Abstract
The marine environment is filled with biotic and abiotic sounds. Some of these sounds predict important events that influence fitness while others are unimportant. Individuals can learn specific sound cues and ‘soundscapes’ and use them for vital activities such as foraging, predator avoidance, communication and orientation. Most research with sounds in elasmobranchs has focused on hearing thresholds and attractiveness to sound sources, but very little is known about their abilities to learn about sounds, especially in benthic species. Here we investigated if juvenile Port Jackson sharks could learn to associate a musical stimulus with a food reward, discriminate between two distinct musical stimuli, and whether individual personality traits were linked to cognitive performance. Five out of eight sharks were successfully conditioned to associate a jazz song with a food reward delivered in a specific corner of the tank. We observed repeatable individual differences in activity and boldness in all eight sharks, but these personality traits were not linked to the learning performance assays we examined. These sharks were later trained in a discrimination task, where they had to distinguish between the same jazz and a novel classical music song, and swim to opposite corners of the tank according to the stimulus played. The sharks’ performance to the jazz stimulus declined to chance levels in the discrimination task. Interestingly, some sharks developed a strong side bias to the right, which in some cases was not the correct side for the jazz stimulus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sound is a reliable cue in aquatic environments. It is highly directional and propagates over large distances with little attenuation or impact from currents (Slabbekoorn et al. 2010). Therefore, it is unsurprising that many aquatic species use acoustic cues for communication and orientation (reviewed in Tyack 1998; Ladich 2015).
Marine mammals are renowned for the use of sound as their primary method of communication, as well as a method to obtain information about the environment, for example, using echolocation or the surf and ice noise to locate the shoreline (reviewed in Richardson et al. 2013). There are also widespread examples of acoustic communication in many fish species across different behavioural contexts, namely during courtship, spawning, agonistic interactions or distressful situations (e.g. Crawford et al. 1986; Myrberg Jr et al. 1986; Ladich and Myrberg 2006; Ladich 2015). In addition, fish can use ambient ‘soundscapes’ as a means of orientation and navigation. Research in coral reef species demonstrated the importance of reef noise as a cue for larvae settling and navigation in juveniles (Simpson et al. 2005; Radford et al. 2011; Huijbers et al. 2012). Interestingly, damselfish Pomacentrus sp. larvae responses to acoustic cues are flexible and cue-dependent (Simpson et al. 2010). Settlement-stage larvae that had experienced either natural reef noise or an artificial tone for some hours moved towards a reef noise chamber in a choice experiment; however, when tested with the tone, the reef noise group responded adversely and moved away from the tone chamber, while the artificial tone group moved towards the tone chamber (Simpson et al. 2010). These results suggest that fish larvae can discriminate different acoustic stimuli, and that recent acoustic experiences influence their behavioural plasticity in the selection of settlement sites.
In the wild, sound is likely associated with important biological events, such as prey and predators, and there are considerable fitness benefits in learning about these sounds (Mann et al. 1997; Tyack 1998; Remage-Healey et al. 2006; Wilson et al. 2008). For example, bottlenose dolphins Tursiops truncatus behaviourally orient toward vocalizations of Gulf toadfish Opsanus beta (Gannon et al. 2005). In turn, the toadfish dramatically reduce their vocalizations and have increased plasma cortisol levels in the presence of low-frequency dolphin sounds, suggesting potential coevolution of dolphins and their prey in a ‘soundscape’ context (Gannon et al. 2005; Remage-Healey et al. 2006). Teleost fish can also learn to associate and discriminate sounds in an artificial setting. Acoustic conditioning for guidance or ranching purposes in the context of fish aquaculture or fisheries has been extensively studied in several freshwater and marine species (Zion et al. 2010; Zion and Barki 2012). In a laboratory experiment, carp Cyprinus carpio were taught to associate a plain tone with a food reward, then to discriminate between the plain tone and a complex musical stimulus, and even to discriminate between two musical stimuli of different genres (Chase 2001). Although music is an artificial auditory stimulus, several experiments have shown similar music perception and categorization between humans and non-human species, including birds, mammals and fish (D’Amato and Salmon 1984; Porter and Neuringer 1984; Hulse et al. 1992; Watanabe and Sato 1999; Chase 2001).
