Abstract
The objective of this study was to investigate the risk factors of wearing-off phenomenon in Parkinson’s disease (PD) and propose safe dosage of levodopa to reduce wearing-off development based on Chinese cohort. Patients with PD who had taken levodopa (l-dopa) for at least 1 month were recruited. Wearing-off was diagnosed based on validated Chinese version of a patient self-rated 9-question Wearing-Off Questionnaire (WOQ-9) and clinical definition. Eleven variables (gender, disease duration at l-dopa initiation, disease duration at assessment, age at onset, age at assessment, H-Y stage, UPDRS III, l-dopa daily total dosage and dosage adjusted to weight, duration of l-dopa treatment, initial drug recipe) were included in our analysis. Univariate analysis, multivariate logistic regression analysis and decision tree classification model(DTC) were used to detect risk factors of wearing-off. Receiver operating characteristic (ROC) curve and DTC were used to investigate cut-off value of l-dopa to best predict wearing-off. Two hundred and thirty-four patients were investigated in our study, among whom 111 developed wearing-off. Patients with wearing-off tended to receive higher l-dopa dosage and endure longer duration of l-dopa treatment. l-Dopa dosage as 281 mg/day and 4.2 mg/kg/day by ROC, as well as 269 mg/day and 3.2 mg/kg/day by DTC were cut-off values for wearing-off. l-Dopa dosage and duration of l-dopa treatment were related to increased wearing-off development. Cumulative l-dopa dosage and l-dopa daily dosage were better predictive of wearing-off. Inadequate evidence was present for delayed l-dopa initiation. l-Dopa daily dosage no more than 275 mg or 4.2 mg/kg was regarded as safe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease is the most common neurodegenerative movement disorder and affects around 1.7 % individuals older than 65 years in China [1]. By far, levodopa ( l-dopa) is the most basic and effective treatment in symptom control [2]. However, its long-term administration may lead to development of motor complications, mainly in terms of wearing-off and dyskinesia, which seriously affect patient’s daily life. Wearing-off phenomenon, also called end of dose phenomenon, was characterized by decline of benefit from each dose of ll-dopa [3]. Frequency of wearing-off development varies among studies, 12–60 % of patients were reported to experience wearing-off by 4–6 years of treatment [4–6]. A multicenter survey showed that in mainland China, the overall prevalence rates of wearing-off were 46.5 % [7]. To reduce its frequency, underlying mechanism and risk factors should be determined.
A body of studies tried to identify risk factors that were related to wearing-off [3, 5, 6, 8–11]. These explorations were important for establishing treatment strategy in clinical process. In STRIDE-PD trial, UPDRS III, age at onset, l-dopa dosage, North American geographic region, female gender were risk factors of wearing-off [6]. Hauser et al. [11] found cumulative l-dopa dose, cumulative l-dopa equivalent dose, and occurrence of dyskinesias were related to wearing-off. Schrag et al. [9] detected disease duration and l-dopa dosage to be its risk factors. l-Dopa dosage was a well-established risk factor in many studies [3, 5, 6, 8–11] and capable of monitoring in clinical process. STRIDE-PD trial specifically measured the effect of l-dopa at four different levels. Their results concluded that 400 mg/day seemed to be the threshold for clinician to obtain, based on prospective assumption. As duration of l-dopa treatment was found to be another risk factor, application of l-dopa was feared by some clinicians and patients [12]. However, a prospective study by Cilia et al. [5] indicated that earlier versus later l-dopa initiation made no difference in either timing or frequency of motor complications development, including wearing-off.
Despite all these important findings, several issues still need to be addressed. Firstly, STRIDE-PD trial concluded 400 mg/day as threshold, which was prospectively defined and arbitrary divided, thus may not be precise enough. Secondly, occurrence of motor complications was relatively less frequent in China, and l-dopa dosage was lower compared to many other countries [13], which highlighted the importance of population-based assessment. Thirdly, as delayed l-dopa initiation did not reduce wearing-off development while longer duration of l-dopa treatment was a risk factor of wearing-off [8, 9], timing of l-dopa initiation remains debated. Thus, further investigation of risk factors and comprehensive interpretation are needed.
