Abstract
Objective
This meta-analysis was performed to evaluate the efficacy and safety of monoclonal antibodies against calcitonin gene-related peptide (CGRP) for episodic migraine prevention.
Methods
MEDLINE, EMBASE, Web of Science, and the Cochrane Library were searched from inception to April 2018. Studies considered to be eligible were randomized controlled trials about efficacy and safety of calcitonin gene-related peptide monoclonal antibody for episodic migraine prevention.
Results
Eight randomized controlled trials involving 2292 patients were included. The outcomes of this meta-analysis presented that CGRP monoclonal antibodies for preventive treatment of episodic migraine significantly reduced the monthly migraine days from baseline [weighted mean difference (WMD) = − 1.52; 95%CI, − 1.92 to − 1.11; Z = 7.40; P < 0.001] and monthly acute migraine-specific medication consumption from baseline [WMD = − 1.45; 95%CI, − 2.17 to − 0.72; Z = 3.93; P < 0.001], as compared with placebo group. CGRP monoclonal antibodies for preventive treatment of episodic migraine significantly increased the ≥ 50% reduction from baseline in migraine days per month [RR = 1.54; 95%CI, 1.38 to1.71; Z = 7.88; P < 0.001]. The adverse events were similar between the CGRP monoclonal antibody group and placebo group (P = 0.998). The outcomes of subgroup analysis showed that erenumab, galcanezumab, and fremanezumab significantly reduced the monthly migraine days from baseline and increased the ≥ 50% reduction from baseline in migraine days per month. Both erenumab and fremanezumab significantly reduced from baseline.
Conclusions
Based on the results of this meta-analysis, CGRP monoclonal antibodies significantly reduced the monthly migraine days and acute migraine-specific medication. CGRP monoclonal antibodies were effective and safe for preventive treatment of episodic migraine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the 2013 International Classification of Headache Disorders-3 (ICHD-3), episodic migraine is at least two cluster periods lasting from 7 days to 1 year (when untreated) and separated by pain-free remission periods of ≥ 1 month [1,2,3]. Calcitonin gene-related peptide (CGRP) is a proinflammatory vasodilating neuropeptide implicated in the pathophysiology of migraine and has become a promising target for treating migraine [4,5,6,7]. Erenumab, eptinezumab, galcanezumab, and fremanezumab are the most studied CGRP monoclonal antibodies. Recently, the efficacy of CGRP monoclonal antibody for episodic migraine prevention has been confirmed in several clinical trials. Therefore, we utilize meta-analysis to improve statistical power and review the efficacy and safety of CGRP monoclonal antibody for episodic migraine.
Methods
This meta-analysis was performed to evaluate the efficacy and safety of monoclonal antibodies against CGRP for episodic migraine prevention.
Search trials
We conducted this post hoc analysis by systematically searching MEDLINE, the Cochrane Library, EMBASE, and Web of Science (from inception up to April 2018) to identify relevant randomized controlled trials (RCTs) by using the following search terms: “monoclonal antibodies against the CGRP-receptor,” “Erenumab,” “Eptinezumab,” “Galcanezumab,” “Fremanezumab,” “episodic migraine,” “randomized controlled trial,” “placebo-controlled.” The reference lists of the initially retrieved articles were also reviewed.
Take the PubMed as an example.
#1 Calcitonin gene-related peptide monoclonal antibody | |
#2 Fremanezumab | |
#3 Galcanezumab | |
#4 Erenumab | |
#5 Eptinezumab | |
#6 episodic migraine | |
#7 randomized controlled trial | |
#8 #1 OR #2 OR #3 OR #4 OR #5 | |
#9 #6 AND #7 AND #8 |
Inclusion criteria
Trials were selected based on the following inclusion criteria: (1) RCTs about calcitonin gene-related peptide monoclonal antibody for episodic migraine prevention; (2) reported at least one of the following outcomes: the reduction of monthly migraine days, ≥ 50% reduction from baseline in migraine days per month, monthly acute migraine-specific medication consumption from baseline and adverse event; (3) no limitations regarding the country, time, or language of publications. Exclusion criteria were as follows: (1) duration of follow-up was less than 7 days and (2) observational trials about episodic migraine.
