Abstract
This study was aimed to determine the risk factors of Carbapenem-resistant Enterobacteriaceae (CRE) nosocomial infections and assess the clinical outcomes. A case-case–control design was used to compare two groups of case patients with control patients from March 2010 to November 2014 in China. Risk factors for the acquisition of CRE infections and clinical outcomes were analyzed by univariable and multivariable analysis. A total of 94 patients with CRE infections, 93 patients with Carbapenem-susceptible Enterobacteriaceae (CSE) infections, and 93 patients with organisms other than Enterobacteriaceae infections were enrolled in this study. Fifty-five isolates were detected as the carbapenemase gene. KPC-2 was the most common carbapenemase (65.5 %, 36/55), followed by NDM-1 (16.4 %, 9/55), IMP-4 (14.5 %, 8/55), NDM-5 (1.8 %, 1/55), and NDM-7 (1.8 %, 1/55). Multivariable analysis implicated previous use of third or fourth generation cephalosporins (odds ratio [OR], 4.557; 95 % confidence interval [CI], 1.971–10.539; P < 0.001) and carbapenems (OR, 4.058; 95 % CI, 1.753-9.397; P = 0.001) as independent risk factors associated with CRE infection. The in-hospital mortality of the CRE group was 57.4 %. In the population of CRE infection, presence of central venous catheters (OR, 4.464; 95 % CI, 1.332–14.925; P = 0.015) and receipt of immunosuppressors (OR, 7.246; 95 % CI, 1.217–43.478; P = 0.030) were independent risk factors for mortality. Appropriate definitive treatment (OR, 0.339; 95 % CI, 0.120–0.954; P = 0.040) was a protective factor for in-hospital death of CRE infection. Kaplan–Meier curves of the CRE group had the shortest survival time compared with the other two groups. Survival time of patients infected with Enterobacteriaceae with a high meropenem MIC (≥8 mg/L) was shorter than that of patients with a low meropenem MIC (2,4, and ≤ 1 mg/L). In conclusion, CRE nosocomial infections are associated with prior exposure to third or fourth generation cephalosporins and carbapenems. Patients infected with CRE had poor outcome and high mortality, especially high meropenem MIC (≥8 mg/L). Appropriate definitive treatment to CRE infections in the patient is essential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbapenems, beta-lactam antibiotics with the broadest spectrum against Gram-negative organisms, used to be considered as the last option for multidrug-resistant Gram-negative bacteria infections [1]. However, the emergence of carbapenem-resistant Enterobacteriaceae (CRE) is a challenge to clinicians and clinical microbiologists because of the limited available antimicrobials to choose. It is well-known that carbapenemases, including the serine carbapenemases (the class A enzymes SME, GES, IMI, NMC, KPC, and the class D enzymes, OXA enzymes) and metallo-carbapenemases (VIM, NDM, IMP, SPM, GIM, and SIM) are a major cause of Enterobacteriaceae resistant to carbapenems [2, 3], although strains with combinations of either ESBL or AmpC and loss of porins may have high MICs of carbapenems [4]. The number of deaths due to carbapenems resistance is considerably high among patients with Enterobacteriaceae infections [5–7]. CRE infections have been reported worldwide, including China [8, 9]. There have been several reports regarding the risk factors for acquisition of CRE [10–13], but little is known on the risk factors and its effect on mortality in China.
Case–control studies have been used frequently to analyze risk factors for antibiotic-resistant organisms in many previous studies [14–16]. However, the selection of patients infected with susceptible organisms as a control group may lead to bias of relative risk because of a distorted estimate of exposure frequency in the source population [17–19]. Control patients and case patients should be selected from the same source population. The base should be thought of as the members of the underlying cohort or source population for the case patients during the time periods when they are likely to become case patients [20, 21]. Therefore, we conducted a case-case–control study to determine risk factors for CRE infection in hospitalized patients, and to estimate the attributed mortality associated with CRE infections in China.