While elasmobranchs are not known to make sounds, they have an inner ear and a lateral line system and their hearing ability has been investigated to some extent (Myrberg 2001; Gardiner et al. 2012; Hart and Collin 2015). Sharks seem to be most sensitive to frequencies below 100 Hz and able to hear sounds up to around 1000 Hz, but so far only a relatively small number of species has been investigated (Gardiner et al. 2012). A few classic field experiments tested whether acoustic signals acted as attractive stimuli to sharks, and pulsed, low-frequency sounds drew large coastal sharks to the speaker’s location (e.g. Myrberg Jr et al. 1972; Nelson and Johnson 1972). Most studies on elasmobranch hearing have focused on frequency range and threshold detection level (i.e. sensitivity) using classical or operant conditioning (e.g. Nelson 1967; Kelly and Nelson 1975), and more recently, auditory-evoked potential techniques (Casper and Mann 2006, 2007, 2009). In the first audiogram obtained of a shark, Kritzler and Wood (1961) conditioned a bull shark Carcharhinus leucas to approach an underwater loudspeaker to obtain a food reward. A similar procedure was used by Nelson (1967) with lemon sharks Negaprion brevirostris, including one individual that was trained in an approach–avoidance discrimination task, in which the shark had to approach the speaker following the presentation of a certain frequency, but avoid it following another frequency (by means of an electric shock). A reliable approach/avoidance response was obtained after 33 shock trials and 50 food trials (Nelson 1967). Similar to teleost fish, elasmobranch hearing abilities have likely been shaped by the biotic and abiotic ambient noise in their environment, and many aspects of their behavioural ecology suggest potential to the use of sounds as reliable signals in the environment, namely in foraging and navigation contexts (Gardiner et al. 2012). In fact, a playback experiment with young lemon sharks Negaprion brevirostris suggests that natural sounds of fish species, including the sounds of distressed prey or healthy prey fleeing after an encounter, induce investigatory behaviours and biting (Banner 1972). In recent years, a growing body of studies have investigated the cognitive abilities of elasmobranchs in greater depth (reviewed in Schluessel 2015), yet the majority used visual stimuli, and a substantial gap remains regarding our knowledge of sharks’ behavioural flexibility to sounds.
This study investigated whether Port Jackson sharks Heterodontus portusjacksoni could learn to associate artificial sound stimuli with a food reward. The first experiment was a food conditioning task with a single artificial sound stimulus, and the second experiment was a dual stimulus discrimination task, retaining the previous sound as one of the stimuli. The Port Jackson shark (PJ) is a benthic, nocturnal species endemic to the southern half of Australia (Last and Stevens 2009). In the east coast of NSW, PJs show a seasonal, long-distance migration from their breeding reef grounds to potential foraging areas (Powter and Gladstone 2009; Bass et al. 2016). Interestingly, these sharks have bisexual philopatry and very high site fidelity during the breeding season, with sporadic displacements between reefs (Bass et al. 2016). We currently know very little about the cognitive abilities of Port Jackson sharks. A single experiment in a laboratory context has shown that PJs can be conditioned to a bubble stream and a LED light with a food reward, and that they are able to remember the association for at least 24 h, and possibly up to 40 days (Guttridge and Brown 2014). Port Jackson sharks are colour blind (Hart et al. 2011), but have very high sensitivity to contrast and light, likely an adaptation to their benthic and nocturnal lifestyle and lower visibility in temperate waters (McFarland 1990; Ryan et al. 2016). The species’ hearing threshold has not been investigated yet, but data from the horn shark Heterodontus francisci, a sister species, suggest they are most sensitive to lower frequencies up to 300 Hz, and that lateral line stimulation is also involved below approximately 100 Hz (Kelly and Nelson 1975; Casper and Mann 2007; Hart and Collin 2015). It seems reasonable that Port Jackson sharks might use sound cues in addition to other senses (e.g. olfaction, lateral line, electromagnetic reception and vision; Gardiner et al. 2012) to navigate between reef areas and locate prey, especially during the night. Port Jackson sharks are also known to have distinct personality traits, similar to many fish species (Budaev and Brown 2011). Byrnes and Brown (2016) found highly repeatable individual differences in boldness and stress reactivity in juvenile PJs, and a correlation between the two personality traits, indicative of a proactive–reactive coping style. Wild adults also showed consistent individual differences in docility scores (Byrnes et al. 2016). Animal personality is likely an important source of behavioural variation that may affect cognitive performance (Carere and Locurto 2011; Sih and Del Giudice 2012; White et al. 2016). For example, shy and less active individuals are generally reactive, less impulsive and sample more information from the environment, and seem to be linked to lower learning ability in associative tasks, but better performance in reversal learning tasks (Dugatkin and Alfieri 2003; Sih and Del Giudice 2012; Trompf and Brown 2014). The study of individual personality traits in elasmobranchs and its ecological and cognitive relevance is in its infancy (Finger et al. 2017), but can be valuable to understand the evolution of personality and of cognitive abilities due to elasmobranchs’ basal position in the vertebrate tree.
In this study, we hypothesized that sharks could learn to associate a sound cue with a reward repeatedly presented in a specific location, and thus would approach the reward zone more quickly and retrieve the reward more often over time. If the sharks were successful in the single stimulus task, they were then exposed to a dual stimulus discrimination task, which retained the previous artificial sound as one of the stimuli. We hypothesized one of three scenarios could occur: (1) the sharks ignore the new stimulus all together, or (2) generalize that sound equals reward, and always choose the reward zone from the previous task; or (3) the sharks learn to respond correctly to each stimulus. We also expected that bolder and more active individuals would be faster in retrieving rewards and in achieving learning criterion in the approach conditioning task, but shyer sharks would perform better in the discrimination task.
