Abstract
Polymerase chain reaction (PCR) for the diagnosis of Clostridium difficile infection (CDI) might result in overdiagnosis. The clinical outcomes of symptomatic CDI patients diagnosed by PCR remain uncertain. We aimed to determine whether patients whose diagnosis of CDI was based on PCR had different characteristics and clinical outcomes than those diagnosed by toxin immunoassay. Consecutive CDI patients, hospitalized at Rabin Medical Center, Beilinson Hospital, Petah Tikva, Israel, between January 2013 and January 2016, were identified retrospectively and included in the study. Diagnosis of CDI was based on PCR or diagnosis by immunoassay for C. difficile toxin. The main outcome was 30- and 90-day all-cause mortality. The PCR group included 165 patients and the immunoassay group included 157 patients. In comparison to the immunoassay group, patients in the PCR group were more likely to be younger, to be independent, to undergo previous abdominal surgery, and to use laxatives. The 30-day mortality rate in the PCR group was significantly lower than that in the immunoassay group, 29/165 (18%) vs 49/157 (31%), respectively; p = 0.028. On multivariate analysis, PCR diagnosis was associated with reduced mortality, OR 0.48 (95% CI 0.26–0.88). PCR-based diagnosis of CDI is associated with reduced all-cause mortality rates. Further studies are needed to determine the management of patients with discrepant immunoassay and PCR diagnosis of CDI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Due to concern of underdiagnosis of Clostridium difficile infection (CDI), highly sensitive molecular methods as polymerase chain reaction (PCR) were developed [1] that may account for a part of the increased incidence of CDI reported in the recent years [2, 3]. One of the proposed methods for diagnosis of CDI by stool samples involves a two-step approach by the use of PCR for discordant results by the C.DIFF QUIK CHEK COMPLETE assay (TechLab, Blacksburg, VA) [4], using the Xpert C. difficile PCR assay (Cepheid, Sunnyvale, CA) [5]. Previous studies have examined the clinical outcomes of patients with CDI according to the C. difficile toxins’ detection method [6,7,8,9,10], and some had showed better clinical outcomes of patients with PCR-based diagnosis of toxin alone [11, 12]. We conducted a retrospective analysis in order to detect differences in the clinical outcomes of patients with PCR-based diagnosis of CDI vs antigen detection-based methods.
Methods
Study design
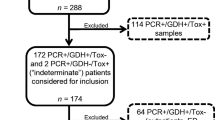
We performed a retrospective data analysis of consecutive hospitalized patients who were diagnosed with CDI. The cohort included hospitalized patients in internal medicine wards, at Rabin Medical Center, Beilinson Hospital, Petah Tikva, Israel (900 bed tertiary care, university-affiliated hospital), from January 2013 to January 2016. We included patients with clinical suspicion of CDI, defined by diarrhea not attributed to any other cause and associated with either a positive C.DIFF QUIK CHEK COMPLETE assay for antigen/toxins for C. difficile (immunoassay group) or patients with C. DIFF QUIK CHEK COMPLETE assay positive for glutamate dehydrogenase (GDH) antigen/negative for toxin, but Xpert C. difficile PCR-positive assay (PCR group). Thus, PCR was performed only on samples from patients with discordant results. The patients were treated for CDI either by metronidazole or oral vancomycin. Diarrhea was defined as passage of three or more unformed stools for at least 2 consecutive days. The decision to test for C. difficile and to treat CDI was made by physicians uninvolved in the study. Patients were included only once in the study, for the first episode fulfilling inclusion criteria. We included only the first sample for each patient. The study was approved by the hospital’s ethics committee.
Data collection
The index point was defined as the day of stool sample received in the laboratory. Data collection was performed by using the electronic patient file. Data on the diarrheal episode, management, and outcomes were collected. A CDI severity index was calculated for each patient based on the presence of each of the following factors: acute kidney injury, hypoalbuminemia, leukocytosis, and active malignancy [13].
Outcomes
Data on the primary and secondary outcomes was extracted from the nationwide electronic medical records. The primary outcome was 30-day all-cause mortality. Secondary outcomes included 90-day all-cause mortality rates, number of patients with recurrent CDI within 90 days of the index point, length of hospital stay (LOS), length of clinical illness, complication of CDI (including need for urgent colectomy, toxic mega-colon, need for ICU transfer), and severe adverse events related to the antibiotic therapy (i.e., severe allergic response, need for drug discontinuation, neuropathy).
Statistical methods
We compared results between the control and PCR group. Dichotomous outcomes were compared using the Pearson Chi-square test. Continuous variables were compared using the student T test or the Mann-Whitney U test, as appropriate. Risk factors for mortality were assessed through univariate analysis (p < 0.05) and then entered into a logistic multivariate analysis using the backward stepwise method. Variables showing high correlation (Spearman’s correlation coefficient > 0.5) were omitted. Odds ratios (OR) were calculated with 95% confidence intervals (CI). Analyses were conducted using IBM SPSS Statistics 20 (IBM, Armonk, NY).
