Abstract
Restless leg syndrome (RLS) is frequently associated with poor mental health and impaired quality of life (QoL), due to discomfort, pain, fatigue, inability to rest, sleep disturbances, and consequently, anxiety and depression. The aim of this study is to address this issue in a community-based cohort of patients with RLS. The present study is a sub-analysis of the community-based prevalence study. In this door-to-door survey, we identified according to four essential IRLSSG diagnostic criteria 107 people with RLS. Clinical characteristics of RLS, including QoL, were obtained from 94 subjects (88 %), who completed the Serbian translation of SF-36. The main finding of our study was that the severity of RLS, in particular frequency of symptoms, negatively influenced majority of the SF-36 domains. The severity of depressive and anxiety symptoms was negatively associated with all domains of SF-36. Age of participants significantly correlated with both physical and mental composite scores. In multivariate linear regression model, higher scores of Hamilton depression (p = 0.001) and anxiety (p = 0.003) Rating scales were the most significant negative contributors of the total SF-36 score in persons with RLS. Suggesting particular role of comorbid depression and anxiety, our results may have a practical implication in terms of different psychosocial treatment and support in addition to the regular therapeutic protocols in RLS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Restless legs syndrome (RLS; also known as Willis-Ekbom disorder) is a sensorimotor-related sleep disorder characterized by distressing deep sensations (tingling, creeping, crawling, burning or aching) in the limbs and irresistible urge to move the legs, often during the evening and nighttime [1, 2]. These symptoms frequently lead to a loss of sleep: more severely affected patients sleep less than 4–5 h per night and experience problems in performing daily activities [3]. Despite wide variations in severity of symptoms among patients [2], RLS in general is frequently associated with poor mental health and impaired quality of life (QoL), due to discomfort, pain, fatigue, inability to rest, sleep disturbances, and consequently, anxiety and depression [3]. Unfortunately, pattern of distribution according to the severity of RLS in general population has not been thoroughly investigated and potential factors that may correlate with severity of the disease are not fully highlighted.
Prevalence studies suggest that RLS affects between 5 and 10 % of the general population in European countries and USA [4, 5]. The International RLS Study Group (IRLS-SG) proposed a set of criteria that clearly defined symptoms of RLS and allowed its more uniform diagnosis worldwide [6]: (1) an urge to move the legs, usually accompanied by an uncomfortable sensation(s); (2) the uncomfortable sensation(s) begins or worsens during periods of rest; (3) the unpleasant sensations are partially or totally relieved by walking/movement; and (4) the urge to move is greater in the evening or night than during the day. To exclude possible alternative diagnoses or “mimickers”, the fifth criterion has been recently added that the symptoms cannot be accounted for as symptoms primary to another medical or behavioral condition [7, 8].
Little is known about the clinical and demographic features associated with the health-related quality of life (HRQoL) in patients with primary RLS [9–11]. RLS symptom severity, usually determined by frequency and severity of symptoms, may broadly range from mild, moderate, severe, to very severe [12]. Clinical series are biased due to the recruitment of the more severe cases with RLS. Therefore, the aim of this cross-sectional study is to address this issue in a community-based cohort of patients with RLS.
Patients and methods
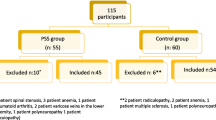
The present study is a sub-analysis of the previously published community-based prevalence study conducted in the region of Sombor (Serbia) [5]. Shortly, between October 2011 and January 2012, a door-to-door survey was carried out to identify people with RLS. Face-to-face interviews were conducted by two specialized nurses who received additional training from neurological and epidemiological experts prior to the study. The study participants answered four specific questions comprising essential criteria for RLS according to the IRLSSG [13], in a brief questionnaire (validated Serbian version) that also collected demographic data. If all four questions answered positively, the respondent was regarded as positive (possible) RLS subject; otherwise, the respondent was considered negative. All four essential IRLSSG diagnostic criteria were met by 107 of 2,112 respondents (5.1 %). In the second step, positive RLS subjects were invited to the city hospital where the possible alternative diagnoses or “mimickers” were excluded after an interview and detailed examination by the movement disorders expert, with a particular emphasize on diabetes mellitus, renal insufficiency, and radicular lesions in the lumbosacral region.