Materials and methods
Study setting
This study was conducted in two large teaching hospitals. The Peking University People’s Hospital (PKUPH) is a 1,500-bed tertiary-care teaching hospital, with approximately 68,000 hospital admissions per year. The Beijing Chao-Yang Hospital of Capital Medical University (BJCYH) is a 1,900-bed tertiary-care teaching hospital with approximately 70,000 hospital admissions annually. Carbapenem-resistant Enterobacteriaceae (CRE) in this study were defined either meropenem- or imipenem-resistant according to the minimum inhibitory concentrations (MICs) breakpoints of the Clinical and Laboratory Standards Institute (CLSI) [22]. The prevalence of CRE infection in PKUPH was 0.6 %, while the prevalence of CRE infection in BJCYH was 0.4 % during the research period.
Study design and patient population
A case-case–control study was conducted to assess the risk factors for the acquisition of CRE infection and clinical outcomes. The study population included adults hospitalized in the two hospitals from October 2010 to November 2014. We confirmed that no solid organ transplant recipients who received organs from executed prisoners were included. Patients from whom strains were isolated within the first 48 h of admission were excluded. Three groups were designated. The CRE group consisted of patients infected with strains either meropenem or imipenem resistant during hospitalization. The carbapenem-susceptible Enterobacteriaceae (CSE) group consisted of patients infected with CSE strains. The control group consisted of all patients infected with organisms other than Enterobacteriaceae during their hospitalization. The CSE group and the control group patients were selected randomly from the source population admitted to the same ward during the same time period (within 30 days).
Data collection and definition
We reviewed the medical records and collected the case information. A standard surveillance form was used to collect the epidemiologic and clinical data, including demographics (sex and age), transfer from other institutions, stay in the ward with previous CRE isolation (within 7 days), underlying diseases (pulmonary disease, malignancy, liver disease, cardiovascular disease, neurologic disease, renal disease, diabetes mellitus, agranulocytosis, tuberculosis, alcohol abuse, and smoking history), APACHE II score (only patient in ICU), medication or intervention therapy prior to a positive culture (≤30 days) (thoracentesis, lumbar puncture, presence of central venous catheters, tracheal cannula, tracheotomy, presence of a Foley catheter, presence of a nasogastric tube, hematopoietic stem cell transplantation, receipt of corticosteroids, receipt of immunosuppressors, ICU stay, and receipt of antibiotics), and antibiotic prescription after the positive culture. Antibiotic variables were collected using binary variables (yes/no) from admission to the hospital. Patients with a blood or any other sterile source culture positive were directly defined to infection. Patients with positive cultures from respiratory, urine and surgical wounds were defined to infections according to the Center for Disease Control and Prevention (CDC) and National Healthcare Safety Network (NHSN) criteria [23]. Mixed infections were defined as more than one pathogen isolated from the same infection site.
The clinical outcomes in this study were defined as follows: 30-day mortality (within 30 days after the first culture positive), in-hospital mortality, presentation with septic shock (septic shock at time of culture), ICU length of stay (ICU LOS), and total hospital length of stay (LOS). ICU LOS and total hospital LOS were defined by duration from first positive culture to discharge. Inappropriate empirical treatment was defined as no active drug treatment given before final culture reports. Any agent treatment less than 48 h was defined as inadequate therapy. Definitive treatment was defined as treatment with antimicrobials for at least 48 h after the susceptibility report became available. Appropriate definitive treatment was defined as at least one active drug treatment given according to the antimicrobial susceptibility testing reports.
Microbiologic methods
Identification and antimicrobial susceptibility testing were performed in the clinical microbiology laboratory using the Vitek 2 automated system (bioMérieux, Marcy l’Etoile, France). Carbapenem (meropenem and/or imipenem) resistance was confirmed by the Etest method, according to the manufacturer’s instructions (AB Biodisk, Solna, Sweden). CRE group isolates were tested for carbapenemase genes (bla KPC, bla NDM and bla IMP) using the polymerase chain reaction (PCR) method [24, 25].
