Abstract
Migraine patients have an increased risk to develop deep white matter hyperintensities (WMH) than the general population. Oxidative stress is believed to play a role in the pathogenesis of migraine. The present study was undertaken to assess oxidant/antioxidant balance of migraineurs with and without WMH. We hypothesized that increased oxidative stress and decreased antioxidant response may play a role in the pathophysiology of WMH in migraineurs. The study included 32 patients in the migraine group and 17 age- and sex-matched healthy subjects without headache in the control group. The migraine group comprised 18 with WMH and 14 without WMH. We evaluated oxidative status with malondialdehyde (MDA) and to determine the activities of antioxidant enzymes: superoxide dismutase, glutathione peroxidase and catalase (CAT) in serum of migraineurs and controls. Comparison of the patient and control groups for oxidative parameters revealed significantly lower level of CAT and higher level of MDA in the patient group. Two-way comparison for CAT and MDA of the migraine with and without WMH and the controls revealed that CAT serum level significantly decreased in migraine patients with WMH than migraine patients without WMH and controls. In this preliminary study, we demonstrated that the levels of CAT were decreased in migraine patients with WMH compared to patients without WMH and controls. These findings suggest that decreased antioxidant response may play a role in the pathophysiology of WMH in migraineurs. Besides, our results encourage the new treatment and follow-up options based on antioxidant systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is a common, chronic, complex, multifactorial brain disorder characterized by recurrent attacks of disabling headache; approximately one-third of patients also have neurological aura symptoms [1]. Migraine patients have an increased risk to develop white matter lesions than the general population [2]. Aradi et al. [3] demonstrated that white matter hyperintensities in migraineurs are characterized by tissue damage with axonal loss, glial hypocellularity, an enlarged extracellular space, and an increased extracellular water fraction. Several pathophysiological mechanisms have been proposed for white matter hyperintensities (WMH) [4–6].
Complex neurobiological changes occur during migraine headache. Currently, the overemphasized theory is of sterile neuroinflammation, which is considered to induce migraine attacks [7]. A number of pathophysiological changes occur in the course of this inflammatory process such as hypoxia due to the vasoconstriction during headache; cytokine-induced stress; oxidative and nitrosative stress caused by peroxynitrates formed during migraine; release of free radicals throughout arachidonic acid formation and psychological stress induced in consequence of oxidative stress [8].
The present study was therefore undertaken to assess oxidative status with malondialdehyde (MDA) as its major indicator, and to determine the activities of antioxidant enzymes: superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT) in serum of migraineurs with and without white matter hyperintensities and compared them with those of age- and sex-matched healthy controls. We hypothesized that increased oxidative stress and decreased total antioxidant defence response may play a role in the pathophysiology of white matter hyperintensities in migraineurs.
Materials and methods
Chemicals
All chemicals for biochemical analyses (MDA, SOD, CAT, GSH-Px) were purchased from Sigma Chemical Co. (St. Louis, MO, USA) and were of analytical grade or the highest grade available.
Experimental design
We recruited patients from the Outpatient Headache Unit of the Department of Neurology, Ankara Education and Research Hospital. Studies were performed in accordance with the approval of the Hospital Ethics Committee. Written informed consent was obtained from each patient. There were 32 patients [29 female (90.6 %), 3 male (9.4 %)] in the migraine group, with a mean age of 33.9 ± 9.5 years; and 17 sex-matched healthy subjects without headache in the control group, composed of 15 (88.2 %) females and 2 (11.8 %) females and their mean age 34.4 ± 8.4 years. The number of migraine patients with WMH was 18 and the mean age was 33.3 ± 9.9 years while the relevant numbers for the group without WMH were 14 and 34.7 ± 9.1 years.
The diagnosis of migraine was made by a neurologist according to International Headache Society Criteria [1]. The control and migraine groups consisted of non-smokers without neurological, chronic or infectious diseases, or iron deficiency. The patients were not taking prophylactic therapy for at least a month and acute attack treatment for a week before taking blood samples. All selected migraine patients underwent detailed clinical investigations to exclude the presence of any medical comorbidity that may itself cause WMH.
