Abstract
Introduction
Plasma chemerin, which has chemotactic and adipogenic functions, is increased in several inflammatory diseases. However, its relationship with multiple sclerosis (MS) has not been explored yet. In this study, we aimed to determine chemerin levels and their possible role in MS.
Methods
Chemerin serum concentrations were evaluated by using ELISA kit in 91 clinically definite MS patients and 52 healthy controls. The mean serum chemerin, insulin, and cholesterol levels were compared. Patients were divided into two groups according to the body mass index (BMI), and the relationships between clinical and metabolic parameters were evaluated.
Results
Serum chemerin levels were 10.46 ± 1.65 ng/mL in MS patients and 10.26 ± 2.14 ng/mL in the control group. No significant difference was found between patients and controls (p = 0.55). We found no difference regarding age, gender, and BMI between two groups (p = 0.053, p = 0.54, p = 0.41). However, female patients with MS had higher chemerin levels than male patients. There were no associations between serum chemerin levels and EDSS score, annualized relapse rate, BMI, insulin resistance, and serum cholesterol levels in MS patients.
Conclusion
In this study, we aimed to determine serum chemerin levels in patients with MS. However, in our study, there was no significant difference between serum chemerin levels of MS patients and healthy controls’. Additionally, chemerin levels were not associated with other metabolic parameters, as well as cognitive dysfunction. Further studies are needed to evaluate the role of chemerin in MS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic, inflammatory, demyelinating disease of the central nervous system with unknown etiology. The underlying pathogenesis of MS is assumed to involve complicated autoimmune mechanisms. The interactions between several genetic and environmental factors have been implicated as risk factors.
Obesity is one of the modifiable risk factors identified to play a role in the development, and also in the prognosis of the disease [1]. Obesity leads to metabolic and endocrine dysfunction. Adipose tissue has been recognized as a complex endocrine organ that regulates metabolism and energy homeostasis. Adipokines are bioactive molecules secreted by adipose tissue that directly influences inflammatory processes. Several studies have focused on the pathogenic roles of adipokines in autoimmune diseases. Previous studies concluded that adipokines are associated with lipid metabolic disorders and play an essential role in metabolism.
Chemerin is a newly identified novel adipokine and has been associated with obesity and metabolic syndrome. Serum concentrations of chemerin are elevated in obesity and metabolic syndrome [2, 3]. Bozaoglu et al. [4] reported that serum chemerin levels were correlated positively with body mass index (BMI), fasting glucose, insulin, triglycerides (TGs), and total cholesterol (TC) and negatively with high-density lipoprotein cholesterol (HDL-C).
Chemerin is considered a proinflammatory adipokine, so it may itself contribute to inflammation in obese subjects [5]. Tomalka et al. [6] reported that increased levels of chemerin are associated with obesity and overweight in patients with MS. Prior studies in MS have suggested that elevated BMI may be associated with more severe disability, cognitive dysfunction, worse quality of life, and more rapid disease progression [7,8,9,10]. Moreover, adequate physical activity, promoting a healthy weight, has been investigated so far as a probable protective factor in MS.
The role of adipokines participate in the mediation of the immune response in MS is currently an active field of research. However, there is no study regarding chemerin has a role in the pathogenic process of MS, in particular, modulating the immune responses of MS patients. Therefore, we aimed to evaluate the association of serum chemerin levels with clinical variables, cognitive functions, and metabolic parameters related to obesity in a group of MS patients.
Materials and methods
Participants
We included patients admitted to the Multiple Sclerosis Unit at the University of Health Sciences, Izmir Bozyaka Education and Research Hospital, between June 2018 and September 2018. All of the patients were diagnosed with relapsing-remitting multiple sclerosis (RRMS) according to the revised McDonald 2010 Criteria. They were randomly selected in order of admission. The inclusion criteria were age over 18 years, disease duration of at least 1 year, Expanded Disability Status Scale (EDSS) scores of 6 or less, and willingness to participate. Patients were excluded if they had any neurological disease other than MS, relapse within three months, a metabolic or endocrine disorder that could negatively affect cognition and metabolic parameters, an active infection that might affect serum chemerin levels, a psychiatric disease that could affect cognitive tests’ performance, and alcohol or drug addiction. Also, subjects with lipemic samples that interfere with biochemical testing due to high lipid concentrations were excluded. The patient group was compared to age and sex-matched healthy controls (HCs). Healthy subjects consisted of the members of the hospital staff. Demographic characteristics and metabolic parameters were recorded.