Statistical analysis
Continuous variables were compared with the Student’s t-test (for normally distributed variables) or the Mann–Whitney U test (for non-normally distributed variables) and presented as the mean ± standard deviation (SD) or median. CRE group and CSE group were compared to the control group using bivariable logistic regression models adjusted for time at risk respectively. Categorical variables were evaluated using the χ2 test or two-tailed Fisher exact test and were presented as percentages in the mortality risk factors analysis in the CRE group. Logistic regression models were used to analyze risk factors for CRE infection and mortality. All variables with a P value <0.10 in univariable analysis were included in the multivariable analysis. Cox proportional hazards regression analysis was performed to estimate survival rate of different case groups and different meropenem MICs levels (MIC ≤ 1 mg/L, MIC = 2,4 mg/L and MIC ≥ 8 mg/L). All variables with a P value <0.20 in univariate analysis were considered as probable predictor variables for the multivariable Cox proportional hazards regression analysis. The days from the first positive culture to death in the hospital within 30 days was displayed in a Kaplan-Meier curves. A log rank test was used to compare different groups.
All tests were two-tailed, with the significance level set at 0.05. SPSS 19 (SPSS Inc., Chicago, IL, USA) was used for data analysis.
Results
Study population and distribution of isolates
Ninety-four patients with CRE infections were identified during the study period. The most common type of infections was respiratory tract infections (46 cases; 49 %), followed by bloodstream infections (18 cases; 19 %), urinary tract infections (13 cases; 14 %), intra-abdominal infections (13 cases; 14 %), and wound and soft tissue infections (4 cases; 4 %). Ninety-three patients with CSE infections and 93 patients of the control group were also included. The infection types of all three groups in the study are listed in Table 1. There was no significant difference in infection types among these groups.
The distribution of Enterobacteriaceae (CRE and CSE) isolation is shown in Table 2. The most common organism isolated was Klebsiella pneumoniae (84 cases; 44.9 %), followed by Escherichia coli (51 cases; 27.3 %), Enterobacter cloacae (26 cases; 13.9 %), Citrobacter freundii (9 cases; 4.8 %), Enterobacter aerogenes (8 cases; 4.3 %), Klebsiella oxytoca (5 cases; 2.7 %), Citrobacter braakii (1 case; 0.5 %), Raoultella ornithinolytica (1 case; 0.5 %), and Raoultella planticola (1 case; 0.5 %).
Carbapenemase production
Of all the 94 CRE isolates, the carbapenemase genes were detected in 84 isolates. Fifty-five isolates produced carbapenemase. KPC-2 was the most common carbapenemase (65.5 %, 36/55), followed by NDM-1 (16.4 %, 9/55), IMP-4 (14.5 %, 8/55), NDM-5 (1.8 %, 1/55), and NDM-7 (1.8 %, 1/55).
Risk factors
Case–control study 1: Analysis of the CRE group versus the control group
The main characteristics of the study population are shown in Table 3. There was no difference in demographic features among the three groups. Case patients were more likely to have neurologic disease (odds ratio [OR], 2.192; 95 % confidence interval [CI] (1.040–4.617); P = 0.039). Among the CRE group cases, there was a greater proportion of patients with central venous catheters (OR, 2.087; 95 % CI, 1.141–3.82; P = 0.017), a tracheal cannula (OR, 3.446; 95 % CI, 1.576–7.537; P = 0.002), and tracheotomy (OR, 2.508; 95 % CI, 1.225–5.132; P = 0.012). In addition, the number of patients who received antibiotics prior to a positive culture was higher than that of the control group, including 3rd or 4th generation cephalosporins (OR, 3.883; 95 % CI, 1.871–8.06; P < 0.001), carbapenems (OR, 5.085; 95 % CI, 2.615–9.889; P < 0.001), and β-Lactam/β-lactamase inhibitor combinations (OR, 2.128; 95 % CI, 1.157–3.915; P = 0.015).
Multivariable analysis revealed that 3rd or 4th generation cephalosporins (OR, 4.557; 95 % CI, 1.971–10.539; P < 0.001) and carbapenems (OR, 4.058; 95 % CI, 1.753–9.397; P = 0.001) were associated with CRE infection (Table 4).