The imaging was achieved with a 1.5 T (General Electric Excite HD) magnetic resonance imaging (MRI) machine in a headache-free state. The scanning protocol included fluid attenuated inversion recovery (FLAIR) images, diffusion-weighted, T2-weighted and T1-weighted imaging scans. The slice thickness was 5 mm with a gap of 2 mm.
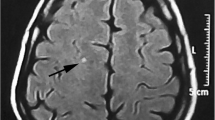
WMH were considered if visible as hyperintense on T2-weighted and FLAIR images, without hypointensity on T1-weighted scans, and were larger than 3 mm. None of the selected migraine patients had infratentorial signal abnormalities. According to brain MRI, migraineurs comprised 18 patients with WMH and 14 patients without WMH. None of the controls had an abnormal brain MRI.
Biochemical analysis
Fasting blood samples were obtained, and malondialdehyde (MDA) levels (nmol/ml) and superoxide dismutase (SOD) (U/ml), catalase (CAT) (IU/ml) and glutathione peroxidase (GSH-Px) (mIU/ml) activities were studied in serum samples.
Malondialdehyde level was measured by thiobarbituric acid-reactive substances (TBARS) method [9]. SOD activity was measured as described [10]. One unit of SOD activity was expressed as the enzyme protein amount causing 50 % inhibition in nitroblue tetrazolium reduction rate. CAT activity was determined by measuring the decrease of hydrogen peroxide (H2O2) absorbance at 240 nm as described [11]. GSH-Px activity was measured by following changes in NADPH absorbance at 340 nm as described [12]. In the activity calculations, extinction coefficients of H2O2 and NADPH were used for CAT and GSH-Px, respectively.
Statistical analysis
All statistical tests were performed by the IBM SPSS Software (version 20, SPSS, Inc., Chicago, IL, USA). Quantitative variables were expressed as mean ± standard deviation (SD) while qualitative variables were expressed as numbers and percentages.
Comparison of the patient and control groups was the unpaired t test, Pearson Chi-square analysis and Fisher’s exact Chi-square test. The standard distribution was checked with the Kolmogorov–Smirnov test. Because the variables did not follow the normal distribution, non-parametric tests were used in subsequent analysis. The Mann–Whitney U tests were utilized to detect any significant differences in each variable obtained from migraineurs and healthy controls. The three-way comparison of the migraine patients with WMH, migraine patients without WMH and control groups for oxidative and antioxidative parameters was with the one-way variance analysis (ANOVA) test and the two-way comparison with the LSD method.
Values of p < 0.05 were accepted as significantly different.
Results
There was no statistically significant difference between the patient and control groups for age and gender. There was also no statistically significant difference between the migraine groups with and without WMH for age and gender. Comparison of the patient and control groups for oxidative stress and antioxidative defence parameters revealed significantly lower level of CAT and higher level of MDA in the patient group (p = 0.025, p = 0.001, respectively). Table 1 presents the comparison of the patient and control groups for the oxidative stress parameter (MDA) and antioxidants (SOD, GSH-Px, CAT).
Comparison of the migraine with and without WMH groups and the controls for SOD, GSH-Px, CAT and MDA showed that the CAT and MDA were statistically significantly different (p = 0.002, p = 0.0001, respectively) (Table 2). Two-way comparison for CAT and MDA of the migraine with and without WMH and the controls revealed that CAT serum level significantly decreased in migraine patients with WMH than migraine patients without WMH and controls (p = 0.012, p = 0.001, respectively). There was no significant difference for MDA between the migraine with and without WMH groups (p = 0.912).
Discussion
Migraine patients have an increased risk to develop deep white matter lesions, subclinical posterior circulation territory infarcts, and infratentorial hyperintense lesions than the general population [2]. Furthermore, the risk of developing those white matter lesions is higher in migraine with aura, female cases, patients with higher headache frequency, longer disease duration and comorbid diseases such as thyroid gland dysfunction and hyperhomocysteinemia [2, 13, 14]. In addition, it appears that brain white matter lesions do not correlate with age [3]. Several pathophysiological mechanisms such as oligemia and focal hypoperfusion related to migraine attacks, immune-based white matter demyelination, mitochondrial dysfunction in neurons, glutamatergic excitotoxicity have been proposed for white matter hyperintensities [4, 6]. MDA is the end product of lipid peroxidation and widely used as a marker of oxidative stress.