Clinical evaluation
We consecutively enrolled 112 RRMS patients. Of the 112 RRMS patients available for analyses, five patients with active infection were excluded. Besides, sixteen patients were also excluded from the study; 8 were currently taking antidepressant medications for depression, 4 had lipemic blood samples, and four had EDSS scores higher than 6. Overall, a total of 91 patients with RRMS were included in the analysis. Patient information, including disease onset, disease course, treatments, relapses, and disability level, was reviewed by two of the neurologists of the MS center. The neurological disability of the patients was assessed with EDSS [11].
Biochemical tests
Blood samples were collected in the early morning after fasting for 12 h. The serum levels of glucose, insulin, TC, LDL-C, TGs, and HDL-C levels were determined by using Beckman Coulter kits in AU 2700 biochemistry autoanalyzer (Beckman Coulter Inc. Brea CA, USA). The insulin resistance was evaluated using the Homeostasis Model Assessment of Insulin Resistance Index (HOMA-IR). The index of insulin resistance, HOMA-IR, was calculated according to the formula as follows: HOMA-IR = fasting serum glucose concentration (mg/dL)/18.1 × fasting serum insulin concentration (uIU/mL)/22.5 [12]. Patients with a value of HOMA-IR ≥ 2.5 were considered to have insulin resistance, as other researchers did [13]. Body mass index (BMI) was calculated as weight (kg) divided by square of height (m2). BMI of 18.5 to 25 kg/m2 was defined as normal weight, BMI of 25.0–29.9 kg/m2 as overweight, and a BMI of 30 kg/m2 or over is obese.
The blood samples for chemerin analysis were collected on the same day. They were centrifuged at 1500×g for 15 min, and serum samples were stored in plastic tubes in deep freezers at − 80 °C until the time of analysis. Serum chemerin concentrations were measured by ELISA methods using a commercial assay (Human Chemerin/RARRES2 ELISA Kit PicoKineTM, catalog number: EK1329, Boster Biological Technology, Pleasanton CA, USA) according to the manufacturer’s instructions. It is a TMB colorimetric sandwich ELISA kit with a range of 0.78–50 ng/mL. Intraassay and interassay precision CV% of the kit were below 7.6% and 9.3%, respectively.
Neuropsychological evaluation
All patients were examined at the time of recruitment and underwent neuropsychological assessment described below. Two examining neurologists with adequate training were assigned to perform the tests. In order to evaluate the cognitive functions of the patients, the Montreal Cognitive Assessment Test (MoCA) and the Symbol Digit Modality Test (SDMT) were performed. The physical activity of the patients was assessed by using the Rapid Assessment of Physical Activity (RAPA), and the presence of depression was investigated using the Beck Depression Inventory (BDI), the fatigue was investigated by using the Fatigue Severity Scale (FSS). Neuropsychological tests were administered in a standardized protocol under the same environmental conditions (room temperature 22–24 °C). All tests were performed early in the morning, 2 h after a mild breakfast. All participants had a rest for at least 10 min before the testing in a silent waiting room.
Montreal Cognitive Assessment Test
Cognitive functions were evaluated using the Montreal Cognitive Assessment (MoCA) test. It is widely used as a screening tool for the presence of cognitive dysfunction in MS patients [14].
Symbol Digit Modality Test
We used the SDMT for cognition [15]. It examines patient attention, concentration, and processing speed [15]. The SDMT has been proposed for the assessment of cognitive impairment in MS [16, 17]. Parmenter et al. [18] found that a score of 55 or lower accurately classified cognitive impairment in MS patients.
Rapid Assessment of Physical Activity
The RAPA is a validated self-reported test able to measure physical activity in adults [19]. We assessed weekly physical activity using the Turkish version of the RAPA Scale [20]. It has nine items ask about different levels of physical activity, as well as strength and flexibility training. The RAPA 1 score categorizes five levels of physical activity. The RAPA 2, evaluates the strength and flexibility training, is scored separately. The cut-off point of the RAPA is 6. Scoring any number less than six is considered a suboptimal level of physical activity. The 6 or 7 score defines an adequate level of health-enhancing physical activity. RAPA test score ≤ 3 corresponds to a sedentary lifestyle or a very light activity level; values ≥ 4 indicates a moderate to a vigorous, active lifestyle [19].