Cut-off value of l-dopa dosage and importance of variables determined by decision tree classification model. Daily total l-dopa dosage and dosage adjusted to weight were separately tested as single variable first, where cut-off value of l-dopa daily dosage as 269 mg/d (a) and 3.2 mg/kg/day (b) were given. Importance of each variable was expressed by normalized importance (upper, percentage) and absolute importance (lower) (c). l-Dopa dosage was the most important factor in wearing-off development
Here, we carried out this cross-section study of 234 Chinese patients with idiopathic Parkinson’s disease, aiming: (1) to detect risk factors in a comprehensive way by univariate analysis, multivariate analysis and decision tree classification (DTC) model; (2) to determine a safe dosage of l-dopa for wearing-off, by receiver operating characteristic (ROC) curve model and DTC model whose application would avoid the limitation resulting from presumed division. DTC model was a type of data mining method, which was a computational process of discovering patterns or classifications using a combination of artificial intelligence, machine learning, statistics, and database systems, and showed accurate prediction as well as some successful alternatives to the traditional statistic approach [14, 15]. ROC and DTC would offer with useful and reliable approach to find a reasonable safe dosage.
Materials and methods
Patients
Patients were recruited in outpatient department of Beijing Tiantan Hospital, Capital Medical University from May 2012 to July 2013. Patients were diagnosed by two experienced expertise in the particular field of movement disorders. Inclusion criteria were established as (1) in accordance with the United Kingdom Parkinson’s Disease Brain Bank (UKPDBB) criteria [16]. (2) Having taken l-dopa drugs for at least 1 month. Exclusive criteria were established as: (1) uncertainly diagnosed or suspicious of parkinsonism syndrome(vascular, drug induced, toxic induced, post-infectious parkinsonism), multiple system atrophy, corticobasal ganglionic degeneration, or supranuclear palsy; (2) with a history of stroke, moderate to severe head trauma, hydrocephalus, brain surgery or brain tumor. All procedures were approved and supervised by the ethics committee of Beijing Tiantan Hospital, and was performed in accordance with the Declaration of Helsinki. Informed consent was obtained either from the participants or their closest relatives.
Demographic profile (gender, age at assessment), disease profile (age at onset, disease severity, disease duration at assessment) and treatment profile (disease duration at l-dopa initiation, l-dopa dosage expressed by daily total dosage and dosage adjusted to weight, initial drug recipe) were collected for each patient. Severity of disease was evaluated by Hoehn and Yahr stage (H-Y stage) and Unified Parkinson’s Disease Rating Scale Part III (UPDRS III). l-dopa dosage was recorded from levodopa drugs only.
Patients were categorized by their presence of motor complications, and group without any motor complications was defined as control group. Wearing-off was diagnosed based on the validated Chinese version of a patient self-rated 9-question Wearing-Off Questionnaire (WOQ-9) [17] and clinical observation. By clinical definition, wearing-off was decreased benefit (less than 4 h) despite of regular l-dopa administration (with effective dosage and at least 3 times a day), as motor functions declined with blood drug concentration [3].
Statistic analysis
Statistic analysis was performed in SPSS 18.0 software (SPSS, inc., Beijing, China). Depending on distribution, numeric variables underwent one-way ANOVA or Mann–Whitney test, and data were expressed as mean value ± SD or median value and interquartile range. Non-numeric variables were analyzed by χ 2 test. Variables detected as a trend (p ≥ 0.05 and <0.2) or significant difference (p < 0.05) were entered in multivariate logistic analysis, otherwise were excluded in this step. Results were presented in terms of odds ratio (OR) and significant value (p). p value less than 0.05 was regarded as significant.