Results
A total of 322 patients, 165 patients in the PCR group and 157 in the immunoassay group, were included. The characteristics of these patients are presented in Table 1. Compared to the immunoassay group, patients in the PCR group were younger (median age 69 vs 75 years, p = 0.007) and independent in their ADLs (46 vs 35%, p = 0.044). The PCR group surpassed the immunoassay group in regard to history of laxative and corticosteroid use, solid organ transplantation, and previous abdominal surgery (22.4 vs 7%, p < 0.01). At presentation, the PCR group patients had lower leukocyte count and higher albumin levels than the immunoassay group patients. As well as, the CDI score was lower for the PCR group (median 1, IQR 0–1 vs 1, IQR 1–2, p = 0.001), difference derived by more patients with a CDI score of 0 (29.7 vs 14.6%, p = 0.007). The management of the patients was similar as evidenced by the similar number of treatment days with metronidazole and vancomycin, ICU admissions, and the need for emergent colectomy.
Primary outcome—30-day all-cause mortality
Thirty-day all-cause mortality rates and other clinical outcomes are presented in Table 2. The 30-day crude mortality rate was 78/322 (24.2%). Unadjusted, the mortality rate in the PCR group (29/165; 17.6%) was significantly lower than that in the immunoassay group (49/157; 31.2%), p = 0.028. Mortality was significantly higher for patients with increased CDI severity score. On multivariable analysis, PCR-based diagnosis of CDI was associated with half the odds for mortality, OR 0.48 (95% CI 0.26–0.88), while age, residence in long-term care facility (LTCF), Charlson comorbidity index, need for ICU admission, and CDI severity score of 3/4 were associated with increased 30-day mortality rates (Table 3).
Secondary outcomes—90-day all-cause mortality, LOS, length of diarrheal illness, complications, recurrence, and adverse events
Ninety-day all-cause mortality rates and other clinical outcomes are presented in Table 2. The 90-day crude all-cause mortality rate was 118/322 (36.8%). Unadjusted, the mortality rate in the PCR group (29.7%) was lower than that in the immunoassay group (44.2%), 49/165 vs 69/157, respectively; p = 0.007. On multivariable analysis, PCR-based diagnosis of CDI was associated with reduced risk for mortality, OR 0.55 (95% CI 0.31–0.97, Table 3). Other factors associated with increased risk of 90-day all-cause mortality were age, residence in LTCF, Charlson comorbidity index, need for ICU admission, and CDI severity score above 2.
LOS, length of diarrheal illness (for all patients and for patients who were discharged alive), and rehospitalization for all causes in the following 3 months were similar between the groups. Severe complication of CDI were noted in 4/165 patients in the PCR group and in 10/157 patients in the immunoassay group, p = 0.10. We observed a trend towards less hospitalization for recurrent CDI (12.7 vs 19.1%, p = 0.117) for patients in the PCR group which were discharged alive (Table 2). No severe adverse events related to the use of metronidazole or vancomycin were noted during the follow-up time.
Discussion
In this study, we demonstrated lower 30- and 90-day all-cause mortality rates in patients with PCR-based diagnosis of CDI, in comparison to patients with antigen-/toxin-positive stool immunoassay. We also demonstrated increased mortality with several “traditional” CDI risk factors as increased age, Charlson comorbidity index, LTCF residency or being independent in ADLs, and higher CDI severity score. We noted a trend for less recurrences of CDI in the PCR group (12.7 vs 19.1%).
Our findings suggest that PCR-based diagnosis of CDI is associated with either a non-CDI-related diarrheal illness or less severe disease and hence the reduced mortality. Patients with PCR-based diagnosis of CDI might represent a carrier state of C. difficile; thus, only a minute amount of toxin is produced which are undetected by the toxin assay. In those patients, several other etiologies for diarrhea might exist, often simultaneously [14]. Our patients in the PCR group were more likely to be under immunosuppression by steroids, chemotherapy, and organ transplantation; they were more likely to use laxatives and undergone previous abdominal surgery (that might affect the intestinal motility). Alternatively, patients with PCR-based diagnosis of CDI might have less severe disease due to small amount of toxin production. This is supported by significantly more patients with CDI severity score of 0/1 in our cohort (70 vs 55%).
Our findings are in concordance with some studies that examined the laboratory confirmation of CDI by PCR and clinical outcomes. Polage et al. [11] examined a similar cohort of patients, discovering that positive PCR patients had less complication and less CDI-related mortality. The toxin-positive assay group had longer duration of diarrhea. Similar to our findings, patients in the toxin-negative/PCR-positive group had been more likely to have several other explanations for the diarrhea. Baker et al. examined the clinical outcomes of patients suspected of CDI by the two-step methods [12]. Mortality rates for that cohort were significantly lower among the toxin-negative/PCR-positive group as well as days of diarrhea and CDI recurrences. Better clinical outcomes for patients with PCR-based diagnosis of CDI alone, however, were not observed in all studies. In a retrospective study of cancer patients with CDI, discordant results between PCR and cytotoxin assay were not associated with increased mortality [10]. Similar results were observed in studies by Guerrero et al. [9], Longtin et al. [6], and Berry et al. [8], in all of which the small number of patients with discordant PCR and toxin assay precluded statistically significant results.