Beck Depression Inventory
Depression was assessed by the BDI commonly used for patients with MS [21]. The BDI is an objective self-report assessment tool, including 21 items rated from 0 to 3. A total score of BDI is calculated by summing the items’ results. The test score can range between 0 and 63, and higher scores determine the severity of depression. Three levels of depression were classified according to the standard cutoffs: 0–13 indicates no depression symptoms, 14–19 mild depression, 20–28 moderate depression, and 29–63 severe depression.
Fatigue Severity Scale
FSS was used to assess the severity of fatigue levels. The FSS, which was published in 1989 by Krupp [22], is validated for use in patients with MS [23]. It is a self-administered questionnaire that has nine items. Each statement is scored on a scale of 1 to 7. The mean FSS score of 4 or higher is defined as fatigue.
Statistical analysis
The statistical analyses were performed using IBM SPSS Statistics for Windows (Version 21.0 Armonk, NY: IBM Corp). The normal distribution of the data was analyzed by examining the Shapiro–Wilk test and histogram graphs. Data on normal distribution are expressed as mean ± SD and were compared using a t test. Categorical variables are expressed as a percentage and were compared using the chi-square test or Fisher’s exact test, as appropriate. Nonparametric variables were analyzed using the Mann–Whitney U test. Spearman correlation analysis was performed to determine the direction and level of relationships among variables. Multivariate logistic regression analysis was used to test the independent association and contribution of HDL-c, EDSS, SDMT, and chemerin with the dependent variable (gender). All tests were considered significant at p < 0.05. A priori sample size was calculated regarding the information that plasma chemerin levels are elevated in overweight/obese patients with multiple sclerosis [6]. For alpha probability error of 0.05 and power of 0.80, a priori sample size was calculated as 114 using G*Power (Ver. 3.1.9.4, Dusseldorf University, Germany). According to the substantial evidence from the literature, medium effect size (a Cohen’s d of 0.50) is targeted. For the main objective of this study, a proposed sample size of 143 subjects (91 patients with MS and 52 healthy controls) would be more adequate. As a result, for alpha probability error of 0.05 and size effect index of Cohen’s d of 0.50, our study has the statistical power calculated as 88.8%. Thus, our proposed sample size of 143 will be more than adequate for the main objective of this study and should also allow for expected attrition and our additional objectives of controlling for possible subgroup analysis.
Results
The study population comprised of 91 MS patients and 52 HCs. The demographical characteristics and clinical features of the study population are presented in Table 1. Eighty-eight patients (96.7%) were currently treated with disease-modifying drugs. The distribution of DMD treatment modalities was as follows: 12 (13.2%) glatiramer acetate, 41 (45%) interferon-β; 15 (16.5%) fingolimod; 3 (3.3%) natalizumab; 9 (9.9%) dimethyl fumarate; and 8 (8.8%) teriflunomide patients.
Association of chemerin with gender, age and BMI
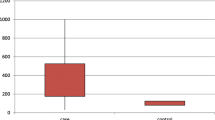
Serum chemerin was measured in 91 MS patients and 52 healthy controls by the ELISA method. Patient and control groups were similar according to age, gender, and BMI. Fifty-three (58.2%) of MS patients were female. We compared serum chemerin levels in male and female MS patients. Regarding chemerin levels, women showed higher levels than men (p = 0.001). Moreover, we determined significant differences in HDL-C levels, SDMT, and EDSS scores among female patients compared to males (Table 2). A multivariate logistic regression analysis with backward stepwise (Wald) method was used to select the set of covariates independently associated with the outcome. Predicted probability estimates were constructed with the regression coefficient estimates from the best-fitting logistic-regression model based on stepwise selection criteria. The risk of elevated serum chemerin levels in women with MS, was about 1.7-folds higher than the males. In addition, among HCs, female subjects had higher chemerin levels than males (p = 0.003). Female preponderance is shown in Fig. 1. However, there was no difference between female MS patients and males regarding age and BMI (p = 0.393, p = 0.584, respectively). Comparisons of variables in MS patients are summarized in Table 2. Chemerin levels did not correlate with age and BMI. According to the BMI index, only 17.5% (16) of MS patients were obese (BMI ≥ 30). Patients with MS were divided into two subgroups according to the BMI status: BMI < 25 and BMI ≥ 25. The results were as follows: in 43 patients who had BMI < 25 and 48 patients had BMI ≥25, chemerin levels were 10.36 ± 1.62 ng/mL and 10.54 ± 1.69 ng/mL, respectively. Chemerin levels were not different in either group (p = 0.802).