Oxidative imbalance has been reported in migraine patients during headache attacks and headache-free period by the findings of various clinical studies [15–19]. Investigations using MDA and NO as the markers of oxidative stress and antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) glutathione reductase (GS-R) and arylesterase revealed at least in part controversial findings in migraine patients.
Tozzi-Ciancarelli et al. [19] found that high concentrations of thiobarbituric acid-reactive substances in the course of attack-free periods in migraine patients with aura. Gupta et al. [8] showed that serum level of MDA was significantly higher in migraine patients in the interictal phase than in controls. Moreover, Tuncel et al. [20] found that MDA levels significantly elevated in the migraine group and SOD activity was significantly higher in the migraine group with aura as compared to without aura. Partly in contrast, Yilmaz et al. [21] found significantly increased platelet MDA and nitrite/nitrate levels only during migraine attacks, but no significant alterations in the interictal phase of migraineurs. Also, a recent study in children with migraine showed significantly decreased MDA levels in migraineurs compared with healthy controls [22]. However, Erol et al. [23] showed that CAT and GSH-Px activities are lower in children and adolescent migraine patients.
In our study, as far as we are aware for the first time in the literature, we investigated the impact of oxidative stress on brain WMH in migraine patients. In agreement with the above-mentioned studies, we found that migraine patients had significantly higher plasma MDA levels which are the end product of lipid peroxidation and one of the markers of oxidative stress.
In addition, we found that migraine patients had significantly lower plasma CAT levels than healthy controls. According to the subgroup analysis, the plasma levels of MDA had increased in both migraine groups, whereas the plasma levels of CAT had only decreased in migraineurs with WMH. Also, Shukla et al. [24] found that migraineurs with a positive family history of migraine had a significantly lower activity of CAT compared with those with a negative family history. A recent study by Seneviratne et al. [25], about investigating clinical and radiological correlates of brain WMH in migraine, reported that family history of migraine significantly associated with brain WMH. When considered from this point of view the results of Shukla et al. seem in agreement with the results of our study.
It has been showed that CAT activity was to be a major enzymatic determinant of cellular resistance to H2O2 toxicity [26]. On the other hand, Karlidag et al. [27] reported that a low serum level of CAT before cardiopulmonary bypass surgery seems to be associated with postoperative delirium disturbance. These results suggested that a low CAT capacity leads the brain to suffer more damage from oxidative stress.
Conclusively, in this preliminary study, we had found increased oxidative stress in the migraine patients with WMH and without WMH and decreased CAT levels only in migraine patients with WMH. Determination of serum CAT concentrations may therefore have a predictive value for the development of WMH. On the other hand, our results encourage the new treatment and follow-up options based on antioxidant defense systems. Further work is required to elucidate the mechanisms underlying these observations.
References
Headache Classification Subcommittee of the International Headache Society (2004) The International Classification of Headache Disorders. Cephalalgia 24(Suppl 1):9–160
Kruit MC, van Buchem MA, Hofman PA, Bakkers JT, Terwindt GM, Ferrari MD et al (2004) Migraine as a risk factor for subclinical brain lesions. JAMA 291(4):427–434
Aradi M, Schwarcz A, Perlaki G, Orsi G, Kovacs N, Trauninger A et al (2013) Quantitative MRI studies of chronic brain white matter hyperintensities in migraine patients. Headache 53(5):752–763
Longoni M, Ferrarese C (2006) Inflammation and excitotoxicity: role in migraine pathogenesis. Neurol Sci 27(Suppl 2):S107–S110
