Abstract
Backgrounds
Beta-2-microglobulin (β2-MG) levels vary in many infectious and autoimmune diseases. We investigated plasma and cerebrospinal fluid (CSF) β2-MG levels in patients with Guillain-Barré syndrome (GBS) and their correlations with clinical parameters.
Methods
CSF samples from 50 patients with GBS including 19 acute inflammatory demyelinating polyneuropathy (AIDP), 6 acute motor axonal neuropathy (AMAN), 10 acute motor-sensory axonal neuropathy (AMSAN), 7 Miller-Fisher syndrome (MFS), and 8 unclassified patients were collected. Moreover, 23 CSF samples from patients with non-inflammatory neurological disorders (NIND) as controls were collected. Plasma samples from 42 enrolled patients and 29 healthy individuals were also collected. The β2-MG levels were measured by immunoturbidimetry on automatic biochemical analyser. Besides, clinical data were extracted from electronic patient documentation system.
Results
CSF levels of β2-MG, lactate dehydrogenase (LDH), and lactate were significantly increased in patients with GBS (p = 0.004, p = 0.041, p = 0.040, respectively), particularly in patients with AIDP (p < 0.001, p = 0.001, p = 0.015, respectively), whereas no statistically significant difference was found in plasma levels of β2-MG. Furthermore, CSF levels of β2-MG were positively correlated with Hughes functional score (r = 0.493, p = 0.032), LDH (r = 0.796, p < 0.001), and lactate (r = 0.481, p = 0.037) but not with protein (r = − 0.090, p = 0.713) in AIDP patients.
Conclusions
CSF β2-MG levels may help identify AIDP and indicate clinical severity. CSF LDH and lactate levels correlate with CSF β2-MG levels; interaction among these biomarkers would need further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Guillain-Barre syndrome (GBS) is an immune-mediated disorder on peripheral nerves and their spinal roots [1], which is characterised by rapidly progressive symmetrical weakness of the limbs or cranial nerve-innervated muscles, usually accompanied by hyporeflexia or areflexia [2]. Currently, GBS is considered to cover a spectrum of clinicopathological subtypes. According to different pathological phenotypes, GBS is divided into two major subtypes: acute inflammatory demyelinating polyneuropathy (AIDP), in which immune attack mainly targets at the myelin sheath and related Schwann-cell components; axonal subtypes, including acute motor axonal neuropathy (AMAN) and acute motor-sensory axonal neuropathy (AMSAN), in which immune injury mainly takes place on the nerve axon membranes [1, 3]. Besides, Miller-Fisher syndrome (MFS), which is characterised by a triad of ataxia, ophthalmoplegia, and areflexia, is recognised as one of the GBS clinical variants [4].
Beta-2-microglobulin (β2-MG), a protein of low molecular weight (11,800), is non-covalently binding to the heavy chain of the major histocompatibility complex (MHC) class I, presenting on the membranes of all nucleated cells, prevailing on lymphocytes and macrophages, and widely existing in various body fluids such as serum, urine, cerebrospinal fluid (CSF), saliva, and colostrum [5,6,7]. Because of the association with immunocytes and MHC class I, β2-MG is essential for the stable expression of antigen-presenting molecules [8]. The change of β2-MG levels may indicate immune cell turnover and immune activation [9, 10]. β2-MG in both CSF and plasma (or serum) have been studied in many neurological disorders, such as meningitis, encephalitis, brain tumours, neurosyphilis, cerebral infraction, Alzheimer’s disease, multiple sclerosis, and central nervous system (CNS) leukaemia [7, 11]. However, there are few studies on CSF and plasma levels of β2-MG in GBS patients. In a small sample study, researchers observed increased CSF β2-MG levels in five patients with GBS [12]. However, Adachi et al. found no elevation of CSF β2-MG levels in GBS patients, whereas the serum β2-MG levels were slightly increased [13].
On the whole, CSF and plasma β2-MG levels and their associations with clinical parameters in GBS patients remain unclear. In this study, we investigated plasma and CSF β2-MG levels in patients with GBS and their correlations with disease severity. In addition, we preliminarily explored the levels of CSF LDH and lactate and their associations with CSF β2-MG in patients with GBS.