Materials and methods
Subjects
Eight juvenile Port Jackson sharks (4 females, 4 males; Table 1), ranging between 35 and 42 cm in total length and hatched in captivity (eggs collected from Jervis Bay, NSW Australia), were used in the study. Sharks were housed at the Sydney Institute of Marine Science (SIMS), Australia, in three 1000-L seawater tanks at ambient temperature for 10 months prior to the experiment. Tanks had continuous circulation of fresh seawater, aeration, a thin layer of sand in the bottom and PVC structures and fake kelp to provide shelter and enrichment. Seawater was pumped directly from Sydney harbour at ambient temperature. Prior to the experiment, sharks were fed small pieces of squid, fish and prawns ad libitum 3 days per week. The experimental tank was adjacent to the housing tanks, and the room had a natural light/dark cycle.
Egg collection occurred under NSW Fisheries permit P08/0010-4.2. This work was approved by the Macquarie University Animal Ethics Committee under ARA 2014-003. At the end of the experiment, all sharks were released at their original site of capture.
Apparatus and stimuli
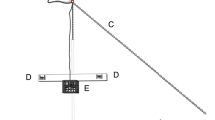
The testing arena (180 × 50 × 50 cm) was placed within a 10-foot circular tank filled with 30 cm of seawater. The arena was divided into a starting compartment (SC; 30 × 50 × 50 cm) and the experimental arena by a sliding Perspex door (Fig. 1). The walls of the SC were blacked out, and a black Perspex door and lid were used to close the SC during acclimation and inter-trial intervals. On the wall opposite to the SC, a small black divider (37.5 cm long) separated a left from a right choice zone. The experimental arena was split into five distinct zones for data analysis (0–4, indicating distance from correct choice zone; Fig. 1). White curtains visually isolated the main circular tank in the room, but there were no special provisions for visual or acoustic isolation in the testing arena. Auditory stimuli comprised two 20-s music clips from Oscar Peterson’s Bossa Beguine and Philip Glass’s Metamorphosis One. The stimuli were chosen based on peak frequency range and tempo (Fig. S1) and the known hearing range of heterodontid sharks (Kelly and Nelson 1975; Gardiner et al. 2012). We chose not to filter low frequencies in the stimuli (lateral line stimulation) since we were not interested in a specific sensory system used in the learning process. Indeed, in a natural setting animals generally use multiple senses simultaneously to gain information and learn about the environment (Shettleworth 2010). Auditory stimuli were fed to an air speaker facing down in a waterproof container partially submerged in the middle of the back wall and on top of the divider by a laptop running a custom Matlab (The MathWorks®, 2004) program using Psychtoolbox-3 (Brainard 1997). Sound was broadcast at 160 dB re 1 µPa. All experimental sessions described below were conducted individually for each shark, once a day in consecutive days during daylight hours and always at the same time. We changed 2/3 of the water in the circular tank between every individual session, and mixed the water in the arena between trials. In the experimental sessions, sharks were fed daily on squid (their preferred food) during the trials using aquarium tongs. Sessions were video recorded with a webcam mounted above the arena.
Experimental set up, showing the starting compartment (start box), the five zones of the experimental arena (0–4, indicating distance to the correct choice zone of experiment 1), positioning of the speaker in the tank and location of the food reward. Note that the correct choice zone was randomly assigned to be on the left- or right-side of the tank for different sharks, thus for some individuals the position of zone 0 and zone 1 is the opposite of this scheme
Experimental procedure
Swimming activity
Swimming activity levels were assessed 2 months before the learning experiment. Sharks were moved from the housing tank to an experimental arena and tested individually. The experimental arena (90 × 50 × 30 cm) was placed within a 10-foot circular tank filled with 30 cm of seawater. The arena was considered to have three equal zones (each 30-cm wide), and we measured the number of times sharks crossed between zones (head and pectoral fins over the demarcation line) over 5 consecutive days. Subjects were given 15 min to acclimate before each trial began, and trials lasted 60 min.
Open-field emergence test and pre-training
Before starting the learning experiments, sharks had to get used to being moved to the experimental tank and fed from the aquarium tongs. We designed the pre-training sessions as open-field emergence trials in the SC to test for boldness (similar to Byrnes and Brown 2016), followed by acclimation and training to feed from aquarium tongs.
At the start of the pre-training session, the shark was placed in the SC (in blackout) for 2 min and allowed to acclimate. Then a sliding door was lifted 20 cm above the floor, the individual was left undisturbed and time until emergence (boldness score) was recorded.
Once the emergence trial was over, the shark could swim freely for 10 min in the experimental tank to settle. After that, we allowed them to retrieve eight free rewards from the aquarium tongs at random time intervals and in random locations in the tank (excluding SC). If the shark did not approach the tongs within 8 min, the reward was removed.
The sharks were deemed ready to start the experiment when they retrieved all eight rewards in less than 60 s each and did not show avoidance behaviour towards the aquarium tongs.
Experiment 1: food approach conditioning
In this experiment, sharks had to learn to associate a jazz sound stimulus with a food reward in a specific location. One group (n = 4) was randomly assigned to associate the stimulus with the left side of the choice zone, and another (n = 4) with the right side of the choice zone (Table 1).
