Abstract
Group B Streptococcus (GBS) is the leading cause of neonatal infections in industrialized countries. Intrapartum antibiotic prophylaxis (IAP) given to colonized parturients is a key step for the prevention of neonatal early-onset infection. We compared the performances of Xpert® GBS polymerase chain reaction (PCR) (Cepheid, Sunnyvale, CA, USA) as a point-of-care system in labor wards to standard culture for intrapartum GBS detection. Pregnant women with a GBS-positive antenatal screening were prospectively included. A vaginal double swab was collected at the time of delivery for point-of-care Xpert® GBS PCR and GBS culture. A total of 565 pregnant women were included. Valid Xpert® GBS results were obtained for 488 (86.4%) women on the first attempt. Repeat testing improved the PCR success to 516 (91.3%) women. Among the 305 women positive for GBS by culture at delivery, only 238 (78.0%) were positive by Xpert® GBS PCR, cycle thresholds being correlated to culture quantification. Among 260 women negative for GBS culture, 56 (21.5%) were positive by Xpert® GBS PCR, including 50 where IAP was initiated before vaginal sampling. Overall, among the 565 women with GBS antenatal positive culture, only 335 (59.3%) were still positive at delivery whatever the technique used, resulting in unnecessary IAP for 40% of them. This large cohort study comparing intrapartum to antepartum GBS detection provides evidence that (i) Xpert® GBS PCR might be a valuable solution for intrapartum GBS detection compared to culture-based strategies and (ii) laboratory training of non-specialized staff is mandatory to reach the performances required for point-of-care tests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Group B Streptococcus (Streptococcus agalactiae, GBS) is the leading cause of neonatal infections in developed countries. Two strategies have significantly reduced the rate of early neonatal GBS colonization and infection [1]: (i) late antenatal GBS screening at 35–37 weeks gestational age (GA) of pregnant women and (ii) intrapartum antibiotic prophylaxis (IAP) for colonized women to reduce vertical transmission of GBS. These strategies have resulted in a substantial decline in GBS early-onset infection incidence in France (0.69 per 1000 live births in 1997 to 0.20 in 2006) [2], as observed in other countries having similar guidelines [3].
The current guidelines from the French High Authority for Health still recommend cultures as the gold standard technique for GBS detection in vaginal samples at 35–37 weeks GA. However, several reports have pinpointed limitations of this strategy, such as: (i) a low sensitivity of antenatal GBS cultures at 35–37 weeks GA to detect GBS colonization [4, 5]; (ii) at least 10% of women diagnosed as antenatal GBS-negative turned out positive at delivery [6]; (iii) the absence of detection in case of premature labor, and, more generally, for women without prenatal care. Therefore, considerable efforts have been made to develop rapid techniques for intrapartum GBS detection, most of them being molecular assays [7,8,9,10,11]. Among them, the Xpert® GBS real-time polymerase chain reaction (PCR) system (Cepheid, Sunnyvale, CA, USA) has the potential to be installed out of the lab, thus allowing diagnostic testing at the point of care, providing results in less than 1 h to allow prompt IAP, which remains the priority. In contrast, culture techniques, which require a minimum of 18 h before giving the first results, are unhelpful for intrapartum GBS detection; however, they remain valid and acceptable for GBS antenatal screening at 35–37 weeks GA for many authors and recommendations [12]. The aim of this study was to assess the performances of Xpert® GBS real-time PCR in labor wards performed by midwives, as compared with intrapartum culture and antenatal screening at 35–37 weeks GA.
Materials and methods
Study design
The study was conducted prospectively in two maternity wards equipped with neonatal intensive care units, belonging to two university hospitals, Louis Mourier (LM) and Cochin Port-Royal (PR), performing approximately 3000 and 5000 deliveries per year, respectively. Between November 2012 and April 2015, eligible pregnant women were prospectively enrolled based on the following criteria: positive GBS vaginal screening at 35–37 weeks (GA), aged ≥ 18 years old. Data were collected with the CleanWEB™ software and included the following characteristics: age, GA at delivery, mode of delivery, antibiotics use in the month before delivery, duration of antibiotic prophylaxis before delivery, duration from the moment of rupture of membranes onward, duration of labor.
Study approval
The study protocol was approved by the Ethical Research Committee (CPP12005) and all women included gave written informed consent.
Vaginal samples collection
According to the recommendations of the French High Authority for Health, a vaginal swab was performed at 35–37 weeks GA for every pregnant woman to screen for GBS vaginal colonization. Samples were sent to the laboratory in Amies transport medium (Copan Diagnostics, Brescia, Italy) for culture. At the time of delivery, another vaginal swab was collected using a double swab (Copan Diagnostics), one dedicated to the Xpert® GBS PCR (Cepheid, Sunnyvale, CA, USA) test performed by midwives in the labor ward and the other sent to the laboratory in Amies transport medium for culture.