Methods
Study population
In this study, a total of 50 patients with GBS admitted to Tianjin Medical University General Hospital, Tianjin, China, from March 2018 to December 2019 were included. Inclusion criteria were meeting levels 1 or 2 of the Seivar’s criteria; detailed documentation of clinical disease course; samples and assay results obtained within 28 days from onset; no previous identified damage of peripheral nerve or preceding immunotherapy in another facility; and no documented comorbidities that would be indicative of altered β2-MG levels (such as CNS leukaemia, any CNS infections, acute or chronic kidney disease). According to the Hadden electrophysiology classification criteria (for AIDP and AMAN) [14] and Sejvar criteria (for AMSAN) [4], GBS patients in this cohort were subclassified into 5 subtypes, including AIDP (n = 19), AMAN (n = 6), AMSAN (n = 10), MFS (n = 7), and unclassified patients (n = 8). Twenty-three patients with non-inflammatory neurological disorders (NIND) were enrolled as controls, whose conventional CSF parameters were within the range of normal reference values. According to the discharge diagnoses, NIND controls included 4 cases with subacute combined degeneration of the spinal cord, 9 cases with chief complaint of dizziness or headache on admission but no detected organic lesions, 6 cases with mental illness, 2 cases with folic acid deficiency neuropathy, 1 case with spinal vascular malformation, and 1 case with cerebral venous sinus thrombosis. Twenty-nine healthy individuals who admitted in the Health Care Center of the hospital were enrolled as healthy controls (HC).
Extraction of clinical data
By reviewing the electronic patient documentation system, we extracted the following parameters: (1) the Hughes functional score (HFS), a neurological evaluation score on general functioning in patients with GBS, ranging from 0 (normal health), 1 (mild neurological symptoms or signs, capable of running), 2 (capable of walking at least 5 m, but incapable of running), 3 (capable of walking 5 m with support or walker), 4 (bed-ridden), 5 (mechanical ventilated), to 6 (dead). HFS was calculated at nadir; (2) detailed electrophysiology reports from two experienced technicians. Nerve conduction studies including motor nerve, sensory nerve, and F-wave analyses were performed in the median, ulnar, tibial, and peroneal nerves (n = 43); (3) conventional CSF parameters. CSF lactate and LDH levels had been measured using commercial diagnostic reagent kit on automatic biochemical analyser; (4) evidence whether the onset of GBS was associated with a preceding infection (diarrhoea or upper respiratory tract infection); (5) evidence whether autonomic nerve was involved; (6) results from tests of anti-ganglioside antibodies (anti-GS-ab, including anti-GM1-ab, anti-GD1b-ab, anti-GQ1b-ab).
Blood and CSF sampling
CSF samples from 50 patients with GBS and 23 patients with NIND were obtained by lumbar puncture before treatment. Blood samples from 42 enrolled patients (16 AIDP, 5 AMAN, 8 AMSAN, 6 MFS, and 7 unclassified patients) and 29 HC were collected in EDTA-coated tubes, and centrifuged at 3000 rpm for 10 min at 4 °C. CSF samples and supernatant were collected, divided into aliquots, and stored at − 80 °C pending analyses. All samples were not thawed or refrozen before analysis.
Measurement of β2-MG concentrations
Plasma and CSF concentrations of β2-MG were measured by immunoturbidimetry, using commercial diagnostic reagent (Medicalsystem Biotechnology Co., Ltd.) on automatic biochemical analyser (Hitachi 008AS). The detection range is 0.8–2.9 mg/L.
Statistical analyses
Continuous data were described by mean ± standard deviation (SD), and discrete variables were described by median value (interquartile, IQR). Mann-Whitney U test was used for continuous data analyses. Kruskal-Wallis test and Bonferroni correction were applied for multiple comparisons. Categorical variables were compared by Fisher’s exact test or chi-square test. Correlation analyses were performed using Spearman correlation test. All statistical analyses were computed with SPSS Statistics version 23.0 (IBM Corporation), and graphs were performed using GraphPad Prism 6.07. Two-tailed p values < 0.05 were considered statistically significant.
Results
Demographic and clinical characteristics of the study population
Demographic and clinical characteristics of the study population are described in Table 1. Since both AMAN and AMSAN were axonal neuropathies, the two subtypes were combined into one group. There were no statistically significant differences in age, gender, preceding infection rate, autonomic nerve involved rate, anti-GS-ab positive rate, CSF dissociation rate, and time interval between lumbar puncture and onset among different groups. Besides, no correlation was found between CSF β2-MG levels and the time of CSF analysis in GBS patients or in the subtypes (p < 0.05).