Receiver operating characteristic (ROC) curve and decision tree classification (DTC) model were applied to determine cut-off value of l-dopa dosage in regarding to wearing-off development. ROC curve set out different cut-off value with its sensitivity and specificity. As another study used ROC to propose starting dose of allopurinol to reduce the risk of allopurinol hypersensitivity syndrome, we found it logical to apply in our study [18]. In our study, positive predictive value and negative predictive value were of greater interest, as they represented likelihood to develop wearing-off above or below threshold dosage. Best predicting dosage in ROC curve model was detected by Youden’s index (maximum value of sensitivity plus specificity minus one). DTC analysis was based on Classification and Regression Tree (CART) and Gini index. For the stopping rule, the maximum tree depth is two levels, and the minimum number of cases for a parent and child node is 30 and 20, respectively. CART analysis is well suited to the generation of clinical decision rules and for either numeric or non-numeric variables. Optimal tree was conducted according to its optimal predictive accuracy. CART stratifies the data by the best binary predictor to create high-risk and low-risk subgroup that demonstrate the greatest gain in overall subgroup homogeneity with respect to the outcome. l-Dopa daily total dosage and l-dopa dosage adjusted to weight were tested separately as single variable. In other words, at child node, data would be divided into subgroups that received different levels of l-dopa dosage, depending on their risk to develop wearing-off. Safe dosage indicated by ROC and DTC was further confirmed according to clinical relevance. And variables which needed further investigation were included in DTC to detect importance of each variable. Importance of each variable was presented in terms of normalized importance and absolute importance.
Results
Summary of variables
Two hundred and thirty-four patients (male:female = 126:108) were investigated in our study, of whom 111 (47.4 %) were recorded for mere wearing-off, 8 (3.4 %) for mere dyskinesia, and 18 (7.7 %) for both. As the number of patients with dyskinesia was too small, only wearing-off was further investigated in our study. In wearing-off group, mean ± SD age at assessment was 62.1 ± 10.2 years old and age of onset was 57.0 ± 10.8 years old. Median disease duration at assessment was 61 months (interquartile range: 48–96), median l-dopa dosage was 387.5 mg/day and 6.1 mg/kg/day.
Risk factors of wearing-off
By comparing wearing-off group to the control, there was no significant difference in gender, age at assessment, age at onset, and initial treatment recipe. H-Y stage and disease duration at l-dopa initiation was regarded a trend (p ≥ 0.05 and <0.2). UPDRS III, disease duration at assessment, l-dopa daily total dosage, l-dopa dosage adjusted to weight and duration of l-dopa treatment showed significant difference. When matching two groups by l-dopa to be their initial treatment, disease duration at assessment (p = 0.053) was excluded to be significant difference but as a trend. Other variables displayed similarly after matching (Table 1).
Six variables which were detected as a trend or significant difference were included in multivariate logistic regression model and DTC. In the former, duration of l-dopa treatment (p = 0.003, OR = 1.024), l-dopa daily total dosage (p < 0.001, OR = 1.004) and dosage adjusted to weight (p < 0.001, OR = 1.282) were risk factors, and overall accuracy was 68 % (Table 2). In the latter, importance of these six variables to wearing-off in a ranking order were: l-dopa daily total dosage (normalized importance: 100 %), l-dopa daily dosage adjusted to weight (77.6 %), duration of l-dopa treatment(15 %), disease duration at assessment (3.7 %), H-Y stage(1.7 %), UPDRS III (1.5 %), and overall accuracy was 69.7 %. Disease duration at l-dopa initiation was also detected in DTC and was not important to wearing-off development (2.0 %) (Fig. 2).
l-Dopa safe dosage
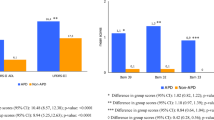
Two methods were applied to indicate cut-off value of l-dopa daily dosage to best predict development of wearing-off. In ROC curve, 281.25 mg/day (sensitivity 93.1 %, specificity 41.2 %, positive predictive value 34.6 %, negative predictive value 80 %, AUC 0.725) and 4.23 mg/kg (sensitivity 81.3 %, specificity 51.5 %, positive predictive value 51.6 %, negative predictive value 71.2 %, AUC 0.716) were detected with largest Youden index (Fig. 1). Negative predictive value 80 % (or 71.2 %) meant 80 % (or 71.2 %) patients would not develop wearing-off blow threshold dosage, and positive predictive value 34.6 % (or 51.6 %) meant 34.6 % (or 51.6 %) patients would develop wearing-off above threshold. The closer AUC was to 1, the better this model performed. Both AUC 0.725 and 0.716 (AUC > 0.7 and <0.9) indicated moderate predictive value.