Risk of bias assessments
The methodological quality for the included trials was assessed independently by two researchers based on the recommendations exemplified in the Cochrane handbook for systematic reviews of interventions and summarized in a domain-based evaluation of the following components: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other biases [8].
Data extraction
Two researchers independently reviewed the full text of the retrieved articles and reported the results in a structured dataset. Disparities between researchers regarding the inclusion of each trial were resolved by a third independent researcher. The information for each study included lead author, publication year, countries of origin, participant characteristics, study design, sample size, clinical follow-up, and outcomes.
The primary end point was to compare the reduction of monthly migraine days between the CGRP group and placebo group from baseline to the final 12th week of the double-blind treatment phase. The second end points included ≥ 50% reduction from baseline in migraine days per month and monthly acute migraine-specific medication consumption from baseline and adverse event.
Statistical analysis
We performed meta-analysis by using the Mantel-Haenszel statistical method. Dichotomous outcomes were analyzed using relative risk (RR) and 95% confidence intervals (CIs). Continuous outcomes were analyzed using weighted mean differences (WMD) and 95% CIs. Based on the practice recommendation of the Cochrane Handbook, trials with zero events in both the intervention and the control groups were not included in the meta-analysis when RR and WMD were calculated.
A random-effects model was used to pool the data, and the statistical heterogeneity between summary data was assessed by the chi-squared test and its extent was quantified by the I2 statistic. Sensitivity analysis was performed by excluding any single hazard ratio from the analysis.
To evaluate the efficacy and safety of different kinds of monoclonal antibodies against CGRP for episodic migraine prevention, we specified subgroups based on the kind of CGRP mABs. Publication bias was assessed by Egger’s test and a visual assessment of a funnel plot.
All meta-analyses were performed by the software program Stata12.0.
P < 0.05 was considered statistically significant.
Results
Study characteristics
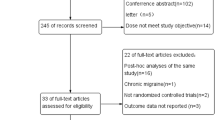
A total of 179 citations were identified from the literature search. Eight RCTs [9,10,11,12,13,14,15,16] involving 2292 patients met the inclusion criteria for this meta-analysis. The other studies were excluded for various reasons (Fig. 1). All studies were published in English from 2014 to 2017. The sample size varied from 133 to 628 patients in those included studies. All of these eight trials were multicenter, double-blind RCTs. Details of the study characteristics are shown in Table 1 and methodological assessments are shown in Table 2.
Quantitative data synthesis
Primary end points
The primary end point was the reduction of monthly migraine days from baseline at the 12th week.
A total of eight trials [9,10,11,12,13,14,15,16] including 2263 patients reported the reduction of monthly migraine days from baseline at 12-week. CGRP mAbs for preventive treatment of episodic migraine significantly reduced the monthly migraine days from baseline [2263 patients, WMD = − 1.52; 95%CI, − 1.92 to − 1.11; Z = 7.40; P < 0.001] (Fig. 2), as compared with placebo group. There was significant heterogeneity in the results (P < 0.001; I2 = 99.1%) while no evidence of publication bias (Egger’s test, P = 0.838) (Fig. 3). The sensitivity analysis (exclusion of any single hazard ratio from the analysis) did not substantively alter the overall result. Besides, sensitivity analysis based on those trials with long duration of follow-up (more than 12 weeks) led to similar outcomes.
The monthly migraine days from baseline: forest plot showing the comparison of the CGRP mAbs group vs. placebo group. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the weighted mean difference (WMD) for all studies
The results of subgroup analysis showed that erenumab, galcanezumab, and fremanezumab significantly reduced monthly migraine days from baseline [(WMD = − 1.63; 95%CI, − 2.31 to − 0.96; Z = 4.76; P < 0.001); (WMD = − 1.10; 95%CI, − 1.18 to − 1.02; Z = 27.84; P < 0.001); (WMD = − 1.83; 95%CI, − 2.55 to − 1.10; Z = 4.95; P < 0.001)], as compared with placebo group. Only one study reported the monthly migraine days from baseline under eptinezumab.