CSF β2-MG levels were increased in patients with GBS, especially in patients with AIDP
CSF β2-MG levels were significantly higher in patients with GBS than those in patients with NIND (Fig. 1, p = 0.004). Similar results were also observed in patients with AIDP but not in other subgroups when compared with NIND (Fig. 1, p < 0.001). In the intergroup comparison, CSF β2-MG levels in patients with AIDP patients were significantly higher than those in the axonal subgroup (Fig. 1, p = 0.015). Besides, there was no significant difference of plasma β2-MG levels in patients with GBS compared to HC (1.66 ± 0.55, 1.40 ± 0.32, p = 0.085).
CSF concentrations of β2-MG in patients with GBS, subtypes of GBS and NIND. CSF, cerebrospinal fluid; β2-MG, beta-2-microglobulin; LDH, lactate dehydrogenase; GBS, Guillain-Barré syndrome; AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy; AMSAN, acute motor-sensory axonal neuropathy; MFS, Miller-Fisher syndrome; Unclassified, unclassified patients; NIND, non-inflammatory neurological disorders. *p < 0.05; **p < 0.01; ***p < 0.001
CSF levels of LDH and lactate were increased in patients with GBS, especially in patients with AIDP
We also compared CSF concentrations of LDH and lactate in patients with GBS. CSF LDH and lactate concentrations were significantly increased in patients with GBS than those in patients with NIND (p = 0.041, p = 0.040, respectively). Across the four different GBS subtypes, CSF levels of LDH and lactate were significantly higher only in patients with AIDP than those in NIND (p = 0.001, p = 0.015, respectively). However, no significant differences of CSF LDH and lactate levels were found among other subgroups.
Correlation between CSF β2-MG levels and plasma β2-MG, CSF LDH, and CSF lactate levels in patients with GBS
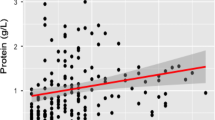
We explored further relationships between CSF β2-MG levels and plasma β2-MG, CSF LDH and CSF lactate levels in patients with GBS. The significant correlation between CSF β2-MG and LDH levels was observed in patients with GBS (r = 0.568, p < 0.001). However, there were no significant correlations between CSF β2-MG levels and plasma β2-MG (r = 0.252, p = 0.107) or CSF lactate levels in patients with GBS (r = 0.233, p = 0.103). Moreover, CSF β2-MG levels were significantly positively correlated with plasma β2-MG, CSF LDH, and CSF lactate levels in AIDP patients (Fig. 2a, r = 0.793, p < 0.001; Fig. 2b, r = 0.796, p < 0.001; Fig. 2c, r = 0.481, p = 0.037). However, there were no significant correlations between CSF β2-MG levels and CSF levels of LDH and lactate in other subgroups.
Correlations between plasma levels of β2-MG, CSF levels of LDH and lactate as well as HFS and CSF β2-MG levels in patients with AIDP. a Correlation between CSF β2-MG and plasma β2-MG levels in patients with AIDP. b Correlation between CSF levels of β2-MG and LDH in patients with AIDP. c Correlation between CSF levels of β2-MG and lactate in patients with AIDP. d Correlation between CSF β2-MG levels and HFS in patients with AIDP. e Correlation between CSF levels of β2-MG and protein in patients with AIDP. CSF, cerebrospinal fluid; β2-MG, beta-2-microglobulin; LDH, lactate dehydrogenase; HFS, Hughes fuctional score; AIDP, acute inflammatory demyelinating polyneuropathy
Correlation between β2-MG levels and other clinical characteristics in patients with GBS
Several studies have confirmed the concentration of β2-MG in CSF was related to the age [15,16,17]. We also confirmed that CSF β2-MG levels were correlated with age in patients with GBS (r = 0.524, p < 0.001), AIDP (r = 0.631, p = 0.004), and NIND (r = 0.644, p = 0.001). In addition, positive correlation between CSF β2-MG levels but not CSF LDH or lactate levels and HFS was observed in patients with AIDP (Fig. 2d, r = 0.493, p = 0.032). However, no correlation was found between CSF β2-MG levels and HFS in patients with GBS (r = 0.169, p = 0.241). Moreover, though in our study CSF protein levels were higher in patients with AIDP (Table 1), no correlation was found between CSF β2MG and protein (Fig. 2e, r = − 0.090, p = 0.713).