GBS culture in the laboratory
Vaginal swabs were cultured on horse blood agar plates incubated under aerobic atmosphere enriched with 5% CO2 and on Granada medium plates (bioMérieux, Marcy L’Étoile, France) incubated under anaerobic atmosphere. All plates were examined after 18–24 h incubation at 37 °C, and incubated for an additional 24 h when negative for GBS. β-Hemolytic orange pigmented colonies and all suspect colonies were identified as GBS by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (Bruker Daltonics, Billerica, MA, USA). When cultures were positive for GBS, a semi-quantitative evaluation was performed as follows: GBS colonies on a single quadrant (+), on two to three quadrants (++), and on four quadrants (+++).
Point-of-care molecular assay
According to the manufacturer’s recommendations, the dedicated swab was transferred to the designated chamber of the Xpert® GBS cartridge, which was then loaded into a Cepheid GeneXpert device located in the labor ward. Beforehand, midwives underwent training to collect samples and perform the Xpert® GBS PCR assay. The real-time PCR was performed for a total assay runtime of around 52 min. PCR results were collected using the Cepheid GeneXpert software. According to the manufacturer’s settings, the test is defined as positive when the cycle threshold (Ct) is > 0 and ≤ 42.
Statistical analysis
We estimated the sensitivity with a 95% confidence interval (CI) for the intrapartum PCR compared with the intrapartum culture (reference standard) using the McNemar test. We decided not to report specificities, as there is a potential problem with the interpretation of the false-positives by PCR. Indeed, as the culture was used as the reference standard, those considered as false-positives by PCR could well be colonized, as the PCR assay may better detect GBS than the culture. Moreover, invalid results or errors were not taken into account for the statistical analysis. Categorical data were analyzed by a chi-square test or a Fisher exact test when necessary (n < 7). Continuous data were studied with Student’s t-test. A p value of < 0.05 was considered significant.
Results
Population study
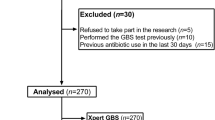
Between November 2012 and April 2015, a total of 565 pregnant women with positive antenatal GBS screening were detected for GBS at delivery [LM n = 301 (53%); PR n = 264 (47%)]. The characteristics of the studied population are shown in Table 1. The median age of the pregnant women was 31 years old. Primigravida and primipara represented 37.0% and 49.6%, respectively, of the parturients. The median GA at the time of delivery was 40.1 weeks. Vaginal delivery was highly preponderant (n = 474; 83.9%). Thirty parturients (5.3%) reported antibiotics intake during the month preceding delivery. The median duration of IAP, labor, and rupture of membranes were 6.7 h, 7.5 h, and 7.1 h, respectively.
Comparison of point-of-care assay to culture
The Xpert® GBS PCR results acquired in the labor ward were compared to those of intrapartum GBS culture (Table 2). Two hundred and ninety-four samples (52.0%) were detected positive by Xpert® GBS PCR, among which 238 (81.0%) and 56 (19.0%) were culture-positive and -negative, respectively. Cts obtained for these 294 samples were correlated to the GBS culture quantification. For GBS culture quantification of (+++) (n = 116), the median Ct was 32.6 (range 25.9–40.4), for (++) and (+) (n = 122), the median Cts were 35.9 (range 29.8–41.1) and 36.5 (range 28.6–40.4), respectively. For the 56 samples detected positive by Xpert® GBS PCR and negative by culture, the median Ct was 38.1 (range 28.6–42). Among these 56 cases, IAP was initiated before vaginal sampling for 50 of them. Furthermore, in one of these 50 cases, the parturient reported receiving β-lactams to treat a pyelonephritis during the month preceding delivery. Two hundred and twenty-two samples (39.3%) were detected negative by PCR, among which 178 (80.2%) and 44 (19.8%) were culture-negative and -positive, respectively. The sensitivity of intrapartum Xpert® GBS PCR and intrapartum culture were not significantly different (84.4% [79.5–88.3] and 81.0% [75.9–85.2], respectively; p = 0.13).