Secondary end points
The secondary end point included ≥ 50% reduction from baseline in migraine days per month and monthly acute migraine-specific medication consumption from baseline and adverse event.
≥ 50% reduction from baseline in migraine days per month
A total of eight RCTs [9,10,11,12,13,14,15,16] including 2206 patients reported ≥ 50% reduction from baseline in migraine days per month. The outcomes of this meta-analysis presented that the CGRP mAbs for preventive treatment of episodic migraine significantly increased the ≥ 50% reduction from baseline in migraine days per month [2206 patients, RR = 1.54; 95%CI, 1.38 to 1.71; Z = 7.88; P < 0.001] (Fig. 4), as compared with placebo group. There was no significant heterogeneity in the results (P = 0.335; I2 = 12.2%) and no evidence of publication bias (Egger’s test, P = 0.081) (Fig. 5). The sensitivity analysis (exclusion of any single hazard ratio from the analysis) did not substantively alter the overall result. Besides, sensitivity analysis based on those trials with long duration of follow-up (more than 12 weeks) led to similar outcomes.
≥ 50% reduction from baseline in migraine days per month: forest plot showing the comparison of the CGRP mAbs group vs. placebo group. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the RR for all studies
The results of subgroup analysis showed that erenumab, galcanezumab, and fremanezumab significantly increased the ≥ 50% reduction from baseline in migraine days per month [(RR = 1.63; 95%CI, 1.40 to 1.90; Z = 6.28; P < 0.001); (RR = 1.37; 95%CI, 1.07 to 1.74; Z = 2.53; P = 0.011); (RR = 1.71; 95%CI, 1.26 to 2.33; Z = 3.44; P < 0.001)], as compared with placebo group. Only one study reported the ≥ 50% reduction from baseline in migraine days per month under eptinezumab.
Monthly acute migraine-specific medication consumption
A total of five trials [9, 10, 12, 15, 16] including 1581 patients reported the monthly acute migraine-specific medication consumption from baseline. CGRP mAbs for preventive treatment of episodic migraine significantly reduced the monthly acute migraine-specific medication consumption from baseline [1581 patients, WMD = − 1.45; 95%CI, − 2.17 to − 0.72; Z = 3.93; P < 0.001] (Fig. 6), as compared with placebo group. There was significant heterogeneity in the results (P < 0.001; I2 = 99.7%) while no evidence of publication bias (Egger’s test, P = 0.481) (Fig. 7). The sensitivity analysis (exclusion of any single hazard ratio from the analysis) did not substantively alter the overall result. Besides, sensitivity analysis based on those trials with long duration of follow-up (more than 12 weeks) led to similar outcomes.
The monthly acute migraine-specific medication from baseline: forest plot showing the comparison of the CGRP mAbs group vs. placebo group. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the weighted mean difference (WMD) for all studies
The results of subgroup analysis showed that both erenumab and fremanezumab significantly reduced the monthly acute migraine-specific medication consumption from baseline [(WMD = − 1.40; 95%CI, − 2.38 to − 0.42; Z = 2.80; P = 0.005); (WMD = − 1.37; 95%CI, − 1.57 to − 1.17; Z = 13.22; P < 0.001)], as compared with placebo group. Only one study reported monthly acute migraine-specific medication consumption from baseline under eptinezumab.