Case–control study 2: Analysis of the CSE group versus the control group
Univariable analysis showed that the CSE group patients were less likely to have neurologic diseases (OR, 2.947; 95 % CI, 1.445–6.012; P = 0.003) compared with the control group. There was no significant difference between the CSE group and the control group in demographic characteristics, invasive procedures, treatments, and procedures prior to a positive culture.
Multivariable analysis showed that neurologic disease (OR, 3.067; 95 % CI, 1.486–6.329; P = 0.002) was associated with CSE infection (Table 4).
Contrasting risk factors for CRE and CSE
When the models examining the risk factors for the recovery of CRE and CSE were compared, the prior receipt of antibiotics, especially 3rd or 4th generation cephalosporins and carbapenems were risk factors for the CRE infection only. The neurologic diseases were risk factors for the CSE infection only.
Outcome study
Univariable analysis was performed to assess the outcomes in this study (Table 5). Among all the patients in this study, 54 (57.4 %) died during their hospitalization in the CRE group, while 15 (16.1 %) and 16 (17.2 %) patients died in the CSE and control group, respectively. In the 30-day mortality analysis, 33 patients died within 30 days after CRE isolation. In contrast, 11 patients died in the CSE group and 16 (17.2 %) patients died in the control group. Patients infected with CRE were significantly more likely to have a longer ICU length of stay and longer hospitalization when compared with that of patients in the CSE and control groups, respectively.
The mean survival time within 30 days for the CRE group was 22.5 days (95 % confidence interval [CI], 20.2–24.8), compared with 26.8 days for the CSE group (95 % CI: 25.1–28.6) and 26.1 days for the control group (95 % CI, 24.1–28.1). Variables associated with 30-day mortality in hospital in Cox proportional hazard model included central venous catheters (hazard ratio [HR], 1.836; 95 % CI, 1.030–3.273; P = 0.040), receipt of immunosuppressors (HR, 2.810; 95 % CI, 1.503–5.254; P = 0.001), and CRE infection (HR, 3.044; 95 % CI,1.424–6.509; P = 0.004). Kaplan-Meier survival analysis was performed in this study according to different case groups (Fig. 1).
Time-adjusted univariable predictors of in-hospital mortality for the CRE group are shown in Table 6. Malignancy (P = 0.031), presence of a central venous catheters (P = 0.011), hematopoietic stem cell transplantation (P = 0.025), receipt of corticosteroids (P = 0.025), receipt of immunosuppressors (P =0.001) and bloodstream infection (0.021) were predictors for in-hospital mortality in the cases of CRE infections. Adjusted multivariable analysis showed that the presence of a central venous catheters (OR, 4.464; 95 % CI, 1.332–14.925; P = 0.015) and receipt of immunosuppressors (OR, 7.246; 95 % CI, 1.217–43.478; P = 0.030) were independent predictors for in-hospital death in CRE infection. While appropriate definitive treatment (OR, 0.339; 95 % CI, 0.120–0.954; P = 0.040) was a protective factor for in-hospital death in CRE infection (Table 7).
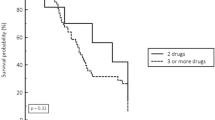
In this study, carbapenems used for treatment included meropenem and imipenem. For CRE infection, serval antibiotic regimens were used. For empirical treatment, monotherapy with carbapenems or other β-lactams were used for the treatment before organism isolation. For definitive treatment, monotherapy or combination therapy were used to combat the infection with at least one active drug according to the antimicrobial susceptibility testing reports, for example, tigecycline, aminoglycosides, and polymyxin. In this study, carbapenems were given as monotherapy or part of combination schemes for 113 patients for the treatment of either CRE or CSE infection. Kaplan–Meier survival curves revealed that patients in the meropenem MIC ≥ 8 mg/L group had a significantly higher 30-day mortality than those in the meropenem MIC = 2,4 mg/L group and MIC ≤ 1 mg/L (P = 0.003; Fig. 2).
Discussion
In this study, a case-case–control study was used to investigate the risk factors for the acquisition of CRE infection and the associated clinical outcomes from two teaching hospitals in China. We found CRE nosocomial infections are associated with prior exposure to 3rd or 4th generation cephalosporins and carbapenems. The presence of central venous catheters and receipt of immunosuppressors are associated with death of patients with CRE infections. Patients infected with CRE had higher 30-day mortality.