In DTC model, 269 mg/day (negative predictive value 81.5 %, positive predictive value 65.6 %) and 3.2 mg/kg (negative predictive value 86.8 %, positive predictive value 62.4 %) were suggested cut-off value with most accurate classification of low-risk and high-risk subgroups to develop wearing-off (Fig. 2).
Discussion
In our cross-section study, we investigated 234 Chinese idiopathic PD patients. Wearing-off was reported in 111 (47.4 %) patients. When analyzing potential risk factors of wearing-off, multivariate and univariate analysis was consistent in three variables, i.e. duration of l-dopa treatment, l-dopa daily dosage adjusted to weight and l-dopa daily total dosage. However, UPDRS III showed statistical difference in univariate analysis, but failed to be risk factor in multivariate analysis. To evaluate its relation as well as other factors’ with wearing-off, DTC succeeded to find l-dopa daily total dosage, l-dopa dosage adjusted to weight, and duration of l-dopa treatment were important in wearing-off, while UPDRS III was not. It is worthy of mentioning that disease-related factors (duration at assessment and severity) were not risk factors in our study, yet their roles in wearing-off could not be ruled out as inevitable interaction among variables and different order of magnitude would affect the result. Still, we were able to conclude that patients with wearing-off tended to receive higher l-dopa dosage and endured longer l-dopa treatment duration.
Disease duration at l-dopa initiation, which represents timing of l-dopa initiation, did not show significant difference in univariate analysis. DTC also denied its relation to wearing-off by showing its low importance in wearing-off development. It was not included in multivariate logistic regression model, for disease duration at l-dopa initiation equaled disease duration at assessment minus duration of l-dopa treatment, and they were considered same variable in multivariate logistic regression analysis. Cilia etc. investigated Ghanaian patients with later l-dopa initiation compared to Italian patients with earlier l-dopa initiation; results showed delayed l-dopa initiation did not change the occurrence or timing of motor complication development [5]. These findings suggested disease duration at l-dopa initiation did not relate to wearing-off. On the other hand, duration of l-dopa treatment was risk factor and was somewhat important. And many other studies showed duration of l-dopa treatment was indeed related to wearing-off [3, 8–10]. This condition meant timing of l-dopa initiation itself was not a risk factor but l-dopa treatment duration had something to do with wearing-off. And it created a dilemma when it comes to whether l-dopa initiation should be delayed. A notion called “cumulative l-dopa dosage” could explain this situation by involving both l-dopa treatment duration and l-dopa dosage. In fact, “cumulative l-dopa dosage” was proven to be a risk factor [11]. In this view, although it seemed duration of l-dopa treatment was related to wearing-off, it is crucial that a possible relation did not mean an important contribution. For instance, when patients with low l-dopa dosage reached required cumulative l-dopa dosage, they tended to endure longer duration of l-dopa treatment. In another word, relation of l-dopa treatment duration with wearing-off could possibly be a byproduct. In fact, DTC model in our study showed that normalized importance of l-dopa total dosage, dosage adjusted to weight, and duration of l-dopa treatment were 100, 77.6 and 15 % respectively, which meant l-dopa daily dosage was 5–7 times more important than duration of l-dopa treatment. Combining those findings, it is reasonable to think l-dopa cumulative dosage and l-dopa daily dosage were more predictive of wearing-off development. And insufficient evidence was provided to suggest duration of l-dopa treatment actually contributed to wearing-off as no such study had ruled out the effect of l-dopa dosage. In addition, an open-labeled, randomized trial showed that patients who started on l-dopa versus levodopa-sparing therapies had very similar long-term outcomes in PDQ-39 mobility scores, dementia occurrence, and death rate [19]. Therefore, we concluded l-dopa initiation should not be delayed.