Our study has some limitations. First, the design of the study is retrospective and subjected to selection bias. However, our center management policy is the same for patients with PCR or antigen/toxin assay-based diagnosis of CDI; therefore, both groups were managed similarly by antibiotics and supportive therapy. Second, this is a single-center experience and other laboratories might encounter different results. Third, our results might be confounded by other unaccounted explanations for the reduced mortality observed in the PCR group. However, our univariate and multivariate analysis of risk factors for mortality is in concordance with other studies of CDI as age, comorbidity, and severity score, thus increasing the validity of our results. Finally, the PCR assay was only performed on samples with discordant GDH/toxin results. Thus, different results might have been encountered if PCR assay was performed systematically and compared with the results obtained from the immunoassay.
In conclusion, PCR-based diagnosis of CDI is associated with reduced all-cause mortality at 30 and 90 days in comparison to patients with antigen-/toxin-positive assay. Future studies should focus on the management of patients with positive PCR and low CDI severity score, since this group might represent patients with diarrhea caused by other conditions. We suggest managing those patients by cessation of the “culprit” antibiotic alone, and withholding specific anti C. difficile therapy until other etiologies for the diarrhea had been excluded.
References
Davies KA, Longshaw CM, Davis GL et al (2014) Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID). Lancet Infect Dis 14:1208–1219
Zilberberg MD, Shorr AF, Kollef MH (2008) Increase in adult Clostridium difficile-related hospitalizations and case-fatality rate, United States, 2000-2005. Emerg Infect Dis 14:929–931
Kang J, Sickbert-Bennett EE, Brown VM, Weber DJ, Rutala WA (2014) Changes in the incidence of health care-associated pathogens at a university hospital from 2005 to 2011. Am J Infect Control 42:770–775
Cheng JW, Xiao M, Kudinha T et al (2015) The role of glutamate dehydrogenase (GDH) testing assay in the diagnosis of Clostridium difficile infections: a high sensitive screening test and an essential step in the proposed laboratory diagnosis workflow for developing countries like China. PLoS One 10:e0144604
Swindells J, Brenwald N, Reading N, Oppenheim B (2010) Evaluation of diagnostic tests for Clostridium difficile infection. J Clin Microbiol 48:606–608
Longtin Y, Trottier S, Brochu G et al (2013) Impact of the type of diagnostic assay on Clostridium difficile infection and complication rates in a mandatory reporting program. Clin Infect Dis: Off Publ Infect Dis Soc Am 56:67–73
Planche TD, Davies KA, Coen PG et al (2013) Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C difficile infection. Lancet Infect Dis 13:936–945
Berry N, Sewell B, Jafri S et al (2014) Real-time polymerase chain reaction correlates well with clinical diagnosis of Clostridium difficile infection. J Hospital Infect 87:109–114
Guerrero DM, Chou C, Jury LA, Nerandzic MM, Cadnum JC, Donskey CJ. Clinical and infection control implications of Clostridium difficile infection with negative enzyme immunoassay for toxin. Clin Infect Dis : Off Publ Infect Dis Soc Am 2011;53:287–290
Kaltsas A, Simon M, Unruh LH et al (2012) Clinical and laboratory characteristics of Clostridium difficile infection in patients with discordant diagnostic test results. J Clin Microbiol 50:1303–1307
Polage CR, Gyorke CE, Kennedy MA et al (2015) Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med 175:1792–1801
Baker I, Leeming JP, Reynolds R, Ibrahim I, Darley E (2013) Clinical relevance of a positive molecular test in the diagnosis of Clostridium difficile infection. J Hospital Infect 84:311–315
Lungulescu OA, Cao W, Gatskevich E, Tlhabano L, Stratidis JG (2011) CSI: a severity index for Clostridium difficile infection at the time of admission. J Hospital Infect 79:151–154
Polage CR, Solnick JV, Cohen SH (2012) Nosocomial diarrhea: evaluation and treatment of causes other than Clostridium difficile. Clin Infect Dis : Off Publ Infect Dis Soc Am 55:982–989
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the institutional research ethics committee of Rabin Medical Center.
Informed consent
The committee waived the need for informed consent.
Rights and permissions
About this article
Cite this article
Avni, T., Babich, T., Ben-Zvi, H. et al. Molecular-based diagnosis of Clostridium difficile infection is associated with reduced mortality. Eur J Clin Microbiol Infect Dis 37, 1137–1142 (2018). https://doi.org/10.1007/s10096-018-3228-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-3228-4