The first three sessions consisted of six trials with a correction procedure, i.e. each trial was repeated up to three times if the shark missed the reward (thus sessions could have up to 24 trials), to maximize exposure to training contingencies in the initial days. Sessions 4 onwards comprised ten regular trials, without the correction procedure.
In all sessions, sharks were given 2 min of acclimation in the SC before starting the trials. The general structure of each training trial was as follows. Once the SC lid was removed and sliding door was opened, the shark was given 30 s to emerge. If 30 s elapsed, the shark was gently ushered into the experimental arena and the SC was closed. The sound stimulus was presented after a random delay (20–40 s), and a food reward was introduced in the choice zone (left or right side) 5 s after stimulus offset. Sharks were given 180 s to consume the reward or the trial was terminated. The shark was then ushered back to the SC, the door and lid were closed, and an inter-trial interval of 30 s preceded the next trial.
For each trial, we recorded the latency to enter the correct choice zone and the latency to eat the reward. We also recorded the position of the shark in the tank 5 s prior to stimulus onset, 5 s after stimulus onset and at reward onset, since we expected that sharks would be conditioned to the sound stimulus if they showed anticipatory behaviour induced by the stimulus, namely changing position in the tank and moving towards the correct choice zone (Guttridge and Brown 2014). The sharks were considered to have entered an area if their head and pectoral fins were over the demarcation line.
To test if the sharks were conditioned to the sound, we ran a probe trial on day 6 and 11 (after five and ten training sessions), and then every second session until reaching criterion. The probe trial was unrewarded, and differed from training trials in that the stimulus was presented at least 40 s after opening of the SC (maximum delay in training trials) and when the shark was resting in the zone furthest away from the choice zone. We recorded the position of the shark at 5 s after stimulus onset and at 5 and 10 s following stimulus offset (corresponding to reward onset and 5 s within reward in training trials). Sharks were considered to have learnt the association between stimulus and reward if they showed directed swimming towards zone 0 induced by the sound stimulus and were in zone 0 at 10 s following stimulus offset in two consecutive probe trials.
Experiment 2: discrimination task
After successful training with a single sound stimulus, sharks were moved to a discrimination task. In this experiment, sharks were presented with either the same jazz stimulus as in experiment 1, where the correct choice zone was also the same as in the previous task (e.g. left side), or a new classical music stimulus, where the correct response was to enter the opposite choice zone (e.g. right side; Fig. S1).
The first three sessions consisted of eight trials with a correction procedure (thus sessions could have up to 32 trials) and session 4 onwards comprised ten regular trials, without correction procedure, all with 2 min of acclimation in the SC before starting the trials. In half of the trials, the stimulus was the jazz music clip, and in the other half the classical music clip. Trials were pseudo-randomized in blocks of two to prevent more than two consecutive trials of the same stimulus.
The general structure of each trial was slightly different to the previous task. The trial began with the removal of the lid and black barrier of the SC, but a transparent barrier kept the shark inside the SC. The stimulus was presented after a random delay (20–40 s) and the transparent barrier was removed at stimulus offset, allowing the shark to make a response by swimming to the choice zone. If the shark made a correct choice, a food reward was introduced in the choice zone and the shark was given 60 s to consume it. After eating the reward, or if the shark entered the wrong choice zone, it was gently ushered back to the SC, both doors and lid were closed and an ITI of 30 s preceded the next trial.
For each trial, we recorded the latency to make a response, if the choice was correct or not and the latency to eat the reward in correct response trials. The learning criterion was set to 80% correct choice in both stimuli in two consecutive sessions.
Data analysis
All statistical analyses were conducted in R v. 3.1.3 (R Core Team 2016). Where applicable, model residuals were inspected and assumptions were met in all cases.
Swimming activity
We tested for agreement repeatability (R) of swimming activity over the five trials using a generalized linear mixed-effects model fitted by PQL (penalized-quasi likelihood) estimation for count data, with individual shark ID as random effect (package rptR, Schielzeth and Nakagawa 2013). We used PQL estimation since it is recommended that repeatability with count data (here number of area crossings) be estimated using multiplicative GLM models (Nakagawa and Schielzeth 2010).
Open-field emergence test
Emergence times for the open-field assay were ln transformed for normality due to heteroscedasticity. Agreement repeatability (R) of emergence times was estimated using a linear mixed-effects model (LMM) with REML estimation, with individual shark ID as random effect (packages lme4 and rptR, Schielzeth and Nakagawa 2013; Bates et al. 2015). An information-theoretic approach was used to build candidate models to examine for other factors that influenced emergence time. Potential fixed effects included in candidate models were total length, sex, housing tank and session number. Models were ranked based on corrected Akaike information criteria (AICc), and differences in AICc and in Akaike model weight were used to choose the best-fit model. The coefficient of determination (R2) was used to assess goodness-of-fit and estimate the amount of variance explained by the model following Nakagawa and Schielzeth (2013), using the package piecewiseSEM (Lefcheck 2016). Inclusion of the random effect in the model was tested by comparing the best-fit model with a null model using parametric bootstrap analysis utilizing exact likelihood ratio tests (5000 simulations; package RLRsim, Scheipl et al. 2008; Bolker et al. 2009). Adjusted repeatability (RA) was then estimated using the best-fit model. Confidence intervals (CI), standard errors (SE) and P values for both R and RA were calculated from parametric bootstrapping of likelihood ratios (1000 simulations; Nakagawa and Schielzeth 2010).