But to keep cumulative l-dopa dosage at a low level, it is crucial for us to keep l-dopa daily dosage low and to define a safe dosage to reduce motor complications. STRIDE-PD trial specifically measured the effect of four dosage levels of l-dopa, where 400 mg/day was considered as threshold merely by empirically presumed division [6]. Here we used analysis models (ROC curve and DTC model) to be more precise. Cut-off dosage as 281 mg/day was given by ROC with negative predictive value 80 % and positive predictive value 34.6 % (AUC = 0.725). It meant in patients receiving no more than 281 mg/day, 80 % of them spared wearing-off. Otherwise 34.6 % would be affected. As we applied DTC model, a similar result as 269 mg/day was given, below which 81.5 % did not develop wearing-off. These dosages were quite similar and in regarding to clinical relevance, we considered a dosage less than 275 mg/day to be threshold for clinicians to obtain. Theoretically, this dosage was sufficient to control symptom. In a mathematical model, de la Fuente-Fernandez postulated that single dose of 100 mg l-dopa tablet taken orally would provide sufficient dopamine even when the whole nigtostriatal system contained zero dopamine [20]. And in PD patient a certain level of dopamine remains. It could be hinted by “honeymoon period”, when low dosage of l-dopa could improve motor symptom dramatically. Dosage adjusted to weight is an important notion in pharmacokinetic, and generally speaking was more reasonable to apply, for body weight alone can be a risk factor and was highly associated with l-dopa concentration in plasma [6, 21]. ROC curve provided 4.2 mg/kg as cut-off value with negative predictive value 71.2 % and positive predictive value 51.6 % and DTC model indicated cut-off point as 3.2 mg/kg. We casted out 3.2 mg/kg to be threshold for such low dosage reduced wearing-off at a cost of symptom control. We therefore proposed 4.2 or 275 mg/day should be determined as safe dosage in clinical process.
Finding risk factors could help us to comprehend and discover underlying mechanism of wearing-off. l-dopa dosage and duration of l-dopa treatment were two factors related to wearing-off development in our study. And l-dopa dosage was the most important, which well fit current theory. Hypothetically, the dopamine concentration in synapse is constant, and oscillation in synapse might be responsible for wearing-off [22]. In those PD patients with wearing-off, dopamine in synapse was higher than control group after exogenous l-dopa administration, indicating presynaptic dysfunction (such as restore dysfunction and presynaptic neuron depletion) may have caused the oscillation [20, 21, 23]. Therefore, high l-dopa dosage would be more likely to lead to dramatic oscillation of dopamine concentration in the synapse.
We acknowledged some limitations in our study. Firstly, our retrospective study was hospital based, and did not contain very large sample. Secondly, as individualized therapy comes on mainstream nowadays, our study did not investigate COMT or MAO-B gene polymorphism when other studies showed susceptibility of these gene polymorphism to the development of wearing-off [24, 25]. Thirdly, although only a few patients were treated with DA or other agents in small dosage, l-dopa dosage should be calculated as LED (l-dopa equivalent dose) [11], which was not involved in our study. Fourthly, subgroups based on genes or onset age or phenotypes are expected to be investigated separately as they may undergo different mechanism and may require different safe dosage. Anyway, dosage determination in our study was simplified and need to be validated with larger sample and detailed investigation, which is also our next step.
In conclusion, we found that l-dopa dosage and l-dopa treatment duration were related to increased development of wearing-off. Cumulative l-dopa dosage and l-dopa daily dosage were better predictive of wearing-off development. Evidence for delayed l-dopa initiation was inadequate. l-dopa daily dosage no more than 275 mg or 4.2 mg/kg was regarded as safe dosage. The reason why our indicated dosage was lower than other studies (such as STRIDE-PD trial) was due to different study approach and different population assessed.