Chemerin, fasting glucose, insulin, and HOMA-IR in MS patients and healthy controls
Chemerin levels in patients with MS were not different compared to HCs (p = 0.304). Likewise, fasting serum glucose, insulin, and HOMA-IR levels were similar in MS patients and controls (p = 0.516, p = 0.670, p = 0.739). Chemerin levels neither correlated with insulin, BMI nor HOMA-IR in patients with MS (r = − 0.018, p = 0.862, r = 0.041, p = 0.702, r = − 0.024, p = 0.820). Besides, chemerin levels neither correlated with insulin, BMI, and HOMA-IR in controls (r = 0.012, p = 0.933, r = 0.01, p = 0.942, r = − 0.014, p = 0.923). The insulin resistance was present only in 20.8% of MS patients and 17.3% of HCs. Therefore, associations of serum chemerin with insulin resistance were not calculated. Regarding gender, there were no significant differences in BMI, insulin levels, and the presence of insulin resistance among MS patients (p = 0.584, p = 0.499, p = 0.627). Although chemerin levels were higher in female MS patients, chemerin did not correlate with BMI, HOMA-IR, and insulin (r = 0.046, p = 0.296, r = − 0.108, p = 0.441, r = − 0.109, p = 0.438). Patients with higher BMI had significant HOMA-IR and insulin levels compared to patients with normal BMI (p = 0.003, p = 0.002).
Association of chemerin with TC, TGs, LDL-C, and HDL-C
Serum chemerin, TC, LDL-C, and HDL-C levels were similar in MS patients and controls (p = 0.304, 0.817, 0.658, 0.326). However, serum TGs were significantly higher in patients with MS (p = 0.012). Chemerin did not show any correlation with TC, LDL-C, and HDL-C and TGs in MS patients. When MS patients were grouped due to gender, female patients with MS had significantly higher values for HDL-C (p < 0.001). Women with MS are more prone to have increased HDL-C levels (OR 1.1-folds) than men (Table 3). When MS patients grouped according to the BMI, MS patients with higher BMI had higher TGs levels than patients with normal BMI (p = 0.022) (Table 2). On the contrary, we did not find any difference in TGs levels among healthy controls when grouped into two according to the BMI (p = 0.516). Also, chemerin levels did not correlate with any metabolic parameter measured among MS patients in either group. Serum TC and LDL-C levels were higher in patients with EDSS ≥ 3 (p = 0.019 and p = 0.011, respectively).
Association of chemerin with EDSS, ARR, and disease duration in patients with MS
The EDSS scores of female MS patients were significantly lower than males (p = 0.027). The risk of increased EDSS scores in men was higher (OR = 1.34) (Table 3). Patients with MS were divided into subgroups according to the EDSS score: EDSS score < 3 and EDSS score ≥ 3. When clinical characteristics were compared according to the EDSS scores, there were no significant differences in most parameters (Table 2). Chemerin levels were not different in either the EDSS group (p = 0.927). Patients with higher EDSS scores had significantly lower education levels and longer disease duration (p = 0.032 and p < 0.001).
Association of chemerin with SDMT, MOCA, BDI, FSS, and RAPA in patients with MS
Female MS patients scored significantly higher in SDMT (p = 0.037). However, female patients had similar MOCA, FSS, and BDI scores with the male. Although chemerin levels were higher in female MS patients, no association was found between chemerin and metabolic markers, disease parameters, and neuropsychological assessments. Besides, patients with higher EDSS scores had significantly lower scores in SDMT (p = 0.001), but higher scores in FSS and BDI (p = 0.02, and p = 0.014, respectively) (Table 2). Chemerin did not show any association with SDMT, MOCA, BDI, and FSS in patients with higher BMI. There were no significant differences in cognitive tests, fatigue, and depression in MS patients with higher BMI compared to patients with lower BMI. Just 21.3% of MS patients were regularly active, according to the RAPA test. There was no significant difference between MS patients and healthy controls regarding physical activity assessed by RAPA 1 test (p = 0.062) (Table 1). Chemerin levels did not differ between patients who had an active or sedentary lifestyle. However, active patients were less fatigued and depressed (p = 0.018, p = 0.012).