Over the past decade, CRE have been recognized as a cause of difficult-to-treat infections associated with high mortality and economic costs in the healthcare settings. Carbapenem resistance in Enterobacteriaceae is a complex issue. It can occur in various Enterobacteriaceae and may be mediated by several mechanisms, including the production of carbapenemases [26]. Since 2001, several studies have reported CRE mediated by carbapenemases in China, especially KPC-1 [25, 27–30]. Our study has showed the same result in carbapenemases distribution. In our previous surveillance of Chinese gram-negative bacilli resistance, we found that the frequency of CRE isolation is increasing [31]. CRE infections have increased in some regions of China, but little is known on the risk factors and the outcomes of carbapenem resistance in Enterobacteriaceae.
Case–control studies have been used to identify risk factors for CRE in many previous studies [14–16]. However, the analysis of patients from whom CSE is isolated as a control group can not represent the entire population. This will cause some deviation to the results. A case-case–control method to analyze the risk factors for CRE acquisition and mortality was used in the present study. Therefore, control patients in our study consisted of patients potentially at risk of CRE infection and were selected from the same wards and time period as case patients, reducing selection bias.
In recent risk factor studies, prior exposure to fluoroquinolones and carbapenems were independent risk factors for CRE infection [10, 32, 33]. Our multivariable analysis showed that previous use of 3rd or 4th generation cephalosporins and carbapenems are predictive factors for CRE infection. Carbapenems were independent risk factors for CRE infection, which is consistent with previous studies. However, unlike other studies, previous use of fluoroquinolones was not found to be associated with CRE acquisition in our analysis.
Schwaber et al. have performed a case-case–control study to assess the risks for carbapenem resistant Klebsiella pneumoniae (CRKP) isolation and a retrospective cohort study to assess mortality in three groups of hospitalized adults (48 patients as the CRKP group, 56 patients as the CSKP group, and 59 patients as the control group). The isolation of CRKP was the only independent predictor of in-hospital mortality [12]. Our study also showed that patients infected with CRE were significantly more likely to have higher in-hospital mortality, longer ICU length of stay and longer hospitalization. In addition, we found that presence of a central venous catheters and receipt of immunosuppressors were independent predictors for in-hospital mortality in CRE infections, which was not found in other previous studies. For the treatment of CRE, appropriate definitive treatment after final antimicrobial susceptibility test can reduce the mortality of CRE infections. This result is consistent with a study recently published in the United States [34]. There was no difference between combination therapy and monotherapy found in our study. This may be due to the limited number of cases in this study.
Recently, Patel et al. made a single-center retrospective matched cohort analysis in adult patients with Enterobacteriaceae infections treated with meropenem, imipenem or doripenem. Eighteen patients infected with Enterobacteriaceae with a carbapenem MIC of 2–8 mg/L were matched (1:1 ratio) based on the pathogen, the source of infection, co-morbidities and disease severity to those with a carbapenem MIC ≤ 1 mg/L [35]. They found that patients infected with Enterobacteriaceae with a carbapenem MIC of 2, 4 or 8 mg/L had higher mortality rates and longer ICU length of stay compared with the matched cohorts with a carbapenem MIC ≤ 1 mg/L. Our study showed that the patients in the meropenem MIC ≥ 8 mg/L had a significantly higher 30-day mortality than those in the meropenem MIC = 2,4 mg/L group and meropenem MIC ≤ 1 mg/L group, which is in accordance with the results discussed above. Both our results were supporting CLSI’s recommendation to lower susceptibility breakpoints for carbapenems. CLSI’s breakpoints were developed largely considering the resistant mechanism, and the clinical data is relatively less, thus our analysis provides the support for the point of clinical data. Some pharmacokinetic/pharmacodynamic studies on carbapenem have demonstrated that a high-dose/prolonged-infusion regimen of a carbapenem would attain a time above the MICs value of 50 % for CRE isolates with MICs up to 4 mg/L, ensuring an acceptable treatment outcome [36]. Some researchers recommend that combination therapy should be considered in the case of serious CRE infections. A carbapenem should be part of the combination regimen if a MIC ≤ 8 mg/L is recorded [37].