Invalid results and control of discrepancies
Forty-nine (8.7%) samples gave invalid or erroneous results due to the presence of mucus (n = 16, 32.7%), inhibitors (n = 20, 40.8%), or because of cartridge intrinsic problems or manipulation errors (n = 13, 26.5%). However, it is important to mention that this is an overall rate that does not take into account the total amount of tests performed before getting a valid result. Indeed, the total number of invalid results was 77, increasing the rate to 13.6%. Discrepant results (samples negative by PCR and positive by culture) were controlled at the laboratory by a trained technician who performed a second Xpert® GBS test (n = 41). Of them, 21 (47.7%) were detected positive, 17 remained negative (38.6%), and three were invalid because of inhibitors (n = 2) or mucus (n = 1). Among the 17 confirmed GBS-negative Xpert® GBS PCR tests, the number of GBS colonies obtained by culture was low, not exceeding a single quadrant (+). For seven of them, the PCR test actually detected a Ct value > 42 (range 42.1–44.7), confirming the low GBS DNA load in these samples. After control in the laboratory, among 305 positive GBS cultures at delivery, 259 were positive by the Xpert® GBS test (Table 3), showing that the sensitivity of PCR detection (92.8% [89.2–95.3]) was significantly higher than that of the intrapartum culture (82.2% [77.5–86.2]); p < 0.001). Finally, among the 565 pregnant women with GBS-positive culture at antenatal screening, only 335 (59.3%) were still positive at delivery, whatever the detection technique used.
Discussion
Currently, in many countries, the most effective method to prevent GBS early-onset disease (EOD) is the use of IAP. A key issue is the accurate identification of GBS-colonized pregnant women in order to select the appropriate at-risk women for whom IAP is beneficial. Therefore, new methods have been developed to identify GBS carriers without temporal delay at the onset of delivery. The aim of this study was to evaluate as a point-of-care system the real-time Xpert® GBS assay for intrapartum GBS detection by midwives in labor wards in a large cohort of 565 pregnant women from two university hospitals. All women included were screened positive for GBS at 35–37 week GA. Considering the clinical characteristics, this cohort is representative of a standard population with low risk factors. As a point-of-care test used in the labor wards, the Xpert® GBS PCR provided valid results in 91.3% of the cases, a rate that has been previously reported [13,14,15,16]. The number of invalid results is an important criterion when assessing the feasibility of a test, especially as a point-of-care technology given the therapeutic implications. Invalid results accounted for 13.6% on the first attempt and 8.7% when it was redone until a valid result was obtained. These rates are quite equivalent to those reported in similar settings. Rates between 10.8% and 13.4% have been reported when assays were performed by midwives in labor wards [14,15,16]; the rate of 8.2% corresponds to that announced by the manufacturer [16]. The sensitivity of the intrapartum Xpert® GBS PCR (84.4%) to detect GBS colonization during labor was not superior to intrapartum cultures (81.0%) when the test was done by midwives in labor wards. The sensitivity of the test became significantly better (91.8%) when discrepant results were controlled by specialized laboratory staff. Nevertheless, it is admitted that, for such a test to be clinically useful, it should have a sensitivity not inferior to 90–95% [11]. These results underline the importance and necessity of fully trained non-laboratory staff to successfully reduce invalid results, especially in the labor ward with high staff turnover.
Compared to GBS culture, the Xpert® GBS test increased intrapartum GBS detection by 19.0%, as, among 294 PCR-positive patients, 56 were culture-negative (Table 3). In these cases, the most likely explanations are low GBS colonization rates or patients who have recently received antimicrobial chemotherapy. Indeed, in 50 cases (89.3%), IAP was initiated before the Xpert® GBS PCR was performed, as the women included were positive at antenatal culture screening, thus known positive on arrival to the labor ward and receiving their antibiotic before the test was performed. Conversely, positive GBS culture associated with negative Xpert® GBS PCR in the labor ward accounted for 19.8% (n = 44/222; Table 2). After control in the laboratory, this rate dropped to 10% (n = 20/198; Table 3), making the PCR test more sensitive than culture. Such discrepant results have already been reported by others, and might be due to several reasons, such as sampling or processing [14, 15, 17]. Of note, among these 20 samples, Cts ranging from 42.0 to 43.2 were obtained for 16, confirming the low amount of GBS DNA and, therefore, leading to negative PCR according to the Ct of 42 fixed by the manufacturer. Changing the Ct to 43 would have improved the sensitivity of the Xpert® GBS test compared to the culture. Increasing the sensitivity will lead to more women receiving IAP without an obvious impact on early-onset infection given the correlation between the importance of the inoculum and the risk of infection.