Clinical characteristics of RLS, including participation in the QoL study, were obtained from 94 out of 107 subjects (88 %) who met diagnostic criteria for RLS (13 participants rejected additional examinations). The patients were included in the study after giving informed consent and filling out a comprehensive questionnaire designed for this study. The study was approved by the Ethical Committee of the School of Medicine, University of Belgrade.
Supplementary investigations performed in all 94 recruited subjects included magnetic resonance imaging (MRI) and/or computed tomography (CT) of the brain and cervical region, electromyoneurography and extensive laboratory analyses, including serum levels of glucose, renal and hepatic functions, iron, ferritin and transferrin. The severity of RLS symptoms occurring over 7 previous days was assessed by the 10-item IRLSSG rating scale (IRLSSG-RS) [12] and classified as mild, moderate, severe and very severe, based on scores from the IRLSSG-RS (mild: 0–10; moderate: 11–20; severe: 21–30; very severe: 31–40). The scale was validated and adapted for Serbian language. Depression and anxiety were assessed using the Hamilton Depression Rating Scale (HDRS) [14] and Hamilton Anxiety Rating Scale (HARS) [15], respectively.
All subjects completed the Serbian translation of SF-36 (http://www.qualitymetric.com SF36 Health Survey (original version) Language Recalls [accessed on 13/05/2006)], which was used as an outcome measure for the HR-QoL [16]. The attending physicians were available for questions and additional explanations. The SF-36 is a generic instrument that provides a profile assessment of HR- QoL in eight domains: physical functioning (PF), role functioning physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role functioning emotional (RE), and mental health (MH). Based on these eight domains, two summary scales have been constructed: the Physical Health Composite (PHC) and the Mental Health Composite (MHC). The total SF-36 score was also calculated. Scoring and the calculations were performed by using the original Ware’s method [16]. Quality of life scores were presented as T scores (mean = 50; SD = 10), which were obtained by linear transformation of raw scores, that facilitates comparisons across the multiple subscales of the SF-36. The items can be summed to give scores ranging from 0 (worst possible health state) to 100 (best possible health state), i.e., higher values indicate better functioning and well-being.
Statistical analysis
Statistical analysis was performed with ANOVA and χ 2 depends on the normality of distribution. Pearson’s correlation coefficient was used to study the relationship between the subscales of the SF-36 and different variables of interest (HDRS, HARS, age at onset, age, duration of disease and the IRLSSG-RS). Spearman rank correlation analysis was conducted to investigate relationships between the IRLSSG-RS severity score, frequency of symptoms and gender and both HDRS and HARS. The linear regression analysis was used to examine how the various variables contribute to the total SF-36 score. Statistical analysis was performed using SPSS software, version 17.0.
Results
Ninety-four subjects with RLS (67 females; 71.3 %) were included in the study (aged 57.9 ± 13.4 years; range 24–86 years). Considering disease severity, 9 patients (9.6 %) had mild, 46 patients (48.9 %) moderate, and 39 (41.5 %) severe and very severe symptoms. The most severely affected subjects were those with significantly more frequent symptoms (p = 0.001) (Table 1).
Significant positive correlation was obtained between the HDRS and the frequency of symptoms (ρ = 0.357; p = 0.001), the IRLLSSG Rating Scale scores (ρ = 0.509; p = 0.001), and female gender (ρ = 0.296; p = 0.004). Significant positive correlations were also observed between the HARS scores and the frequency of symptoms (ρ = 0.386; p = 0.001), the IRLLSSG Rating Scale scores (ρ = 0.535; p = 0.001); and female gender (ρ = 0.296; p = 0.004). Sleep disturbances, tiredness or daytime somnolence due to RLS, assessed by two IRLSSG Rating Scale items, were significantly more common among RLS sufferers with one or more episodes per week when compared to those with less than one episode of RLS per week (Table 2). Those with more frequent RLS symptoms also had significantly higher HDRS (p = 0.001) and HARS (p = 0.002) scores (Table 2).