Several limitations should be noted in our study. The sample size of the index groups was relatively small. Therefore, the power of both the risk factor and the outcome studies was limited. The majority of the patients in this study received a standard dose of carbapenem therapy (equivalent of meropenem 1000 mg every 8 hours). However, the small sample size limited our ability to evaluate the influence of different dosing strategies on clinical outcomes. Infection control practices were also critical variables to assess the risk factors for CRE acquisition. Our outcomes did not apply to the institutions with a high prevalence of CRE isolation or CRE outbreaks associated with insufficient infection control practices. Furthermore, a molecular epidemiology investigation of CRE was not carried out, although different drug resistance mechanisms may influence treatment outcomes.
In conclusion, our study expounded prior exposure to 3rd or 4th generation cephalosporins, and carbapenems were independent risk factors associated with CRE nosocomial infections in two teaching hospitals in China. Presence of a central venous catheters and receipt of immunosuppressors were independent predictors for in-hospital mortality in CRE infections. Patients infected with high meropenem MICs (over 8 mg/L) Enterobacteriaceae had poor outcome and high mortality. Appropriate definitive treatment for the CRE infections patient is essential.
References
Temkin E, Adler A, Lerner A, Carmeli Y (2014) Carbapenem-resistant Enterobacteriaceae: biology, epidemiology, and management. Ann NY Acad Sci 1323:22–42
Queenan AM, Bush K (2007) Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 20:440–458, Table of contents
Nordmann P, Cuzon G, Naas T (2009) The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis 9:228–236
Doumith M, Ellington MJ, Livermore DM, Woodford N (2009) Molecular mechanisms disrupting porin expression in ertapenem-resistant Klebsiella and Enterobacter spp. clinical isolates from the UK. J Antimicrob Chemother 63:659–667
Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ (2014) Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis 20:1170–1175
Gupta N, Limbago BM, Patel JB, Kallen AJ (2011) Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 53:60–67
Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP (2008) Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 29:1099–1106
Chen S, Feng W, Chen J et al (2014) Spread of carbapenemase-producing enterobacteria in a southwest hospital in China. Ann Clin Microbiol Antimicrob 13:42
Kumarasamy KK, Toleman MA, Walsh TR et al (2010) Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 10:597–602
Bogan C, Kaye KS, Chopra T et al (2014) Outcomes of carbapenem-resistant Enterobacteriaceae isolation: matched analysis. Am J Infect Control 42:612–620
Kofteridis DP, Valachis A, Dimopoulou D et al (2014) Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization: a case-case–control study. J Infect Chemother 20:293–297
Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y (2008) Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother 52:1028–1033
Vardakas KZ, Matthaiou DK, Falagas ME, Antypa E, Koteli A, Antoniadou E (2015) Characteristics, risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections in the intensive care unit. J Infect 70:592–599
Correa L, Martino MD, Siqueira I et al (2013) A hospital-based matched case–control study to identify clinical outcome and risk factors associated with carbapenem-resistant Klebsiella pneumoniae infection. BMC Infect Dis 13:80
Jeong SJ, Yoon SS, Bae IK, Jeong SH, Kim JM, Lee K (2014) Risk factors for mortality in patients with bloodstream infections caused by carbapenem-resistant Pseudomonas aeruginosa: clinical impact of bacterial virulence and strains on outcome. Diagn Microbiol Infect Dis 80:130–135
Kritsotakis EI, Tsioutis C, Roumbelaki M, Christidou A, Gikas A (2011) Antibiotic use and the risk of carbapenem-resistant extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae infection in hospitalized patients: results of a double case–control study. J Antimicrob Chemother 66:1383–1391