Adverse events
The total adverse events were the composite of nasopharyngitis, infection, sinusitis, constipation, arthralgia, fatigue, nausea, back pain, migraine, hypertension, et al. A total of eight RCTs [9,10,11,12,13,14,15,16] including 2284 patients reported the adverse events. The outcomes of this meta-analysis presented that the adverse events after CGRP mAbs were similar with placebo group [2284 patients, RR = 1.00; 95%CI, 0.92 to 1.08; Z = 0.00; P = 0.998] (Fig. 8). There was no significant heterogeneity in the results (P = 0.367; I2 = 8.2%) and no evidence of publication bias (Egger’s test, P = 0.212) (Fig. 9). The sensitivity analysis (exclusion of any single hazard ratio from the analysis) did not substantively alter the overall result. Besides, sensitivity analysis based on those trials with long duration of follow-up (more than 12 weeks) led to similar outcomes.
Adverse events: forest plot showing the comparison of the CGRP mAbs group vs. placebo group. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the RR for all studies
The results of subgroup analysis showed that erenumab, galcanezumab, and fremanezumab shared the same risk of adverse events when compared with placebo group [(RR = 0.98; 95%CI, 0.87 to 1.11; Z = 0.30; P = 0.763); (RR = 1.05; 95%CI, 0.91 to 1.22; Z = 0.66; P = 0.509); (RR = 1.00; 95%CI, 0.92 to 1.08; Z = 0.01; P = 0.990)]. Only one study reported adverse events under eptinezumab.
Discussion
In our meta-analysis, CGRP monoclonal antibodies for preventive treatment of episodic migraine significantly reduced the monthly migraine days from baseline and monthly acute migraine-specific medication consumption from baseline at the 12th week when compared with placebo group. CGRP monoclonal antibodies for preventive treatment of episodic migraine significantly increased the ≥ 50% reduction from baseline in migraine days per month. The adverse events were similar between the CGRP monoclonal antibody group and placebo group. The outcomes of subgroup analysis showed that erenumab, galcanezumab, and fremanezumab significantly reduced the monthly migraine days from baseline and increased the ≥ 50% reduction from baseline in migraine days per month. Both erenumab and fremanezumab significantly reduced monthly acute migraine-specific medication consumption from baseline. Only one study reported the clinical outcomes about eptinezumab. Therefore, the effectiveness and safety of eptinezumab for episodic migraine still need further research.
Former studies have explored the potential benefit of CGRP monoclonal antibodies for preventive treatment of episodic migraine and the results showed that CGRP monoclonal antibody significantly reduced migraine frequency, especially for galcanezumab [9,10,11,12,13,14,15,16]. A systematic review including four studies reported that CGRP monoclonal antibody reduced the monthly migraine days from baseline in multiple dose subgroups [17]. The outcomes of our study which included eight RCTs were consistent with these former findings. Besides, the subgroup analysis of this meta-analysis presented the benefits of erenumab, galcanezumab, and fremanezumab for episodic migraine prevention. Therefore, this meta-analysis confirmed and extended the results from previous reports [18,19,20].
CGRP monoclonal antibody, as a new preventive treatment for migraine, significantly reduced the monthly migraine days and acute migraine-specific medication in this meta-analysis. However, whether it can improve the quality of life for patients with migraine may be more important and is still uncertain. Most of the included patients were adults that ranging from 18 to 65 years old. Whether patients who are younger than 18 years or older than 65 years old also obtain the same benefits from CGRP monoclonal antibody remains unknown. Actually, a high proportion of migraine patients in clinical practice present one or more comorbidities, such as fibromyalgia [21,22,23], cardiovascular disease [24,25,26], pelvic pain [27], and low back pain [28, 29]. However, the effect of CGRP monoclonal antibody on these comorbidities is still unclear and needs further research. Several studies reported differing degrees of headache activity at baseline. However, the effectiveness of CGRP monoclonal antibody on different degrees of headache remains unknown and needs further research.