Mean scores of the SF-36 questionnaire, as well as the influence of symptoms frequency on different SF 36 items, are presented on Table 3. Shortly, frequency of the RLS symptoms influenced all the SF-36 subdomains, except PF, RF, RE, and BP.
Gender, symptoms expression in hands, and time during the day when the symptoms occurred, did not influence QoL in our patients. Employment influences RF (p = 0.014), GH (p = 0.003), and PHC domains (p = 0.002), as well as the total score (p = 0.014). Family history influenced VT (p = 0.028), while adequate sleep influenced RF (p = 0.059) and RE (p = 0.033) domains. Treatment influenced MH scale (p = 0.035).
According to correlation analysis (Table 4), age of participants correlated with PF, RP, VT, MH, GH and PHC, as well as the total SF-36 score. The HDRS statistically significantly correlated with all domains, while the HARS correlated with all domains except BP. Statistically significant correlations are also observed between IRLSSG-RS and all domains of SF-36, except PF. Disease duration correlated only with PF and SF (Table 4).
To determine factors statistically significantly associated with total quality of life in the persons with RLS, we performed univariate logistic regression analyses with the total SF-36 score as dependent variable, while independent variables were different demographic and clinical factors studied (Table 5). All variables univariately statistically significant (p < 0.05) entered in the multivariate logistic regression model. This analysis showed that higher scores of HDRS [standardized β coefficient = −0.443; 95 % confidence interval (CI), −1.939, −0.627; p = 0.001) and HARS (standardized β coefficient = −0.348; 95 % CI, −1.728, −0.363; p = 0.003] were the most significant negative contributors of the total SF-36 score in persons with RLS.
Discussion
The main finding of our study was that the severity of RLS, in particular frequency of symptoms, negatively influenced majority of the SF-36 domains, except PF, RF, and BP. The severity of depressive symptoms was negatively associated with all domains; the same was true for anxiety, with an exception for BP. Finally, age of participants significantly correlated with PF, RP, and RE domains, as well as both composite scores (Table 4). Our data are in accordance with the previous studies, suggesting that patients with more severe RLS symptoms have more severely impaired QoL [10, 17, 18]. As in our study, Abetz et al. [9] also found that increased RLS severity had a significant effect on all areas of functioning measured by the SF-36, with the exceptions of PF and general health, who did not discriminate between severity levels (Table 3). Winkelman et al. [19] conducted a large polysomnografic study and reveled that subjects with RLS had poorer HR-QoL (measured by the SF-36) in all physical domains, as well as in the Mental Health and Vitality domains. In a study that used the Restless Legs Syndrome Quality of Life questionnaire (RLS-QoL), which comprised social functioning, daily functioning, and emotional well-being, symptom severity significantly affected QoL [20].
There are several limitations of our study that make it difficult to compare our data with the data of other studies. First, this is the lack of standards for the SF-36 in the Serbian general population. For instance, Happe et al. [17] compared RLS patients with the age-matched general population using the EuroQoL (EQ-5D) and found considerably lower scores in the former group. In another study [18], comparison of the QoL of RLS patients with QoL of healthy controls, as well as patients with other chronic diseases (hypertension, diabetes type 2, and osteoarthritis in the knee) revealed that only patients with osteoarthritis were doing worse than RLS patients. Abetz et al. [9] also showed that RLS patients had significantly lower scores on all 8 scales of the SF-36 when compared with those suffering from hypertension, other cardiovascular conditions, diabetes mellitus or osteoarthritis. Second, we enrolled and personally examined participants from a community-based study prevalence study that used door-to-door survey. One may expect that the RLS symptoms in our cohort are less severe than in clinically based studies [17, 18]. Indeed, in our study, severe symptoms were present in about a third of patients, but only 6.5 % had very severe symptoms. Finally, like in few previous studies [4, 19, 21], we used the SF-36, while some other studies dealing with the QoL in RLS used different questionnaires like the EQ-5D [17], the disease-specific RLS-QoL [20], or the visual analog scales [22].