Discussion
Currently, very little is known about the impact of chemerin on inflammatory activity and neurological deterioration in MS. In the present study, we investigated whether serum chemerin had any association with obesity, particularly in MS and healthy controls. Elevated circulating chemerin in MS patients was described in a recent study [6]. However, in this study, there was no difference concerning serum chemerin levels among the patient subgroups and the healthy controls. Besides, chemerin levels did not differ in obese and overweight compared to normal weighted MS patients. On the contrary, Tomalka et al. [6] reported that plasma chemerin levels were elevated in overweight and obese patients with MS. Notably, we found no associations between chemerin levels and fasting serum glucose levels, insulin levels, and insulin resistance at all. Tomalka et al. [6] further supported this finding.
Chemerin has been associated with inflammation and metabolic syndrome, which are probable risk factors for developing MS. Hedstrom and coworkers [24]suggested that inflammatory and immunologic mechanisms in obesity may potentially explain the association between adolescent obesity and increased risk of MS.To our knowledge, the role of chemerin in obesity and dysfunction of lipid metabolism is not thoroughly investigated in the course of MS. However, in our study, there was no difference regarding chemerin levels and insulin resistance among MS patients grouped according to the BMI status.
Many factors impact the production levels of adipokines, such as gender, age, and BMI [25]. However, we found no differences between MS patients and healthy individuals in terms of age, gender, and BMI. Our results further showed that female MS patients exhibited higher chemerin levels than males. However, there are conflicting results in this issue. Our finding was in accordance with several studies that detected higher chemerin levels in women [2, 4, 26]. A study has found that women expressed significantly more chemerin mRNA than men [27]. This finding might be explained by the sex-related variations in chemerin mRNA expression. Sex differences in circulating adipokine levels have been reported before. Although female patients had higher chemerin levels than males, chemerin did not correlate with either metabolic parameters in our study. Gender preponderance may be due to the metabolic dysfunction of adipose tissue and the influence of sex hormones.
In the present study, patients with MS had higher TGs levels than controls. Notably, serum TGs levels were significantly higher in overweight and obese MS patients than patients with normal weight. Serum chemerin levels were significantly associated with TGs in overweight and obese MS patients. Furthermore, obese MS patients had insulin resistance and exhibited higher insulin levels compared to normal weighted MS patients. However, we found that MS patients with higher BMI had similar chemerin levels compared to patients with normal BMI. Contrary to our presented findings, Sledzinski et al. [28] reported that BMI was the main predictor of serum chemerin concentration.
Obesity during adolescence has been associated with the future risk of MS in females [29, 30]. Additionally, several studies reported that serum chemerin concentration increases in obesity and metabolic syndrome and is correlated positively with BMI, fasting glucose, insulin, TGs, and TC and negatively with HDL-C cholesterol [2, 3]. On the contrary, our findings revealed that chemerin was not related to serum lipids, whereas a high BMI did not influence chemerin levels. Another study suggested that disability progression was related to higher LDL-C, TC, and TGs in patients with MS [31]. However, we found no association between chemerin levels and relapse rate, EDSS scores, and disease duration in MS patients. Cognitive test scores, depression, and fatigue were not related to chemerin levels either.
In the present study, we did not observe a relationship between BMI and physical activity level. No significant differences in physical activity and capacity were detected between obese and nonobese MS patients. Our findings indicate that MS patients are largely inactive according to the RAPA test. Here, we observed that there were no differences between physically active and sedentary patients regarding the EDSS score, relapse rate, and disease duration.