Discussion
β2-MG is a component of MHC class I molecule which plays an important role in T cell–mediated immune activation by participating in antigen presentation. There is evidence that cells lack of β2-MG significantly reduced the immunogenicity to CD8+ T cells [18]. Therefore, we consider that β2-MG may be a protein related to T lymphocyte activation. Besides, CSF β2-MG levels were increased in autoimmune diseases, such as multiple sclerosis, neuro-Behcet’s disease, and neurosarcoidosis, in which T cells play a role [12, 19,20,21]. Thus, we investigated the CSF and plasma levels of β2-MG in patients with GBS, and found that CSF β2-MG levels in GBS patients were significantly increased in our study. We suspect this finding may result from the immune activation which occurred on proximal nerve roots. It has been demonstrated that dorsal root ganglia and dorsal roots are in direct contact with the CSF in the subarachnoid space [22], and this physiological structure creates a pathway for inflammatory signaling molecules affected by local nerve injury entering into CSF. Besides, in the pathogenesis of GBS, the breakdown of blood-nerve-barrier (BNB) and blood-CSF-barrier (BCB) may be a key event [23, 24]. Previous study supported the theory that protein can leak from blood through the blood-nerve barrier [25]. In our study, the strong correlation of β2-MG between CSF and plasma in AIDP patients may be also attributed to the dysfunction of BNB and BCB. However, although we found plasma β2-MG levels were increased in patients with GBS, the trend was not statistically significant. This may be due to the limited sample size of our study.
Our study showed CSF β2-MG levels were significantly increased, especially in patients with AIDP but not in other subgroups. It has been demonstrated that antibodies and complement are involved in the pathogenesis of GBS [26,27,28]. However, unlike in other subtypes, specific antibody biomarkers in AIDP have not been identified [1]. Previous study has showed that T cells are abundant in early lesions of AIDP, and circulating activated T cells and serum soluble IL-2 receptor concentrations are increased in the acute stage [29], indicating that T cell–mediated immunity directed against unknown antigens on the surface of Schwann cells or the myelin sheath may play a great part in the pathogenesis of AIDP [30]. One hypothesis suggests that activated T cells penetrate the endothelium and release cytokines that activated endoneurial macrophages which ultimately invade myelin by the subsequent release of toxic mediators [30, 31]. Though another hypothesis suggests that an antibody-mediated attack fixes complement to the outer layer of the Schwann cell membrane and causes vesicular dissolution [27], the two mechanisms are not mutually exclusive. Therefore, we suppose the T cell–mediated immune response may be related to the increase of CSF β2-MG levels in patients with AIDP. Moreover, in our study, we found CSF β2-MG levels were positively related to the HFS at nadir in patients with AIDP. This suggests that β2-MG may have potential value to be a marker of disease severity of AIDP.
LDH is a tetrameric enzyme that increases the rate of inter-conversion of pyruvate to lactate. It is an accepted marker for cellular damage, released by all types of cells after loss of membrane integrity [32]. It is generally considered that poor CNS oxygenation or high metabolic activity may increase lactate concentration in the CSF [33, 34]. However, recent studies reported that during the rapid proliferation of antigenic stimulated T cells, glucose was utilised for aerobic glycolysis, which resulted in the generation of extracellular lactate [35]. This phenomenon has been demonstrated in some autoimmune diseases such as rheumatoid arthritis [36]. In our study, CSF LDH levels were also increased in patients with GBS and AIDP, which was consistent with previous study [37]. Besides, we also found that CSF lactate levels were significantly increased in patients with GBS, especially in AIDP. There were significant correlations between CSF LDH, lactate levels, and β2-MG levels in patients with AIDP, which may be the secondary effects of immune attack and immune stimulation. The poor correlation between CSF β2-MG and protein also supports the increase of CSF β2-MG which may not be simply due to a leakage of the proteins through BCB and BNB, but the presence of some degree of intrathecal immune activation. However, we did not find a correlation between CSF LDH or lactate levels and the HFS at nadir in patients with AIDP, suggesting that these two indicators lack specificity in patients with AIDP.