In our study, the HDRS scores statistically significantly correlated with all the SF-36 domains, similar to the HARS and the IRLS, with the exception of BP. Cho et al. [18] identified depression, severity of symptoms and gender (females) as factors significantly associated with SF-36 scores in the RLS patients; the QoL patients was significantly worse in those with more severe depression. Happe et al. [17] also reported anxiety/depression among the main contributors of decreased QoL in RLS. Fatigue [23], insomnia [24, 25] and daytime somnolence [26], frequently observed in RLS, are also independent risk factors for depression. In patients with more frequent RLS patients, we observed significantly more common expression of sleep disturbances, tiredness or daytime somnolence (Table 2). In the same group of patients, the HDRS and HARS scores were higher and significantly positively correlated with the frequency of symptoms, disease severity and female gender. Abetz et al. [9] also found that depression and anxiety in RLS significantly positively correlated with these three variables. Therefore, in parallel with the treatment of basic RLS symptoms, identification and treatment of anxiety/depression may be beneficial approach in this disorder. As some other authors [9], we found that therapy for RLS influenced emotional well-being of patients. However, all current medications induce side effects that may reduce the extent to which QoL symptoms are improved (i.e. adverse effects further affect QoL) [27].
Age of our patients also correlated with several domains of the SF-36. It has been already shown that the older patients reported that RLS symptoms affected their daily functioning, impaired ability to perform tasks, had detrimental effects on cognitive functions, and led to the feelings of hopelessness, with irritability and short tempered behavior [20]. However, age at onset of RLS symptoms correlated with only with physical functioning. Despite hypothesis that early-onset RLS produced greater deficits in QoL than late-onset RLS, several studies failed to find influence of this variable on the QoL in RLS [9, 17].
As recently suggested by Kalloo et al. [27], we may also conclude in this community-based cohort that RLS has significant influence on patients QoL that “extends beyond sleep, affecting their social lives and emotional and psychological health”. Suggesting particular role of comorbid depression and anxiety, our results may have a practical implication, i.e., that different psychosocial treatment and support could be added to the regular therapeutic protocols in RLS patients. Additionally, the severity of the disease should not also be underestimated in the planning of treatment approach. We used generic SF-36 instrument, but further use of the disease-specific instruments for measuring specific problems in patients with RLS, as well as treatment effects, could be more useful.