Experiment 1: food approach conditioning
Lack of motivation was apparent in some trials throughout the single stimulus task. Port Jackson sharks are a benthic species, and in most trials sharks would typically show directed swimming in the bottom of the tank towards the choice zones; however, on occasion we observed an odd vertical swimming at the water surface in a circular pattern, without approaching the choice zone. These null trials were excluded from the analysis (ranging from 5.2 to 25.9% of trials per individual shark; Fig. S2). A Mann–Whitney U test was used to test if the overall proportion of null trials of the sharks that did not learn the task was different from those who learnt.
The information-theoretic approach described for the open-field emergence test was also used to examine the latency to enter the choice zone and latency to eat the reward in the Food approach conditioning task. The average emergence time per individual was taken as a boldness score and used as a fixed effect. We first explored the optimal structure of the random components (comparing random intercept models with random intercept and slope models) before testing the fixed effects (Zuur et al. 2009). Wald tests were used to test the significance of fixed effects (Bolker et al. 2009; Zuur et al. 2009).
To test for anticipatory behaviour induced by the sound stimulus, we assigned a binary response for the presence/absence of each shark in zone 0 and used generalized linear mixed-effects models (GLMM) to compare the presence in zone 0 in different time periods of the trial: (1) 5 s prior to stimulus onset and 5 s during stimulus onset; and (2) 5 s prior to stimulus onset and reward onset.
Experiment 2: discrimination task
The latency to make a choice and eat the reward, the percentage of correct choices and the percentage of left and right choices were recorded for each individual. The learning criterion consisted of eight correct responses out of ten consecutive trials for each of the stimuli presented (Binomial test: P = 0.044). Individual results are provided to show intraspecific variation. Discrete Time Markov Chain (DTMC) transition probability matrices between trials (t − 1) and t were estimated for each individual shark to investigate if choice/stimulus and outcome in one trial would influence choice on the following trial (package markovchain, Spedicato et al. 2016). We computed transition matrices for sessions 1–5 (initial days of the task) and for the overall task to explore rule formation over time. Confidence intervals of individual transition matrices should be considered cautiously due to low raw counts of transition steps.
Results
Swimming activity
Swimming activity was highly variable between individuals, ranging between 0.02 and 23.4 zone changes per minute (median = 3.38). The frequency of changes between zones was repeatable across sessions within individuals: sharks that were more active in the first session were generally more active in subsequent sessions (R = 0.690 (0.088), 95% CI 0.496–0.840, P = 0.001).
Open-field emergence test and pre-training
Four sharks ran three open-field emergence trials and pre-training sessions, and the remaining four sharks ran four open-field emergence trials and pre-training sessions (Table 1). On the last day of pre-training, all sharks approached and aggressively bit the tongs.
Emergence time ranged from 1.54 to 180.23 s, with a median of 12.56 s. The best-fit model had only sex as fixed effect: males were shyer (took longer to emerge) than females. Sex accounted for 54.2% of the variance in emergence times and shark ID for 6.8% of the variance. Inclusion of shark ID did not significantly improves the model (LRT = 0, P = 0.267), but we chose to keep it since personality traits are inherently individually based. Boldness was repeatable across trials within individuals [R = 0.626 (0.196), 95% CI 0.092–0.847, P = 0.001]: sharks that were faster to emerge in the first trial were generally faster to emerge in subsequent trials (Fig. 2). However, no repeatability was found when sex was included as fixed effect [RA = 0.157 (0.170), 95% CI 0–0.542, P = 1].
Experiment 1: food approach conditioning
Latency to enter choice zone and eat reward
Sharks entered the choice zone on 86.5% of the trials, and consumed the reward 78.3% of the times. The model that best explained changes in latency to enter the choice zone had only session number as significant fixed effect (latency decreased over sessions; F = 19.243, df = 1, P < 0.001; Fig. 3a). The inclusion of shark ID as random intercept increased the strength of the model (LRT = 9.814, P < 0.001), and from observation of individual regressions and AICc scores we also included a random slope in the final model. Session accounted for 25.1% of the variance in latency to enter the choice zone and random effects for 20.4% of the variance. The model that better explained changes in latency to eat reward had both session and sex as fixed effects (latency decreased over sessions, and females were slower to eat the rewards compared to males; session: F = 29.984, df = 1, P = 0.0014; sex: χ2 = 7.380, df = 1, P = 0.007; Fig. 3b). The inclusion of shark ID as random intercept increased the strength of the model (LRT = 1.100, P = 0.04), and we also included a random slope in the final model. The fixed effects accounted for 41.5% of the variance in latency to eat the reward and random effects for 11.7% of the variance.