References
Zhang ZX et al (2005) Parkinson’s disease in China: prevalence in Beijing, Xian, and Shanghai. Lancet 365(9459):595–597
Poewe W et al (2010) Levodopa in the treatment of Parkinson’s disease: an old drug still going strong. Clin Interv Aging 5:229–238
Benbir G et al (2006) A hospital-based study: risk factors in development of motor complications in 555 Parkinson’s patients on levodopa therapy. Clin Neurol Neurosurg 108(8):726–732
Ahlskog JE, Muenter MD (2001) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 16(3):448–458
Cilia R et al (2014) The modern pre-levodopa era of Parkinson’s disease: insights into motor complications from sub-Saharan Africa. Brain 137(Pt 10):2731–2742
Warren Olanow C et al (2013) Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov Disord 28(8):1064–1071
Chen W et al (2014) Prevalence of wearing-off and dyskinesia among the patients with Parkinson’s disease on levodopa therapy: a multi-center registry survey in mainland China. Translational Neurodegeneration 3(1):26
Kum WF et al (2009) Risk factors in development of motor complications in Chinese patients with idiopathic Parkinson’s disease. J Clin Neurosci 16(8):1034–1037
Schrag A, Quinn N (2000) Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain 123(Pt 11):2297–2305
Juri-Claveria C et al (2007) Risk factors associated with the development of motor complications in Parkinson’s disease. A study in a Chilean population. Rev Neurol 45(2):77–80
Hauser RA, McDermott MP, Messing S (2006) Factors associated with the development of motor fluctuations and dyskinesias in Parkinson disease. Arch Neurol 63(12):1756–1760
Kurlan R (2005) “Levodopa phobia”: a new iatrogenic cause of disability in Parkinson disease. Neurology 64(5):923–924
Zhang ZX et al (2014) Chinese culture permeation in the treatment of Parkinson disease: a cross-sectional study in four regions of China. BMC Res Notes 7:65
Bellazzi R, Zupan B (2008) Predictive data mining in clinical medicine: current issues and guidelines. Int J Med Inform 77(2):81–97
Podgorelec V et al (2002) Decision trees: an overview and their use in medicine. J Med Syst 26(5):445–463
Hughes AJ et al (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184
Chan A et al (2011) A validation study of the Chinese wearing off questionnaire 9-symptom for Parkinson’s disease. Clin Neurol Neurosurg 113(7):538–540
Stamp LK et al (2012) Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum 64(8):2529–2536
Gray R et al (2014) Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet 384(9949):1196–1205
de la Fuente-Fernandez R et al (2004) Presynaptic mechanisms of motor fluctuations in Parkinson’s disease: a probabilistic model. Brain 127(Pt 4):888–899
Muller T et al (2000) Levodopa in plasma correlates with body weight of parkinsonian patients. Parkinsonism Relat Disord 6(3):171–173
de la Fuente-Fernandez R (2013) Imaging of dopamine in PD and implications for motor and neuropsychiatric manifestations of PD. Front Neurol 4:90
de la Fuente-Fernandez R et al (2001) Biochemical variations in the synaptic level of dopamine precede motor fluctuations in Parkinson’s disease: PET evidence of increased dopamine turnover. Ann Neurol 49(3):298–303
Wu H et al (2014) Catechol-O-methyltransferase Val158Met polymorphism: Modulation of wearing-off susceptibility in a Chinese cohort of Parkinson’s disease. Parkinsonism Relat Disord 20(10):1094–1096
Hao H et al (2014) Association of Catechol-O-Methyltransferase and monoamine oxidase B gene polymorphisms with motor complications in parkinson’s disease in a Chinese population. Parkinsonism Relat Disord 20(10):1041–1045
Acknowledgments
We appreciate Beijing Municipal Science and Technology Commission, Beijing Health System, Beijing Nature Science Foundation and Beijing Municipal Commission of Education for the financial support. This research was supported by a grant from Beijing Municipal Science and Technology Commission, China (Grant No. Z111107058811012), a grant from High Level Technical Personnel Training Project of Beijing Health System, China (Grant No. 2011-3-022), a grant from Beijing Nature Science Foundation and Beijing Municipal Commission of Education, China (Grant No. kz20120025028), and a grant from National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2013BAI09B03).
Conflict of interest
No conflict exists among authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, H., Fang, J., Li, F. et al. Risk factors and safe dosage of levodopa for wearing-off phenomenon in Chinese patients with Parkinson’s disease. Neurol Sci 36, 1217–1223 (2015). https://doi.org/10.1007/s10072-015-2078-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-015-2078-4