Sparaco M, Feleppa M, Lipton RB, Rapoport AM, Bigal ME (2006) Mitochondrial dysfunction and migraine: evidence and hypotheses. Cephalalgia 26(4):361–372
Tajti J, Pardutz A, Vamos E, Tuka B, Kuris A, Bohar Z et al (2011) Migraine is a neuronal disease. J Neural Transm 118(4):511–524
Goadsby PJ, Charbit AR, Andreou AP, Akerman S, Holland PR (2009) Neurobiology of migraine. Neuroscience 161(2):327–341
Gupta R, Bhatia MS, Banerjee B (2010) Oxidative stress in migraine: mechanisms and response to treatment. Indian J Priv Psychiatry 4(2):30–34
Dahle LK, Hill EG, Holman RT (1962) The thiobarbituric acid reaction and the autoxidations of polyunsaturated fatty acid methyl esters. Arch Biochem Biophys 98:53–261
Durak I, Canbolat O, Kavutcu M, Ozturk HS, Yurtarslani Z (1996) Activities of total, cytoplasmic, and mitochondrial superoxide dismutase enzymes in sera and pleural fluids from patients with lung cancer. J Clin Lab Anal 10(1):17–20
Aebi H (1974) Catalase. In: Bergmeyer HU (ed) A methods of enzymatic analysis. Academic Press, New York, pp 673–677
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Kruit MC, van Buchem MA, Launer LJ, Terwindt GM, Ferrari MD (2010) Migraine is associated with an increased risk of deep white matter lesions, subclinical posterior circulation infarcts and brain iron accumulation: the population-based MRI CAMERA study. Cephalalgia 30(2):129–136
Swartz RH, Kern RZ (2004) Migraine is associated with magnetic resonance imaging white matter abnormalities: a meta-analysis. Arch Neurol 61(9):1366–1368
Bolayir E, Celik K, Kugu N, Yilmaz A, Topaktas S, Bakir S (2004) Intraerythrocyte antioxidant enzyme activities in migraine and tension-type headaches. J Chin Med Assoc 67(6):263–267
Ciancarelli I, Tozzi-Ciancarelli MG, Di Massimo C, Marini C, Carolei A (2003) Urinary nitric oxide metabolites and lipid peroxidation by-products in migraine. Cephalalgia 23(1):39–42
Gupta R, Pathak R, Bhatia MS, Banerjee BD (2009) Comparison of oxidative stress among migraineurs, tension-type headache subjects, and a control group. Ann Indian Acad Neurol 12(3):167–172
Shimomura T, Murakami F, Kotani K, Ikawa S, Kono S (1999) Platelet nitric oxide metabolites in migraine. Cephalalgia 19(4):218–222
Tozzi-Ciancarelli MG, De Matteis G, Di Massimo C, Marini C, Ciancarelli I, Carolei A (1997) Oxidative stress and platelet responsiveness in migraine. Cephalalgia 17(5):580–584
Tuncel D, Tolun FI, Gokce M, Imrek S, Ekerbicer H (2008) Oxidative stress in migraine with and without aura. Biol Trace Elem Res 126(1–3):92–97
Yilmaz G, Surer H, Inan LE, Coskun O, Yucel D (2007) Increased nitrosative and oxidative stress in platelets of migraine patients. Tohoku J Exp Med 211(1):23–30
Bockowski L, Sobaniec W, Kulak W, Smigielska-Kuzia J (2008) Serum and intraerythrocyte antioxidant enzymes and lipid peroxides in children with migraine. Pharmacol Rep 60(4):542–548
Erol I, Alehan F, Aldemir D, Ogus E (2010) Increased vulnerability to oxidative stress in pediatric migraine patients. Pediatr Neurol 43(1):21–24
Shukla R, Barthwal MK, Srivastava N, Sharma P, Raghavan SA, Nag D et al (2004) Neutrophil-free radical generation and enzymatic antioxidants in migraine patients. Cephalalgia 24(1):37–43
Seneviratne U, Chong W, Billimoria PH (2013) Brain white matter hyperintensities in migraine: clinical and radiological correlates. Clin Neurol Neurosurg 115(7):1040–1043
Spitz DR, Adams DT, Sherman CM, Roberts RJ (1992) Mechanisms of cellular resistance to hydrogen peroxide, hyperoxia, and 4-hydroxy-2-nonenal toxicity: the significance of increased catalase activity in H2O2-resistant fibroblasts. Arch Biochem Biophys 292(1):221–227
Karlidag R, Unal S, Sezer OH, Bay Karabulut A, Battaloglu B, But A et al (2006) The role of oxidative stress in postoperative delirium. Gen Hosp Psychiatry 28(5):418–423
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aytaç, B., Coşkun, Ö., Alioğlu, B. et al. Decreased antioxidant status in migraine patients with brain white matter hyperintensities. Neurol Sci 35, 1925–1929 (2014). https://doi.org/10.1007/s10072-014-1864-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-1864-8