Anticipatory behaviour
The best-fit GLMM models had trial time period (5 s before the song, 5 s during the song and reward onset) and session as fixed effects and individual shark ID as random effect. Sharks were observed significantly more times in zone 0 at 5 s during the song (Fig. 4a) or at reward onset (Fig. 4b) compared to 5 s before the song, and the presence in zone 0 increased over sessions, suggesting the sharks formed an association between the song and food delivered in a specific location (before song/during song: timestamp, χ2 = 31.406, df = 1, P < 0.001; session, z = 4.614, df = 1, P < 0.001; before song/reward onset: timestamp, χ2 = 49.724, df = 1, P < 0.001; session, z = 5.532, df = 1, P < 0.001).
Probe trials
Five out of eight sharks reached learning criterion after an average of 13.6 training sessions, with small individual variation (median 12; range 12–18; Table 1). The remaining three sharks did not pass a single probe trial after 16 training sessions, and were excluded due to time constraints. Interestingly, all three showed quick, steady latencies to enter the correct choice zone, with an average of 26.19 ± 15.01 s (median 20.99 s) over the last two probe trials, but did it irrespective of the sound stimulus. In addition, we found that the three sharks that failed the probe trials were as motivated during training, showing a low proportion of null trials similar to the group of sharks that learnt the task (W = 9, P = 0.764).
Experiment 2: discrimination task
Sharks were presented with the same jazz stimulus and a novel classical music stimulus. Task participation was high, with sharks showing a response in 98.8% of the trials on average (individual participation from 96.6 to 99.7%). Sharks were also fast in their response (median ± IQR: 12.44 ± 7.41 s), and in retrieving the reward if choice was correct (median ± IQR: 5.69 ± 2.93 s).
Learning curves are shown in Fig. 5 for each shark individually (a–e), and DTMC transition probabilities are depicted in Fig. 6. The sharks’ performance to the previously learned jazz stimulus was low in the initial sessions of this task. After a mean of 31.4 sessions (median 33; range 26–34; Table 1), none of the five sharks learned to discriminate the jazz and the classical music stimulus.
Performance of individual sharks (a–e) over training sessions during experiment 2. The proportion of correct choices to the Jazz stimulus is given by the grey circles and dotted line, and for the classical music stimulus by the black circles and full line (each session comprised five trials per stimulus). Left panel: sharks had to enter the right-side choice zone with Jazz; right panel: sharks had to enter the right-side choice zone with classical music. Note that none of the five sharks reached learning criterion
DTMC transition probability of choosing the right-side zone in trial t over a sessions 1–5 and b all sessions of experiment 2, depending on choice and outcome of trial (t − 1): shark chose the left-side and was wrong (white bars); chose the left-side and was correct (white dashed bars); chose the right-side and was wrong (grey bars); or chose the right-side and was correct (grey dashed bars). Dashed horizontal lines mark chance levels. Note that S359 and S415 were originally trained to the left-side zone with the Jazz stimulus. Only one shark (S363) chose a zone randomly during sessions 1–5 (a) and another (S388) over all sessions, with all others showing an overall bias to the right-side zone
Choice/outcome DTMC transition probabilities during the initial sessions suggest only one shark was choosing a zone randomly (S363; Fig. 6a), with all others showing an overall bias to the right-side zone—even those successfully trained to the left-side with the jazz stimulus in experiment 1 (S359, S415, Fig. 5b, d). Individual variation in choice strategy was also seen: S359 had a right-side bias but went left on half the trials following no reward on the left-side, and S375 and S415 predominantly choose the left-side after being rewarded on the right (Fig. 6a). Choice/outcome DTMC matrices over all experimental sessions suggest that S388’s choice was random, while the remaining four sharks were predominantly biased to choose the right-side zone regardless of the stimulus and outcome of the previous choice (Fig. 6b).
Discussion
Our results show that juvenile Port Jackson sharks learnt to associate an artificial sound with a food reward. However, not all sharks were able to learn the association. None were successful when required to discriminate between two sound cues and all developed strong side biases. We observed repeatable individual differences in activity and boldness, yet these personality traits were not linked to the sharks’ learning performance.
In this study, five sharks learned to associate a sound stimulus with a food reward. In agreement with our predictions, the sharks became faster in approaching the correct choice zone and in retrieving the reward, and showed anticipatory behaviour induced by the stimulus. Interestingly, the three sharks that failed to learn the association were consistent in entering the correct choice area early in the trial, but paid no attention to the sound stimulus. In this task, the food reward was always accessible in the same location after a short random interval. If we exclude the sound cue, the task resembles a place-learning task with a variable interval (VI) schedule of reinforcement—the shark makes an operant response (enter the choice area), and a food reward is delivered at random time intervals. It is possible these sharks ignored the sound cue and were responding to the VI place-learning task, and indeed their steady rate of response is characteristic of VI schedules (Ferster and Skinner 1957).