There are some limitations in our study. First, the sample size of this study is small and we did not obtain samples from patients with GBS after treatment. Second, we did not explore the exact mechanisms underlying the interaction of β2-MG and T cell activation. Further studies are needed to explore the CSF and plasma levels of β2-MG and their underlying mechanisms in patients with GBS.
Conclusions
The main findings of this retrospective study were that CSF β2-MG levels were significantly increased in patients with AIDP and positively correlated with HFS, suggesting that CSF β2-MG may be a potential biomarker for AIDP and may have potential value to predict the disease severity. Moreover, CSF LDH and lactate levels were elevated and correlated with CSF β2-MG levels in patients with AIDP. The mechanisms underlying these interactions need further exploration.
Availability of data and material
The data that support the findings of this study are available on reasonable request from the corresponding author.
Code availability
Not applicable for this study.
References
Willison HJ, Jacobs BC, van Doorn PA (2016) Guillain-Barre syndrome. Lancet 388(10045):717–727. https://doi.org/10.1016/S0140-6736(16)00339-1
van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA (2014) Guillain-Barre syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol 10(8):469–482. https://doi.org/10.1038/nrneurol.2014.121
Wang Y, Sun S, Zhu J, Cui L, Zhang HL (2015) Biomarkers of Guillain-Barre syndrome: some recent progress, more still to be explored. Mediat Inflamm 2015:564098–564012. https://doi.org/10.1155/2015/564098
Sejvar JJ, Kohl KS, Gidudu J, Amato A, Bakshi N, Baxter R, Burwen DR, Cornblath DR, Cleerbout J, Edwards KM, Heininger U, Hughes R, Khuri-Bulos N, Korinthenberg R, Law BJ, Munro U, Maltezou HC, Nell P, Oleske J, Sparks R, Velentgas P, Vermeer P, Wiznitzer M, Brighton Collaboration GBSWG (2011) Guillain-Barre syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 29(3):599–612. https://doi.org/10.1016/j.vaccine.2010.06.003
Talal N, Grey HM, Zvaifler N, Michalski JP, Daniels TE (1975) Elevated salivary and synovial fluid beta2-microglobulin in Sjogren's syndrome and rheumatoid arthritis. Science 187(4182):1196–1198. https://doi.org/10.1126/science.46621
Cejka J, Van Nieuwkoo JA, Mood DW, Kithier K, Radl J (1976) Beta2-microglobulin in human colostrum and milk: effect of breast feeding and physico-chemical characterization. Clin Chim Acta 67(1):71–78. https://doi.org/10.1016/0009-8981(76)90218-7
Starmans JJ, Vos J, van der Helm HJ (1977) The beta 2-microglobulin content of the cerebrospinal fluid in neurological disease. J Neurol Sci 33(1-2):45–49. https://doi.org/10.1016/0022-510x(77)90180-0
Gobin SJ, Biesta P, Van den Elsen PJ (2003) Regulation of human beta 2-microglobulin transactivation in hematopoietic cells. Blood 101(8):3058–3064. https://doi.org/10.1182/blood-2002-09-2924
Alvarez-Cermeno JC, Villar LM, Roy G, Ferreira A, Bootello A, Gimeno A, Gonzalez-Porque P (1987) Increased beta 2-microglobulin in CSF of multiple sclerosis. J Neurol Neurosurg Psychiatry 50(9):1238. https://doi.org/10.1136/jnnp.50.9.1238
Becker JW, Reeke GN Jr (1985) Three-dimensional structure of beta 2-microglobulin. Proc Natl Acad Sci U S A 82(12):4225–4229. https://doi.org/10.1073/pnas.82.12.4225
Adachi N (1991) Beta-2-microglobulin levels in the cerebrospinal fluid: their value as a disease marker. A review of the recent literature. Eur Neurol 31(4):181–185. https://doi.org/10.1159/000116674
Hallgren R, Terent A, Venge P (1982) Lactoferrin, lysozyme, and beta 2-microglobulin levels in cerebrospinal fluid: differential indices of CNS inflammation. Inflammation 6(3):291–304. https://doi.org/10.1007/BF00916410