Finally, at the time of delivery, only 59.3% of women detected GBS-positive at 35–37 GA were still positive whatever the test, confirming that GBS colonization is intermittent during pregnancy [5, 12, 14, 18, 19]. According to the recommendations, all these women who were screened GBS-positive at 35–37 weeks GA received IAP, even those detected GBS-negative intrapartum according to our protocol. The risk of infection is clearly correlated with the presence of GBS [1]; therefore, it can be considered that IAP has been given unnecessarily to 40% of women. At a time where the proper use of antibiotic prescription has become a major issue considering the potential impacts on the newborn’s microbiota and on the selection of multiresistant bacteria, intrapartum PCR GBS detection must, therefore, be considered as a valuable alternative solution.
They are several limitations of our study. First, we have not collected the time elapsed between the rupture of the membranes, the vaginal sampling, and the realization of the Xpert® GBS test. The availability of this information could have explained some of the discrepancies observed between the Xpert® GBS PCR results to those of GBS culture. Second, in this study, only women screened GBS-positive in antepartum were included, while those who were negative and becoming positive at delivery were not studied. Therefore, we could not determine the rate of women who turned positive and who would have benefited from an IAP. Third, a cost–benefit analysis has not been performed in our study. However, such analyses have already been published by Haberland et al. [20] and El Helali et al. [21], demonstrating the cost-effectiveness of intrapartum PCR-based strategies compared to antepartum culture screening, since they generate more benefits per birth, result in fewer courses of IAP, and, eventually, in fewer cases of EOD. Although the cost of such tests restricts their implementation and routine use on many maternity wards, their effectiveness and associated benefits will very likely contribute to their popularization and favor their reimbursement by health care insurances in the near future.
In conclusion, this study is one of the largest cohorts of pregnant women detected GBS-positive in antepartum screening for which intrapartum GBS detection was performed. We provide evidence that: (i) the Xpert® GBS PCR assay as a point-of-care test might be a valuable solution for intrapartum GBS detection compared to culture-based strategies; (ii) training of non-specialized staff is absolutely mandatory to reach the acceptable sensitivity required for such a molecular test used as point of care in labor wards.
References
Benitz WE, Gould JB, Druzin ML (1999) Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics 103:e77
Jourdan-Da Silva N, Antona D, Six C, Georges S, Goulet V, Judlin P, Lévy-Bruhl D (2008) Neonatal group B streptococcus infections in France: incidence from 1997 to 2006 and current prevention practices in maternity wards. Bull Epidemiol Hebd 14:110–113
Edmond KM, Kortsalioudaki C, Scott S, Schrag SJ, Zaidi AKM, Cousens S et al (2012) Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet 379:547–556. https://doi.org/10.1016/S0140-6736(11)61651-6
Edwards RK, Clark P, Duff P (2002) Intrapartum antibiotic prophylaxis 2: positive predictive value of antenatal group B streptococci cultures and antibiotic susceptibility of clinical isolates. Obstet Gynecol 100:540–544
Goodman JR, Berg RL, Gribble RK, Meier PR, Fee SC, Mitchell PD (1997) Longitudinal study of group B streptococcus carriage in pregnancy. Infect Dis Obstet Gynecol 5:237–243. https://doi.org/10.1155/S1064744997000409
Puopolo KM, Madoff LC, Eichenwald EC (2005) Early-onset group B streptococcal disease in the era of maternal screening. Pediatrics 115:1240–1246. https://doi.org/10.1542/peds.2004-2275
Block T, Munson E, Culver A, Vaughan K, Hryciuk JE (2008) Comparison of carrot broth- and selective Todd-Hewitt broth-enhanced PCR protocols for real-time detection of Streptococcus agalactiae in prenatal vaginal/anorectal specimens. J Clin Microbiol 46:3615–3620. https://doi.org/10.1128/JCM.01262-08
Ke D, Ménard C, Picard FJ, Boissinot M, Ouellette M, Roy PH et al (2000) Development of conventional and real-time PCR assays for the rapid detection of group B streptococci. Clin Chem 46:324–331
Montague NS, Cleary TJ, Martinez OV, Procop GW (2008) Detection of group B streptococci in Lim broth by use of group B streptococcus peptide nucleic acid fluorescent in situ hybridization and selective and nonselective agars. J Clin Microbiol 46:3470–3472. https://doi.org/10.1128/JCM.00858-08