There were several strengths of the current study. We evaluated a large group of RRMS patients and compared them to HCs, including the measurement of chemerin. However, our study has potential limitations. First of all, we analyzed serum chemerin levels once during the MS course. The cross-sectional design of the study prevented us from determining the variability of the chemerin levels in time. Finally, we could not mention the role and contribution of chemerin on disease course and progression. Secondly, patients were evaluated with current body weight. We are not able to differentiate the effect of weight status before the diagnosis of disease course and chemerin concentrations. Third, subjects with MS were divided into only two groups according to the BMI with the cutoff score 25. Therefore, overweight MS patients were evaluated together with obese patients and were compared to patients with normal weight. We did not include a third group defined as obese. The main reason for this division concerned our desire to differentiate patients according to the BMI, as obese patients were relatively a few in the whole patient group. Fourth, we included healthy subjects among the members of hospital staff. This selection could be inappropriate for the comparison. However, two groups were age, sex, and physical activity matched. Future studies with larger cohorts should examine whether chemerin levels differ between obese and normal-weight MS patients. Finally, further research investigating the effect of chemerin on the disease course is needed.
In conclusion, chemerin was not related to lipid metabolism, disease progression, and cognitive dysfunction. Finally, we suggest that chemerin or obesity is not directly involved in the prognosis and progression of MS. This issue needs to be investigated in further studies. In conclusion, our findings may raise new questions for exploring the obesity-related factors in the pathogenesis of MS.
References
Amato MP, Derfuss T, Hemmer B, Liblau R, Montalban X, Soelberg Sørensen P, Miller DH, 2016 ECTRIMS Focused Workshop Group (2017) Environmental modifiable risk factors for multiple sclerosis: report from the 2016 ECTRIMS focused workshop. Mult Scler 6:1352458516686847
Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D (2007) Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 148:4687–4694
Ernst MC, Sinal CJ (2010) Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab 21:660–667
Bozaoglu K, Segal D, Shields KA, Cummings N, Curran JE, Comuzzie AG, Mahaney MC, Rainwater DL, VandeBerg JL, MacCluer JW, Collier G, Blangero J, Walder K, Jowett JBM (2009) Chemerin is associated with metabolic syndrome phenotypes in a Mexican-American population. J Clin Endocrinol Metab 94(8):3085–3088
Sell H, Divoux A, Poitou C, Basdevant A, Bouillot JL, Bedossa P, Tordjman J, Eckel J, Clément K (2010) Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J Clin Endocrinol Metab 95:2892–2896
Tomalka-Kochanowska J, Baranowska B, Wolinska-Witort E, Uchman D, Litwiniuk A, Martynska L, Kalisz M, Bik W, Jan Kochanowski J (2014) Plasma chemerin levels in patients with multiple sclerosis. Neuro Endocrinol Lett 35(3):218–223
Owji M, Ashraf-Ganjouei A, Sahraian MA, Bidadian M, Ghadiri F, Naser Moghadasi A (2019) The relationship between cognitive function and body mass index in multiple sclerosis patients. Mult Scler Relat Disord 32:37–40
Briggs FBS, Thompson NR, Conway DS (2019) Prognostic factors of disability in relapsing remitting multiple sclerosis. Mult Scler Relat Disord 30:9–16
Marck CH, Neate SL, Taylor KL, Weiland TJ, Jelinek GA (2016) Prevalence of comorbidities, overweight and obesity in an international sample of people with multiple sclerosis and associations with modifiable lifestyle factors. PLoS One 11(2):e0148573
Tettey P, Simpson S Jr, Taylor B, Blizzard L, Ponsonby AL, Dwyer T, Kostner K, van der Mei I (2014) An adverse lipid profile is associated with disability and progression in disability, in people with MS. Mult Scler 20(13):1737–1744
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Ld I-R, Pisarchyk L, Pérez-Luque EL, Garay-Sevilla ME, Malacara JM (2014) Whole-body and hepatic insulin resistance in obese children. PLoS One 9(11):e113576
Dagenais E, Rouleau I, Demers M, Jobin C, Roger E, Chamelian L, Duquette P (2013) Value of the MoCA test as a screening instrument in multiple sclerosis. Can J Neurol Sci 40(3):410–415