None of the five sharks that successfully associated the jazz sound with reward learnt the discrimination between jazz and classical music. The possibility that the sharks could not acoustically distinguish the two stimuli cannot be ruled out, but is unlikely given the many differences in the sonograms. In addition, if that were the case we would expect them to maintain the response previously learnt regardless of the stimulus. This was not observed, as all sharks showed a reduction in performance for the jazz stimulus in the first sessions of the discrimination task. Decreased performance to a previously learnt association during the initial stages of a new task is commonly observed in operant conditioning before individuals acquire the new discrimination, which suggests an attempt at rule formation in our sharks.
Interestingly, most individuals developed a bias to the right-side choice zone after a few sessions in the discrimination task. While we did not directly test for laterality and side bias in a choice scenario, we found no prior preference to spend time on the left- or right-side of a rectangular arena in any of the sharks used in this experiment (Vila Pouca and Brown, in review). Together with the fact that some of the sharks were successfully trained to the left-side choice zone in the food conditioning task, it seems that the side bias only developed when the task was too difficult to learn. Strong side bias are often seen in animal learning experiments with two-choice responses, and perhaps arise from an animal’s default-option when facing indecision, which yields a higher payoff compared to random choice.
In this experiment, sharks took a median of 12 sessions to learn the food approach conditioning task, which amounts to approximately 120 trials in total, before beginning the discrimination task. This overall number of trials ran before starting the discrimination task is very low compared to the training sessions of birds, rats and other fish (D’Amato and Salmon 1984; Porter and Neuringer 1984; Chase 2001), and might explain their poor performance. For example, in a music discrimination experiment, koi carp were given 40 days of 50-trial sessions with one of the stimulus before they began the discrimination phase (Chase 2001). In addition, these koi carp had been serving as experimental subjects for 5 years (as is the case for a great number of subjects in animal cognition experiments), while our sharks were naïve to learning experiments.
Sharks can respond to artificial magnetic fields (Meyer et al. 2005), as well as weak electric fields (Jordan et al. 2011), thus another option to consider is that the magnetic and/or electric field created by the speaker acted as a cue, which the sharks might have used to learn the task. We were unable to test this hypothesis; it would have been valuable to assess the sharks’ response to a sound stimulus outside their hearing ability (thus to the speakers’ electromagnetic field alone), or start the discrimination training with an easier discrimination, such as the jazz stimulus versus a plain tone. Another potential issue to consider is background noise in the tank, reverberation and signal distortion (however, from visual comparison of the in-water sonograms and the original stimuli, these factors do not seem to have been extreme).
Associative learning has been widely investigated in many species of teleost fish, comprising a large range of tasks and multiple sensory modalities (e.g. visual, tactile or auditory; Brown et al. 2011). Studies using auditory stimuli have shown teleost fish use acoustic cues for communication and orientation, and can learn both with natural or artificial sounds (Chase 2001; Simpson et al. 2010; Ladich 2015). A few studies have investigated associative learning skills with auditory stimuli in large costal shark species, including a single lemon shark that was trained to approach a speaker following one frequency, but avoid it with another frequency (Nelson 1967). Our study is the first to assess acoustic conditioning in a benthic elasmobranch, and to examine if boldness and swimming activity were linked to any learning performance assays in the food approach conditioning task. We found repeatable within-individual emergence times and activity levels which is consistent with previous studies (Byrnes and Brown 2016; Byrnes et al. 2016); however, neither of the two traits were correlated with latency to enter the choice zone, latency to eat the reward, or anticipatory behaviour in our eight sharks. Far more research in multiple species and with a greater sample size is needed to properly address this topic in elasmobranch fishes.
In conclusion, this study shows that benthic elasmobranchs can learn an association task with a sound stimulus. Underwater sounds are likely ecologically relevant cues to benthic species, especially nocturnal ones, to aid in locating prey and in navigating between reef areas. Further studies should investigate preferential behaviour and associative learning using natural reef sounds, including those made by fish and crustaceans living there.