Riedlinger J, Beqaj SH, Milish MA, Young S, Smith R, Dodd M et al (2010) Multicenter evaluation of the BD Max GBS assay for detection of group B streptococci in prenatal vaginal and rectal screening swab specimens from pregnant women. J Clin Microbiol 48:4239–4241. https://doi.org/10.1128/JCM.00947-10
Di Renzo GC, Melin P, Berardi A, Blennow M, Carbonell-Estrany X, Donzelli GP et al (2015) Intrapartum GBS screening and antibiotic prophylaxis: a European consensus conference. J Matern Fetal Neonatal Med 28:766–782. https://doi.org/10.3109/14767058.2014.934804
Hiller JE, McDonald HM, Darbyshire P, Crowther CA (2005) Antenatal screening for group B Streptococcus: a diagnostic cohort study. BMC Pregnancy Childbirth 5:12. https://doi.org/10.1186/1471-2393-5-12
Buchan BW, Faron ML, Fuller D, Davis TE, Mayne D, Ledeboer NA (2015) Multicenter clinical evaluation of the Xpert GBS LB assay for detection of group B Streptococcus in prenatal screening specimens. J Clin Microbiol 53:443–448. https://doi.org/10.1128/JCM.02598-14
El Helali N, Nguyen J-C, Ly A, Giovangrandi Y, Trinquart L (2009) Diagnostic accuracy of a rapid real-time polymerase chain reaction assay for universal intrapartum group B streptococcus screening. Clin Infect Dis 49:417–423. https://doi.org/10.1086/600303
Mueller M, Henle A, Droz S, Kind AB, Rohner S, Baumann M et al (2014) Intrapartum detection of Group B streptococci colonization by rapid PCR-test on labor ward. Eur J Obstet Gynecol Reprod Biol 176:137–141. https://doi.org/10.1016/j.ejogrb.2014.02.039
Park JS, Cho D-H, Yang JH, Kim MY, Shin SM, Kim E-C et al (2013) Usefulness of a rapid real-time PCR assay in prenatal screening for group B streptococcus colonization. Ann Lab Med 33:39–44. https://doi.org/10.3343/alm.2013.33.1.39
de Tejada BM, Pfister RE, Renzi G, François P, Irion O, Boulvain M et al (2011) Intrapartum Group B streptococcus detection by rapid polymerase chain reaction assay for the prevention of neonatal sepsis. Clin Microbiol Infect 17:1786–1791. https://doi.org/10.1111/j.1469-0691.2010.03378.x
Davies HD, Miller MA, Faro S, Gregson D, Kehl SC, Jordan JA (2004) Multicenter study of a rapid molecular-based assay for the diagnosis of group B Streptococcus colonization in pregnant women. Clin Infect Dis 39:1129–1135. https://doi.org/10.1086/424518
Valkenburg-van den Berg AW, Sprij AJ, Oostvogel PM, Mutsaers JAEM, Renes WB, Rosendaal FR et al (2006) Prevalence of colonisation with group B Streptococci in pregnant women of a multi-ethnic population in The Netherlands. Eur J Obstet Gynecol Reprod Biol 124:178–183. https://doi.org/10.1016/j.ejogrb.2005.06.007
Haberland CA, Benitz WE, Sanders GD, Pietzsch JB, Yamada S, Nguyen L et al (2002) Perinatal screening for group B streptococci: cost–benefit analysis of rapid polymerase chain reaction. Pediatrics 110:471–480
El Helali N, Giovangrandi Y, Guyot K, Chevet K, Gutmann L, Durand-Zaleski I (2012) Cost and effectiveness of intrapartum group B streptococcus polymerase chain reaction screening for term deliveries. Obstet Gynecol 119:822–829. https://doi.org/10.1097/AOG.0b013e31824b1461
Acknowledgements
The sponsor was Assistance Publique-Hôpitaux de Paris (APHP) (Department of Clinical Research). We thank Florence Artiguebieille, Valerie Fauroux, Farah Ketroussi, and Chahrazed Guettouche from URC-CIC Cochin-Necker, AP-HP, Paris, for the management of the clinical trial. We thank all the technical and medical staff of the maternity wards.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CPo has received reimbursement for attending meetings from bioMérieux, Cepheid, and has received research funding from Institut Mérieux. CPl has received reimbursement from Cepheid for attending the European Congress of Clinical Microbiology and Infectious Diseases, which took place in Amsterdam from 9 to 12 April 2016.
This work was partially funded by a grant from APHP (P111008), Institut Mérieux, and the IRT Bioaster. Cepheid had no role in the data collection, data analysis, data interpretation, or writing of the report.
Research involving human participants
The study protocol was approved by the Ethical Research Committee (CPP12005).
Informed consent
All women included gave written informed consent.
Rights and permissions
About this article
Cite this article
Plainvert, C., El Alaoui, F., Tazi, A. et al. Intrapartum group B Streptococcus screening in the labor ward by Xpert® GBS real-time PCR. Eur J Clin Microbiol Infect Dis 37, 265–270 (2018). https://doi.org/10.1007/s10096-017-3125-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-3125-2