Adachi N, Tshukagoshi H, Murakami F, Kanai M (1978) beta2-Microglobulin levels in cerebrospinal fluid--the levels in various neurological diseases and their comparison with those in serum (author's transl). Rinsho Shinkeigaku 18(6):351–357
Hadden RD, Cornblath DR, Hughes RA, Zielasek J, Hartung HP, Toyka KV, Swan AV (1998) Electrophysiological classification of Guillain-Barre syndrome: clinical associations and outcome. Plasma Exchange/Sandoglobulin Guillain-Barre Syndrome Trial Group. Ann Neurol 44(5):780–788. https://doi.org/10.1002/ana.410440512
Lofberg H, Grubb AO, Sveger T, Olsson JE (1980) The cerebrospinal fluid and plasma concentrations of gamma-trace and beta2-microglobulin at various ages and in neurological disorders. J Neurol 223(3):159–170. https://doi.org/10.1007/BF00313180
Kepplinger B, Baran H, Kainz A, Ferraz-Leite H, Newcombe J, Kalina P (2005) Age-related increase of kynurenic acid in human cerebrospinal fluid - IgG and beta2-microglobulin changes. Neurosignals 14(3):126–135. https://doi.org/10.1159/000086295
Haarmann A, Hahnel L, Schuhmann MK, Buttmann M (2018) Age-adjusted CSF beta2-microglobulin and lactate are increased and ACE is decreased in patients with multiple sclerosis, but only lactate correlates with clinical disease duration and severity. J Neuroimmunol 323:19–27. https://doi.org/10.1016/j.jneuroim.2018.07.001
Wang D, Quan Y, Yan Q, Morales JE, Wetsel RA (2015) Targeted disruption of the beta2-microglobulin gene minimizes the immunogenicity of human embryonic stem cells. Stem Cells Transl Med 4(10):1234–1245. https://doi.org/10.5966/sctm.2015-0049
Svatonova J, Borecka K, Adam P, Lanska V (2014) Beta2-microglobulin as a diagnostic marker in cerebrospinal fluid: a follow-up study. Dis Markers 2014:495402–495406. https://doi.org/10.1155/2014/495402
Matejcikova Z, Mares J, Prikrylova Vranova H, Klosova J, Sladkova V, Dolakova J, Zapletalova J, Kanovsky P (2015) Cerebrospinal fluid inflammatory markers in patients with multiple sclerosis: a pilot study. J Neural Transm (Vienna) 122(2):273–277. https://doi.org/10.1007/s00702-014-1244-9
Us O, Lolli F, Baig S, Link H (1989) Intrathecal synthesis of beta-2-microglobulin in multiple sclerosis and aseptic meningo-encephalitis. Acta Neurol Scand 80(6):598–602. https://doi.org/10.1111/j.1600-0404.1989.tb03934.x
Joukal M, Klusakova I, Dubovy P (2016) Direct communication of the spinal subarachnoid space with the rat dorsal root ganglia. Ann Anat 205:9–15. https://doi.org/10.1016/j.aanat.2016.01.004
Kanda T, Yamawaki M, Iwasaki T, Mizusawa H (2000) Glycosphingolipid antibodies and blood-nerve barrier in autoimmune demyelinative neuropathy. Neurology 54(7):1459–1464. https://doi.org/10.1212/wnl.54.7.1459
Mata S, Galli E, Amantini A, Pinto F, Sorbi S, Lolli F (2006) Anti-ganglioside antibodies and elevated CSF IgG levels in Guillain-Barre syndrome. Eur J Neurol 13(2):153–160. https://doi.org/10.1111/j.1468-1331.2006.01161.x
Ziganshin RH, Ivanova OM, Lomakin YA, Belogurov AA Jr, Kovalchuk SI, Azarkin IV, Arapidi GP, Anikanov NA, Shender VO, Piradov MA, Suponeva NA, Vorobyeva AA, Gabibov AG, Ivanov VT, Govorun VM (2016) The pathogenesis of the demyelinating form of Guillain-Barre Syndrome (GBS): proteo-peptidomic and immunological profiling of physiological fluids. Mol Cell Proteomics 15(7):2366–2378. https://doi.org/10.1074/mcp.M115.056036
Hafer-Macko C, Hsieh ST, Li CY, Ho TW, Sheikh K, Cornblath DR, McKhann GM, Asbury AK, Griffin JW (1996) Acute motor axonal neuropathy: an antibody-mediated attack on axolemma. Ann Neurol 40(4):635–644. https://doi.org/10.1002/ana.410400414