Limitations
Firstly, due to the limited trials in this meta-analysis, we only included eight RCTs. However, all of these eight trials were international, multicenter, and high-quality studies. Secondly, several studies reported multiple dose groups. Goadsby et al. [9] reported erenumab 70 and 140 mg, Tepper et al. [10] reported erenumab 70 and 140 mg, Sun et al. [11] reported erenumab 7, 21, and 70 mg, Dodick et al. [12] only reported eptinezumab 1000 mg, Skljarevski et al. [13] reported galcanezumab 5, 50, 120, and 300 mg, Dodick et al. [14] only reported galcanezumab 150 mg, Cohen et al. [15] reported fremanezumab 225 and 657 mg, and Bigal et al. [16] reported fremanezumab 225 and 675 mg. Due to the complexity and difference of the dose about CGRP mAbs, the heterogeneity will be greatly increased if mix all the doses together. Therefore, in order to reduce the heterogeneity among these trials, we choose the most commonly dose for each kind of CGRP mAbs. Such as, the common dose of erenumab was 70 mg, the common dose of galcanezumab was 120 mg, the common dose of fremanezumab was 225 mg, and the common dose of eptinezumab was 1000 mg. Thirdly, in this meta-analysis, there were one study reported 3-month follow-up, six studies reported 12th week follow-up (close to 3 months), and one study reported 6-month follow-up. The sensitivity analysis based on those trials with long follow-up (more than 12 weeks) led to similar outcomes. Due to the limitation of the included studies, the long-term safety of CGRP monoclonal antibody still needs further research. Fourthly, the potential for publication bias was assessed by Egger’s test and a visual assessment of a funnel plot in this meta-analysis. Although the P > 0.05 in Egger’s test, the funnel plots were asymmetric. Potential conflicts of interest, whether outcomes and analyses, have been standardized and extent of trial registration may need to be considered. Fifthly, recently, erenumab, eptinezumab, galcanezumab, and fremanezumab are the most studied CGRP monoclonal antibodies. However, among these included eight RCTs, only one RCT provided the relative information about eptinezumab. Therefore, the efficacy and safety about eptinezumab for episodic migraine remain unknown and need further research. Sixthly, there was significant heterogeneity in the result about the monthly acute migraine-specific medication consumption from baseline. However, no evidence of publication bias was found and the sensitivity analysis did not substantively alter the overall result.
Taken as a whole, CGRP monoclonal antibodies significantly reduced the monthly migraine days and acute migraine-specific medication, increased ≥ 50% reduction from baseline. CGRP monoclonal antibodies were effective and safe for preventive treatment of episodic migraine.
References
Headache Classification Committee of the International Headache Society (2013) The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 33:629–808
Lipton RB, Manack Adams A, Buse DC, Fanning KM, Reed ML (2016) A comparison of the Chronic Migraine Epidemiology and Outcomes (CaMEO) study and American Migraine Prevalence and Prevention (AMPP) study: demographics and headache-related disability. Headache 56:1280–1289
Goadsby PJ, Sprenger T (2010) Current practice and future directions in the prevention and acute management of migraine. Lancet Neurol 9:285–298
Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J (2002) CGRP may play a causative role in migraine. Cephalalgia 22:54–61
Ho TW, Edvinsson L, Goadsby PJ (2010) CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 6(10):573–582
Russell FA, King R, Smillie SJ, Kodji X, Brain SD (2014) Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94(4):1099–1142
Kaiser EA, Russo AF (2013) CGRP and migraine: could PACAP play a role too? Neuropeptides 47(6):451–461
Higgins JP, Green S Cochrane handbook for systematic reviews of interventions (version 51.0). [cited 2012 Jan 5]. Available at: http://www.cochrane-handbook.org.
Goadsby PJ, Reuter U, Hallström Y, Broessner G, Bonner JH, Zhang F, Sapra S, Picard H, Mikol DD, Lenz RA (2017) A controlled trial of erenumab for episodic migraine. N Engl J Med 377(22):2123–2132
Tepper S, Ashina M, Reuter U et al (2017) Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 16(6):425–434
Sun H, Dodick DW, Silberstein S et al (2016) Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 15(4):382–390
Dodick DW, Goadsby PJ, Silberstein SD et al (2014) Safety and efficacy of ALD403, an antibody to calciton in gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 13(9):1100–1107
Skljarevski V, Oakes TM, Zhang Q, Ferguson MB, Martinez J, Camporeale A, Johnson KW, Shan Q, Carter J, Schacht A, Goadsby PJ, Dodick DW (2017) Effect of different doses of galcanezumab vs placebo for episodic migraine prevention: a randomized clinical trial. JAMA Neurol 75:187–193. https://doi.org/10.1001/jamaneurol.2017.3859.