Harris AD, Karchmer TB, Carmeli Y, Samore MH (2001) Methodological principles of case–control studies that analyzed risk factors for antibiotic resistance: a systematic review. Clin Infect Dis 32:1055–1061
Kaye KS, Harris AD, Samore M, Carmeli Y (2005) The case-case–control study design: addressing the limitations of risk factor studies for antimicrobial resistance. Infect Control Hosp Epidemiol 26:346–351
Schechner V, Temkin E, Harbarth S, Carmeli Y, Schwaber MJ (2013) Epidemiological interpretation of studies examining the effect of antibiotic usage on resistance. Clin Microbiol Rev 26:289–307
Wacholder S, McLaughlin JK, Silverman DT, Mandel JS (1992) Selection of controls in case–control studies. I. Principles. Am J Epidemiol 135:1019–1028
Wacholder S, Silverman DT, McLaughlin JK, Mandel JS (1992) Selection of controls in case–control studies. II. Types of controls. Am J Epidemiol 135:1029–1041
CLSI. Clinical and Laboratory Standards Institute (2014) Performance standards for antimicrobial testing: twentieth informational supplement, CLSI document. M100-S24. Wayne, PA
Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332
Wang X, Li H, Zhao C et al (2014) Novel NDM-9 metallo-beta-lactamase identified from a ST107 Klebsiella pneumoniae strain isolated in China. Int J Antimicrob Agents 44:90–91
Yang Q, Wang H, Sun H, Chen H, Xu Y, Chen M (2010) Phenotypic and genotypic characterization of Enterobacteriaceae with decreased susceptibility to carbapenems: results from large hospital-based surveillance studies in China. Antimicrob Agents Chemother 54:573–577
Centers for Disease Control and Prevention (CDC) (2013) Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep 62:165–70
Hawkey PM, Xiong J, Ye H, Li H, M’Zali FH (2001) Occurrence of a new metallo-beta-lactamase IMP-4 carried on a conjugative plasmid in Citrobacter youngae from the People’s Republic of China. FEMS Microbiol Lett 194:53–57
Wei ZQ, Du XX, Yu YS, Shen P, Chen YG, Li LJ (2007) Plasmid-mediated KPC-2 in a Klebsiella pneumoniae isolate from China. Antimicrob Agents Chemother 51:763–765
Yu YS, Du XX, Zhou ZH, Chen YG, Li LJ (2006) First isolation of blaIMI-2 in an Enterobacter cloacae clinical isolate from China. Antimicrob Agents Chemother 50:1610–1611
Zhang X, Lou D, Xu Y et al (2013) First identification of coexistence of blaNDM-1 and blaCMY-42 among Escherichia coli ST167 clinical isolates. BMC Microbiol 13:282
Wang Q, Zhao CJ, Wang H et al (2013) Antimicrobial resistance of gram-negative bacilli isolated from 13 teaching hospitals across China. Zhonghua Yi Xue Za Zhi 93:1388–1396
Ahn JY, Song JE, Kim MH et al (2014) Risk factors for the acquisition of carbapenem-resistant Escherichia coli at a tertiary care center in South Korea: a matched case–control study. Am J Infect Control 42:621–625
Teo J, Cai Y, Tang S et al (2012) Risk factors, molecular epidemiology and outcomes of ertapenem-resistant, carbapenem-susceptible Enterobacteriaceae: a case-case–control study. PLoS One 7:e34254
Qureshi ZA, Paterson DL, Potoski BA et al (2012) Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 56:2108–2113
Patel TS, Nagel JL (2015) Clinical outcomes of Enterobacteriaceae infections stratified by Carbapenem MICs. J Clin Microbiol 53:201–205
Daikos GL, Markogiannakis A (2011) Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 17:1135–1141
Axel H, Stephan G (2014) Treatment of infections caused by carbapenem-resistant enterobacteriaceae. Curr Treat Options Infect Dis 4:425–438
Acknowledgments
We thank Professor Fengxue Zhu and Doctor Zengli Xiao from Intensive Care Unit of Peking University People’s Hospital for providing APACHE II scores.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the National Natural Science Foundation (81572036).
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Wang, Q., Zhang, Y., Yao, X. et al. Risk factors and clinical outcomes for carbapenem-resistant Enterobacteriaceae nosocomial infections. Eur J Clin Microbiol Infect Dis 35, 1679–1689 (2016). https://doi.org/10.1007/s10096-016-2710-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2710-0