References
Banner A (1972) Use of sound in predation by young lemon sharks, Negaprion brevirostris (Poey). Bull Mar Sci 22:251–283
Bass NC, Mourier J, Knott NA, Day J, Guttridge T, Brown C (2016) Long-term migration patterns and bisexual philopatry in a benthic shark species. Mar Freshw Res 68:1414–1421
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. arXiv preprint. arXiv:1406.5823
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Brainard DH (1997) The psychophysics toolbox. Spatial Vis 10:433–436
Brown C, Laland K, Krause J (2011) Fish cognition and behavior. Wiley, Oxford
Budaev S, Brown C (2011) Personality traits and behaviour. In: Brown C, Laland K, Krause J (eds) Fish cognition and behavior. Wiley-Blackwell, Oxford, p 472
Byrnes EE, Brown C (2016) Individual personality differences in Port Jackson sharks Heterodontus portusjacksoni. J Fish Biol 89:1142–1157
Byrnes EE, Vila Pouca C, Chambers SL, Brown C (2016) Into the wild: developing field tests to examine the link between elasmobranch personality and laterality. Behav 153:1777–1793
Carere C, Locurto C (2011) Interaction between animal personality and animal cognition. Curr Zool 57:491–498
Casper BM, Mann DA (2006) Evoked potential audiograms of the nurse shark (Ginglymostoma cirratum) and the yellow stingray (Urobatis jamaicensis). Environ Biol Fishes 76:101–108
Casper BM, Mann DA (2007) Dipole hearing measurements in elasmobranch fishes. J Exp Biol 210:75–81
Casper B, Mann D (2009) Field hearing measurements of the Atlantic sharpnose shark Rhizoprionodon terraenovae. J Fish Biol 75:2768–2776
Chase AR (2001) Music discriminations by carp (Cyprinus carpio). Anim Learn Behav 29:336–353
Crawford JD, Hagedorn M, Hopkins CD (1986) Acoustic communication in an electric fish, Pollimyrus isidori (Mormyridae). J Comp Physiol A 159:297–310
D’Amato MR, Salmon DP (1984) Processing of complex auditory stimuli (tunes) by rats and monkeys (Cebus apella). Anim Learn Behav 12:184–194
Dugatkin LA, Alfieri MS (2003) Boldness, behavioral inhibition and learning. Ethol Ecol Evol 15:43–49
Ferster CB, Skinner BF (1957) Schedules of reinforcement. Appleton-Century-Crofts, East Norwalk, CT, USA
Finger JS, Dhellemmes F, Guttridge TL (2017) Personality in elasmobranchs with a focus on sharks: Early evidence, challenges, and future directions. In: Vonk J, Weiss A, Kuczaj SA (eds) Personality in nonhuman animals. Springer International Publishing, Cham, pp 129–152
Gannon DP, Barros NB, Nowacek DP, Read AJ, Waples DM, Wells RS (2005) Prey detection by bottlenose dolphins, Tursiops truncatus: an experimental test of the passive listening hypothesis. Anim Behav 69:709–720
Gardiner JM, Hueter RE, Maruska KP, Sisneros JA, Casper BM, Mann DA, Demski LS (2012) Sensory physiology and behavior of elasmobranchs. Biol Sharks Relat 1:349–401
Guttridge TL, Brown C (2014) Learning and memory in the Port Jackson shark, Heterodontus portusjacksoni. Anim Cogn 17:415–425
Hart NS, Collin SP (2015) Sharks senses and shark repellents. Integr Zool 10:38–64
Hart NS, Theiss SM, Harahush BK, Collin SP (2011) Microspectrophotometric evidence for cone monochromacy in sharks. Naturwissenschaften 98:193–201
Huijbers CM, Nagelkerken I, Lössbroek PAC, Schulten IE, Siegenthaler A, Holderied MW, Simpson SD (2012) A test of the senses: fish select novel habitats by responding to multiple cues. Ecology 93:46–55
Hulse SH, Takeuchi AH, Braaten RF (1992) Perceptual invariances in the comparative psychology of music. Music Percept 10:151–184. https://doi.org/10.2307/40285605
Jordan LK, Mandelman JW, Kajiura SM (2011) Behavioral responses to weak electric fields and a lanthanide metal in two shark species. J Exp Mar Biol Ecol 409:345–350
Kelly JC, Nelson DR (1975) Hearing thresholds of the horn shark, Heterodontus francisci. J Acoust Soc Am 58:905–909
Kritzler H, Wood L (1961) Provisional audiogram for the shark, Carcharhinus leucas. Science 133:1480–1482
Ladich F (2015) Sound communication in fishes. Animal signals and communication series, vol. 4. Springer, Vienna
Ladich F, Myrberg A (2006) Agonistic behavior and acoustic communication. Commun Fish 1:121–148
Last PR, Stevens JD (2009) Sharks and rays of Australia. CSIRO Publishing, Australia, 644p
Lefcheck JS (2016) piecewiseSEM: piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol Evol 7:573–579
Mann DA, Lu Z, Popper AN (1997) A clupeid fish can detect ultrasound. Nature 389:341–341
McFarland W (1990) Light in the sea: the optical world of elasmobranchs. J Exp Zool 256:3–12
Meyer CG, Holland KN, Papastamatiou YP (2005) Sharks can detect changes in the geomagnetic field. J R Soc Interface 2:129–130
Myrberg AA Jr, Ha SJ, Walewski S, Banbury JC (1972) Effectiveness of acoustic signals in attracting epipelagic sharks to an underwater sound source. Bull Mar Sci 22:926–949
Myrberg AA Jr, Mohler M, Catala JD (1986) Sound production by males of a coral reef fish (Pomacentrus partitus): its significance to females. Anim Behav 34:913–923
Myrberg AA (2001) The acoustical biology of elasmobranchs. Environ Biol Fishes 60:31–46
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev Camb Philos Soc 85:935–956
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Nelson DR (1967) Hearing thresholds, frequency discrimination, and acoustic orientation in the lemon shark, Negaprion brevirostris (Poey). Bull Mar Sci 17:741–768
Nelson DR, Johnson RH (1972) Acoustic attraction of Pacific reef sharks: effect of pulse intermittency and variability. