Abstract
The aim of this study was to determine the prevalence and predictive features of bacteremia among patients evaluated in the emergency department for urinary tract infection. Of the 350 patients with symptomatic urinary tract infection included in this retrospective study, 53 (15%; 95%CI 11.6–19.4%) were bacteremic. Five variables were independently associated with bacteremia: residence at home rather than in an institution (OR 4; 95%CI 1.5–10.7), presence of an indwelling urinary catheter (OR 3.3; 95%CI 1.3–8.8), presence of band forms in the blood count (OR 3.3; 95%CI 1.5–7.2), shaking chills (OR 2.3; 95%CI 1.1–4.8), and neutrophilia (OR 1.1; 95%CI 1.04–1.15). These easily assessable parameters may assist in the diagnosis of bacteremic urinary tract infection and the selection of empiric antibiotic treatment, thus potentially improving a patient’s prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary tract infection (UTI) is one of the most common infectious diseases [1]. The majority of patients with urinary tract infection are treated in the community as outpatients, while patients admitted to the emergency department are usually more seriously ill. In previous studies, UTI was found to be the source of bacteremia in 24–56% of bacteremic elderly patients [2] and in 30–40% of patients with community-acquired bacteremia [3–6]. Bacteremic UTI is associated with prolonged hospitalization and higher rates of complications and mortality compared with non-bacteremic UTI [7, 8].
Identification of patients with increased risk of bacteremia, based upon readily available clinical and laboratory features, could help target patients who will more likely benefit from broader spectrum, intravenous, antibiotic therapy. Published studies that addressed the issue of risk factors for bacteremia among patients with UTI found inconsistent results [8, 9]. The purpose of the study presented here was to determine the prevalence and predictive features of bacteremia among patients evaluated for UTI in the emergency department.
Materials and methods
In this study we retrospectively screened the medical records of all consecutive patients who were admitted to the emergency department of Shaare Zedek Medical Center during a 5-month period (August–December 2004) and had urine cultures drawn. Included in the study were patients above the age of 16 years who had a positive urine culture, clinical symptoms and/or signs supporting the diagnosis of UTI, and from whom at least one blood culture was obtained. As a matter of hospital policy, blood cultures are drawn upon a physician’s clinical suspicion of a more serious infection. One or more of the following urinary symptoms were considered suggestive of UTI: dysuria, frequency, urgency, macroscopic hematuria, flank pain, back pain, suprapubic pain or costovertebral tenderness on physical examination. In patients older than 65 years, the following non-specific symptoms and signs were also considered suggestive of clinically significant UTI if no other obvious infectious source was identified: nausea, vomiting, loss of appetite, shortness of breath, abdominal tenderness, mental or functional deterioration, fever or hypothermia [10]. A questionnaire was used to collect data on demographic, historical, clinical and laboratory variables.
Urine specimens were collected for culture from midstream urination, an indwelling catheter, supra-pubic aspiration (SPA), or one-time catheterization. The urine was incubated on a dip-slide (Hy-labs, Rehovot, Israel) [11] or collected in a sterile container; laboratory work-up was performed in accordance with established guidelines [12–14].
Blood was cultured using a set of two bottles, one for aerobic and one for non-aerobic pathogens. The culture bottles were incubated in the Bactec 9240 system (Becton Dickinson Diagnostic Instrument Systems, Sparks, MD, USA) for 5 days, or until positive. A positive urine culture was defined according to the method of collection: isolation of a single organism ≥105 cfu/ml if obtained by midstream sampling or via an indwelling catheter; or isolation of a single organism ≥103 cfu/ml if obtained by SPA or one-time catheterization. In young women presenting with symptoms of acute UTI, a positive culture was defined as the isolation of a single organism ≥102 cfu/ml. If two pathogens were isolated, the culture was considered positive if each pathogen’s quantitative growth was as specified previously. Isolation of three or more pathogens was considered indicative of contamination unless urine was collected by SPA or one-time catheterization, or if the blood culture was found positive for at least one of these organisms.
Bacteremic UTI was defined by isolation of a blood culture pathogen identical to the one isolated from the urine culture, or by isolation of a blood culture pathogen known to be commonly associated with UTI regardless of the urine culture result after other sources of infection were excluded.
Data were entered into Epi-Info 2002 version 2.2 (CDC, Atlanta, GA, USA). Data were analyzed using this program and SPSS version 11.0 (SPSS, Chicago, IL, USA). Bivariate analysis of categorical variables was performed using either the chi-square or Fisher’s exact test, where applicable. The Student’s t-test or the Mann–Whitney test (for variables with an abnormal distribution) was used to compare continuous variables. All variants that had a p value of ≤0.1 in the bivariate analysis and had potential clinical value were entered as covariates into a logistic regression model. Logistic regression was used to identify independent predictive features of bacteremic UTI.
Results and discussion
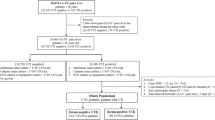
During the 5-month study period, 1,910 urine samples were obtained for culture from patients admitted to Shaare Zedek Medical Center’s emergency department; 842 (44%) of them were positive. Six hundred and fifteen patients above the age of 16 years had a positive urine culture and also had a blood culture drawn. Of these, 350 (57%) patients met the inclusion criteria. Bacteremia was confirmed in 53 patients (15%; 95% confidence interval [CI] 11.6–19.4%). Escherichia coli was the causative pathogen isolated most frequently from both bacteremic and non-bacteremic patients (58 and 48%, respectively). No statistically significant association was found between any bacterial strain and the presence of bacteremia.
Demographic, clinical and laboratory predictive features for bacteremia in patients with UTI are shown in Table 1. Interestingly, admission from home rather than from a chronic-care facility was more frequently associated with bacteremia (p = 0.005). A history of shaking chills was the only symptom variable associated with bacteremia (p = 0.0001) in the bivariate analysis. No association was found between bacteremia and diabetes mellitus or chronic renal failure and immunosuppressive state (data not shown). Although patients with bacteremia were found to have a higher pulse rate, lower systolic blood pressure, and higher body temperature, all of which reached statistical significance (data not shown), these variables were not included in the logistic regression model since the differences were not clinically significant. Bacteremic patients were more severely ill according to the Systemic Inflammatory Response Syndrome (SIRS) classification, but this was not found to be predictive of bacteremia in the multivariate analysis (data not shown).
Variables that were found to have both a statistically and clinically significant association with bacteremia in the bivariate analysis were entered as covariates into the logistic regression model. The results of the multivariate analysis are shown in Table 2.
The current study had two objectives, namely to determine (a) the prevalence and (b) the predictive features of bacteremia among patients evaluated in the emergency department for UTI. These issues may have a significant impact on the choice of empiric treatment and consequently on the course and prognosis of the disease. Of the 350 patients included in the study, 53 (15%; 95%CI 11.6–19.4%) were bacteremic. Five variables were found to be independently associated with bacteremia.
Previously published studies that addressed the issue of bacteremia among patients with UTI have reported rates of 16–32% for bacteremia in hospitalized patients with UTI [8, 15]. Subjective clinical evaluations regarding the presence of bacteremia are frequently inaccurate [8]. Studies addressing the issue of predicting bacteremia caused by UTI have reached inconsistent conclusions. While Richard et al. [9] emphasized the absence of fever and leukocytosis in 15% of patients with bacteremic UTI, Leibovici et al. [8] identified the following five independent predictors of bacteremia in hospitalized patients with UTI: fever, leukocytosis, high creatinine levels, presence of diabetes mellitus and a low serum albumin level. Varying inclusion criteria, as well as differences in the definitions of UTI and positive blood cultures could have contributed to the different results found in their studies compared to ours. We assume that the lower prevalence of bacteremia in patients living in chronic-care facilities may be due to the close medical supervision of these patients and their earlier referral to hospital compared to individuals living at home. Since our study only included patients from whom both urine and blood cultures were obtained, it was possibly biased towards patients with more serious disease and may have resulted in a higher percentage of bacteremia that was likewise biased.
In conclusion, bacteremia occurs in a significant proportion of patients with UTI evaluated in emergency departments. Our study identified five easily assessable variables that were independently associated with bacteremia: residence at home rather than in an institution, presence of an indwelling urinary catheter, presence of band forms in the blood count, shaking chills, and neutrophilia. Our findings may contribute to improving the identification of patients with an increased likelihood of bacteremia, thereby allowing physicians to target these patients for broader spectrum, intravenous, antibiotic treatment that will probably improve the prognosis. Patients without these predictive features for bacteremia could be safely treated with agents that have a narrower spectrum, which would keep expenses low and help prevent the emergence of resistant strains. Further studies are required before the risk factors for bacteremia in patients with UTI identified in this study can be routinely used in the decision-making process for appropriate antibiotic selection.
References
Nicolle LE (2001) Epidemiology of urinary tract infections. Infect Med 18:153–162
Richardson JP (1993) Bacteremia in the elderly. J Gen Intern Med 8:89–92
Lark RL, Saint S, Chenoweth C, Zemencuk JK, Lipsky BA, Plorde JJ (2001) Four-year prospective evaluation of community-acquired bacteremia: epidemiology, microbiology, and patient outcome. Diagn Microbiol Infect Dis 41:15–22
Mylotte JM, Kahler L, McCann C (2001) Community-acquired bacteremia at a teaching versus a nonteaching hospital: impact of acute severity of illness on 30-day mortality. Am J Infect Control 29:13–19
Whitelaw DA, Rayner BL, Willcox PA (1992) Community-acquired bacteremia in the elderly: a prospective study of 121 cases. J Am Geriatr Soc 40:996–1000
Siegman-Igra Y, Fourer B, Orni-Wasserlauf R, Golan Y, Noy A, Schwartz D, Giladi M (2002) Reappraisal of community-acquired bacteremia: a proposal of a new classification for the spectrum of acquisition of bacteremia. Clin Infect Dis 34:1431–1439
Jerkeman M, Braconier JH (1992) Bacteremic and non-bacteremic febrile urinary tract infection—a review of 168 hospital-treated patients. Infection 20:143–145
Leibovici L, Greenshtain S, Cohen O, Wysenbeek AJ (1992) Toward improved empiric management of moderate to severe urinary tract infections. Arch Intern Med 152:2481–2486
Richard JA, Patrick WM (1996) Bacteremic urinary tract infection in older people. J Am Geriatr Soc 44:927–933
Nickel JC, Pidutti R (1992) A rational approach to urinary tract infections in older patients. Geriatrics 47:49–55
Rosenberg M, Berger SA, Barki M, Goldberg S, Fink A, Miskin A (1992) Initial testing of a novel urine culture device. J Clin Microbiol 30:2686–2691
Clarridge JE, Johnson JR, Pezzlo MT (1998) Laboratory diagnosis of urinary tract infections. In: Cumitech 2B. American Society for Microbiology, Washington, DC
Aspevall O, Hallander H, Gant V, Kouri T (2001) European guidelines for urinalysis: a collaborative document produced by European clinical microbiologists and clinical chemists under ELCM in collaboration with ESCMID. Clin Microbiol Infect 7:173–178
Morgan MG, McKenzie H (1993) Controversies in laboratory diagnosis of community acquired urinary tract infection. Eur J Clin Microbiol Infect Dis 12:491–504
Senay H, Goetz MB (1991) Epidemiology of bacteremic urinary tract infections in chronically hospitalized eldery men. J Urol 145:1201–1204
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bahagon, Y., Raveh, D., Schlesinger, Y. et al. Prevalence and predictive features of bacteremic urinary tract infection in emergency department patients. Eur J Clin Microbiol Infect Dis 26, 349–352 (2007). https://doi.org/10.1007/s10096-007-0287-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-007-0287-3