeneous electro-Fenton oxidation of p-nitrophenol with a reusable fluffy clump steel wire. Desalin Water Treat 1–11. doi:10.1080/19443994.2015.1070758
Tezcan Un U, Koparal AS, Bakir Ogutveren U (2013) Fluoride removal from water and wastewater with a bach cylindrical electrode using electrocoagulation. Chem Eng J 223:110–115. doi:10.1016/j.cej.2013.02.126
Vasconcelos VM, Ponce-de-León C, Nava JL, Lanza MRV (2015) Electrochemical degradation of RB-5 dye by anodic oxidation, electro-Fenton and by combining anodic oxidation-electro-Fenton in a filter-press flow cell. J Electroanal Chem. doi:10.1016/j.jelechem.2015.07.040
Wang C-T, Hu J-L, Chou W-L, Kuo Y-M (2008) Removal of color from real dyeing wastewater by electro-Fenton technology using a three-dimensional graphite cathode. J Hazard Mater 152:601–606. doi:10.1016/j.jhazmat.2007.07.023
Worlddyevariety.com/basic-dyes/basic-black-3.html (Last accessed: October 21th 2015)
Zhang G, Yang F, Gao M, Fang X, Liu L (2008) Electro-Fenton degradation of azo dye using polypyrrole/anthraquinonedisulphonate composite film modified graphite cathode in acidic aqueous solutions. Electrochim Acta 53:5155–5161. doi:10.1016/j.electacta.2008.01.008
Zhou M, Yu Q, Lei L, Barton G (2007) Electro-Fenton method for the removal of methyl red in an efficient electrochemical system. Sep Purif Technol 57:380–387. doi:10.1016/j.seppur.2007.04.021
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gökkuş, Ö. Oxidative degradation of Basic Black 3 by electro-generated Fenton’s reagent using carbon fiber cathode. Clean Techn Environ Policy 18, 1525–1534 (2016). https://doi.org/10.1007/s10098-016-1134-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-016-1134-y