Smith A (1991) Symbol digit modalities test. Western Psychological Services, Los Angeles
Deloire MS, Bonnet MC, Salort E, Arimone Y, Boudineau M, Petry KG, Brochet B (2006) How to detect cognitive dysfunction at early stages of multiple sclerosis? Mult Scler 12:445–452
Sepulcre J, Vanotti S, Hernandez R, Sandoval G, Cáceres F, Garcea O, Villoslada P (2006) Cognitive impairment in patients with multiple sclerosis using the Brief Repeatable Battery Neuropsychology test. Mult Scler 12:187–195
Parmenter BA, Weinstock-Guttman B, Garg N, Munschauer F, Benedict RHB (2007) Screening for cognitive impairment in multiple sclerosis using the symbol digit modalities test. Mult Scler 13:52–57
Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB (2006) The rapid assessment of physical activity (RAPA) among older adults. Prev Chronic Dis 3:1–8
Çekok FK, Kahraman T, Kalkışım M, Genç A, Keskinoğlu P (2017) Cross-cultural adaptation and psychometric study of the Turkish version of the rapid assessment of physical activity. Geriatr Gerontol Int 17(11):1837–1842
Minden SL, Feinstein A, Kalb RC, Miller D, Mohr DC, Patten SB, Bever C Jr, Schiffer RB, Gronseth GS, Narayanaswami P (2014) Evidence based guideline: assessment and management of psychiatric disorders in individuals with MS: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 82:174–181
Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD (1989) The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 46:1121–1123
Armutlu K, Korkmaz N, Keser I, Sumbuloglu V, Akbiyik DI, Guney Z, Karabudak R (2007) The validity and reliability of the Fatigue Severity Scale in Turkish multiple sclerosis patients. Int J Rehabil Res 30:81–85
Hedstrom AK, Lima Bomfim I, Barcellos L, Gianfrancesco M, Schaefer C, Kockum I, Olsson T, Alfredsson L (2014) Interaction between adolescent obesity and HLA risk genes in the etiology of multiple sclerosis. Neurology 82(10):865–872
Lausten-Thomsen U, Christiansen M, Louise Hedley P, Esmann Fonvig C, Stjernholm T, Pedersen O, Hansen T, Holm JC (2016) Reference values for serum leptin in healthy non-obese children and adolescents. Scand J Clin Lab Invest 76(7):561–567
Gu P, Jiang W, Lu B, Shi Z (2014) Chemerin is associated with inflammatory markers and metabolic syndrome phenotypes in hypertension patients. Clin Exp Hypertens 36:326–332
Alfadda AA, Sallam RM, Chishti MA, Moustafa AS, Fatma S, Alomaim WS, Al-Naami MY, Bassas AF, Chrousos GP, Jo H (2012) Differential patterns of serum concentration and adipose tissue expression of chemerin in obesity: adipose depot specificity and gender dimorphism. Mol Cell 33(6):591–596
Sledzinski T, Korczynska J, Hallmann A, Kaska L, Proczko-Markuszewska M, Stefaniak T, Sledzinski M, Swierczynski J (2013) The increase of serum chemerin concentration is mainly associated with the increase of body mass index in obese, non-diabetic subjects. J Endocrinol Investig 36(6):428–434
Munger KL, Chitnis T, Ascherio A (2009) Body size and risk of MS in two cohorts of US women. Neurology 73(19):1543–1550
Munger KL, Bentzen J, Laursen B, Stenager E, Koch-Henriksen N, Sørensen TI, Baker JL (2013) Childhood body mass index and multiple sclerosis risk: a long-term cohort study. Mult Scler 19(10):1323–1329
Weinstock-Guttman B, Zivadinov R, Mahfooz N, Carl E, Drake A, Schneider J, Teter B, Hussein S, Mehta B, Weiskopf M, Durfee J, Bergsland N, Ramanathan M (2011) Serum lipid profiles are associated with disability and MRI outcomes in multiple sclerosis. J Neuroinflammation 8:127
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The present study was performed under the ethical standards of the 1964 Declaration of Helsinki and its later amendments. The Ethical Committee of the University of Health Sciences, Bozyaka Education and Research Hospital approved the study protocol (Ethical approval license: Reference Number: 04, Date 16.05.2018). Informed consent was obtained from all individuals who participated in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koskderelioglu, A., Gedizlioglu, M., Eskut, N. et al. Impact of chemerin, lipid profile, and insulin resistance on disease parameters in patients with multiple sclerosis. Neurol Sci 42, 2471–2479 (2021). https://doi.org/10.1007/s10072-020-04847-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04847-y