References
Hening WA (2002) Restless legs syndrome: a sensorimotor disorder of sleep/wake motor regulation. Curr Neurol Neurosci Rep 2:186–196
Hening W, Allen R, Earley C, Kushida C, Picchietti D, Silber M (1999) The treatment of restless leg syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Review. Sleep 22:970–999
Earley CJ (2003) Clinical practice. Restless legs syndrome. N Engl J Med 348:2103–2109
Allen RP, Walters AS, Montplaisir J, Hening W, Myers A, Bell TJ, Ferini-Strambi L (2005) Restless legs syndrome prevalence and impact: REST general population-based study. Arch Intern Med 165:1286–1292
Pekmezovic T, Jovic J, Svetel M, Kostic VS (2013) Prevalence of restless legs syndrome among adult population in a Serbian district: a community-based study. Eur J Epidemiol 28:927–930
Walters AS (1995) Toward a better definition of the restless legs syndrome. The International Restless Legs Syndrome Study Group. Mov Disord 10:634–642
International RLS Study Group (2011) Revised diagnostic criteria. Available at: http://irlssg.org/diagnostic-criteria/. Accessed 1 Oct 2012
Hening WA, Allen RP, Washburn M, Lesage SR, Earley CJ (2009) The four diagnostic criteria for restless leg syndrome are unable to exclude confounding conditions (“mimics”). Sleep Med 10:976–981
Abetz L, Allen R, Follet A, Washburn T, Earley C, Kirsch J, Knight H (2004) Evaluating the quality of life of patients with restless legs syndrome. Clin Ther 26:925–935
McCrink L, Allen RP, Wolowacz S, Sherrill B, Connolly M, Kirsch J (2007) Predictors of health-related quality of life in sufferers with restless legs syndrome: a multi-national study. Sleep Med 8:73–83
Kushida C, Martin M, Nikam P, Blaisdell B, Wallenstein G, Ferini-Strambi L et al (2007) Burden of restless legs syndrome on health-related quality of life. Qual Life Res 16:617–624
Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP (2003) Trenkwalder C; International Restless Legs Syndrome Study Group. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med 4:121–132
Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J (2003) Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med 4:101–119
Hamilton M (1967) Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6:278–286
Hamilton M (1959) The assessment of anxiety states by rating. Br J Med Psychol 32:50–55
Ware JE, Snow KK, Kosinski M (1993) SF-36 health survey manual and interpretation guide. Nimrod Press, Boston
Happe S, Reese JP, Stiasny-Kolster K, Peglau I, Mayer G, Klotsche J et al (2009) Assessing health-related quality of life in patients with restless legs syndrome. Sleep Med 10:295–305
Cho YW, Kim DH, Allen RP, Earley CJ (2012) Assessing health-related quality of life in patients with restless legs syndrome in Korea: comparison with other chronic medical diseases. Sleep Med 13:1158–1163
Winkelman J, Redline S, Baldwin CM, Resnick HE, Newman AB, Gottlieb DJ (2009) Polysomnographic and health-related quality of life correlates of restless legs syndrome in the Sleep Heart Health Study. Sleep 32:772–778
Cuellar N, Strumpf NE, Ratcliffe SJ (2007) Symptoms of restless legs syndrome in older adults: outcomes on sleep quality, sleepiness, fatigue, depression, and quality of life. J Am Geriatr Soc 55:1387–1392
Abetz L, Vallow SM, Kirsch J, Allen RP, Washburn T, Earley CJ (2005) Validation of the restless legs syndrome quality of life questionnaire. Value Health 8:157–167
Dodel R, Happe S, Peglau I, Mayer G, Wasem J, Reese H, Giani G, Geraedts G, Trenkwalder C, Oerther W, Stiasny-Kolster K (2010) Health economic burden of patients with restless legs syndrome in a German ambulatory setting. Pharmacoeconomics 28:381–393
Addington AM, Gallo JJ, Ford DE, Eaton WW (2001) Epidemiology of unexplained fatigue and major depression in the community: the Baltimore ECA follow-up, 1981–1994. Psychol Med 31:1037–1044
Breslau N, Roth T, Rosenthal L, Andreski P (1996) Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry 39:411–418
Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ (1997) Insomnia in young men and subsequent depression. The Johns Hopkins precursors study. Am J Epidemiol 146:105–114
Ford DE, Kamerow DB (1989) Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? J Am Med Assoc 262:1479–1484
Kalloo A, Gamaldo CE, Kwan AB, Salas RE (2013) The impact of restless legs syndrome/willis-Ekbom disorder on quality of life. Eur Neurol Rev 8:97–104
Acknowledgments
This work was supported by the Ministry of Education and Science of the Republic of Serbia (Grant Numbers 175090 and 175087).
Conflict of interest
The authors have no any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Svetel, M.V., Jovic, J.S., Pekmezovic, T.D. et al. Quality of life in patients with primary restless leg syndrome: community-based study. Neurol Sci 36, 1345–1351 (2015). https://doi.org/10.1007/s10072-015-2103-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-015-2103-7