Hafer-Macko CE, Sheikh KA, Li CY, Ho TW, Cornblath DR, McKhann GM, Asbury AK, Griffin JW (1996) Immune attack on the Schwann cell surface in acute inflammatory demyelinating polyneuropathy. Ann Neurol 39(5):625–635. https://doi.org/10.1002/ana.410390512
Hughes RA, Cornblath DR (2005) Guillain-Barre syndrome. Lancet 366(9497):1653–1666. https://doi.org/10.1016/S0140-6736(05)67665-9
Hughes RAC, Hadden RDM, Gregson NA, Smith KJ (1999) Pathogenesis of Guillain–Barré syndrome. J Neuroimmunol 100(1-2):74–97. https://doi.org/10.1016/s0165-5728(99)00195-2
Kieseier BC, Kiefer R, Gold R, Hemmer B, Willison HJ, Hartung HP (2004) Advances in understanding and treatment of immune-mediated disorders of the peripheral nervous system. Muscle Nerve 30(2):131–156. https://doi.org/10.1002/mus.20076
Creange A, Sharshar T, Planchenault T, Christov C, Poron F, Raphael JC, Gherardi RK (1999) Matrix metalloproteinase-9 is increased and correlates with severity in Guillain-Barre syndrome. Neurology 53(8):1683–1691. https://doi.org/10.1212/wnl.53.8.1683
Schmidt H, Otto M, Niedmann P, Cepek L, Schroter A, Kretzschmar HA, Poser S (2004) CSF lactate dehydrogenase activity in patients with Creutzfeldt-Jakob disease exceeds that in other dementias. Dement Geriatr Cogn Disord 17(3):204–206. https://doi.org/10.1159/000076357
Mariani CL, Nye CJ, Tokarz DA, Green L, Lau J, Zidan N, Early PJ, Guevar J, Munana KR, Olby NJ, Miles S (2019) Cerebrospinal fluid lactate in dogs with inflammatory central nervous system disorders. J Vet Intern Med 33(6):2701–2708. https://doi.org/10.1111/jvim.15606
Portero M, Martinez de Merlo E, Perez C, Benito M, Daza MA, Fragio C (2019) Cerebrospinal fluid and blood lactate concentrations as prognostic biomarkers in dogs with meningoencephalitis of unknown origin. Vet J 254:105395. https://doi.org/10.1016/j.tvjl.2019.105395
Palmer CS, Ostrowski M, Balderson B, Christian N, Crowe SM (2015) Glucose metabolism regulates T cell activation, differentiation, and functions. Front Neurol 6:1. https://doi.org/10.3389/fimmu.2015.00001
Yang Z, Matteson EL, Goronzy JJ, Weyand CM (2015) T cell metabolism in autoimmune disease. Arthritis Res Ther 17:29. https://doi.org/10.1186/s13075-015-0542-4
Nussinovitch M, Prais D, Finkelstein Y, Harel D, Amir J, Volovitz B (2002) Lactic dehydrogenase isoenzymes in cerebrospinal fluid of children with Guillain-Barre syndrome. Arch Dis Child 87(3):255–256. https://doi.org/10.1136/adc.87.3.255
Acknowledgements
We were grateful to all the participants in this study.
Funding
This work was supported by grants from National Natural Science Foundation of China (grant numbers 81771363) and the Natural Science Foundation of Tianjin (grant numbers 18JCQNJC81700).
Author information
Authors and Affiliations
Contributions
Ming-Qi Liu: experimental design, manuscript writing, data analysis. Jing Wang: data collection and collation. Chen-Na Huang: data collection. Yuan Qi: experimental design, manuscript revising. Lin-Jie Zhang: manuscript revising, acquisition of funding. Ming Yi: manuscript revising. Sheng-Hui Chang: data analysis. Li-Sha Sun: technical help, experiment implementation. Li Yang: experimental design, acquisition of funding.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The study was approved by the ethics committee of Tianjin Medical University General Hospital and followed the principles outlined in the Declaration of Helsinki for all human experimental investigations.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, MQ., Wang, J., Huang, CN. et al. Elevated cerebrospinal fluid levels of beta-2-microglobulin in patients with Guillain-Barré syndrome and their correlations with clinical features. Neurol Sci 42, 4249–4255 (2021). https://doi.org/10.1007/s10072-021-05108-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05108-2