Dodick DW, Goadsby PJ, Spierings ELH et al (2014) Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol 13:885–892
Cohen JM, Dodick DW, Yang R, Newman LC, Li T, Aycardi E, Bigal ME (2017) Fremanezumab as add-on treatment for patients treated with other migraine preventive medicines. Headache 57(9):1375–1384
Bigal ME, Dodick DW, Rapoport AM et al (2015) Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 14(11):1091–1100
Hong P, Wu X, Liu Y (2017) Calcitonin gene-related peptide monoclonal antibody for preventive treatment of episodic migraine: a meta analysis. Clin Neurol Neurosurg 154:74–78
Khan S, Olesen A, Ashina M et al (2017) CGRP, target for preventive therapy in migraine and cluster headache: systematic review of clinical data. Cephalalgia 333102417741297.
Mitsikostas DD, Reuter U (2017) Calcitonin gene-related peptide moncoclonal antibodies for migraine prevention: comparisons across randomized controlled studies. Curr Opin Neurol 30(3):272–280
Bigal ME, Dodick DW, Krymchantowski AV (2016) TEV-48125 for the preventive treatment of chronic migraine: efficacy at early time points. Neurology 87(1):41–48
Giamberardino MA, Affaitati G, Martelletti P et al (2015) Impact of migraine on fibromyalgia symptoms. J Headache Pain 17(1):28
de Tommaso M, Sciruicchio V, Delussi M, Vecchio E, Goffredo M, Simeone M, Barbaro MGF (2017) Symptoms of central sensitization and comorbidity for juvenile fibromyalgia in childhood migraine: an observational study in a tertiary headache center. J Headache Pain 18(1):59
Cho SJ, Sohn JH, Bae JS, Chu MK (2017) Fibromyalgia among patients with chronic migraine and chronic tension-type headache: a multicenter prospective cross-sectional study. Headache 57(10):1583–1592
Ferrante E (2013) Modified Valsalva test differentiates primary from secondary cough headache. J Headache Pain 14(1):1–2
Adelborg K, Szépligeti SK, Holland-Bill L et al (2018) Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ 360:k96
Lipton RB, Reed ML, Kurth T, Fanning KM, Buse DC (2017) Framingham-based cardiovascular risk estimates among people with episodic migraine in the US population: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache 57(10):1507–1521
Karp BI, Sinaii N, Nieman LK, Silberstein SD, Stratton P (2011) Migraine in women with chronic pelvic pain with and without endometriosis. Fertil Steril 95(3):895–899
Kakisaka Y, Ohara T, Katayama S, Suzuki T, Hino-Fukuyo N, Uematsu M, Kure S (2013) Another case of lower back pain associated with migraine: the importance of specific questions. J Child Neurol 28(5):680
Yoon MS, Manack A, Schramm S, Fritsche G, Obermann M, Diener HC, Moebus S, Katsarava Z (2013) Chronic migraine and chronic tension-type headache are associated with concomitant low back pain: results of the German Headache Consortium study. Pain 154(3):484–492
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was not registered.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Yuhan Zhu is the first author and Yanyan Liu is the co-first author.
Rights and permissions
About this article
Cite this article
Zhu, Y., Liu, Y., Zhao, J. et al. The efficacy and safety of calcitonin gene-related peptide monoclonal antibody for episodic migraine: a meta-analysis. Neurol Sci 39, 2097–2106 (2018). https://doi.org/10.1007/s10072-018-3547-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3547-3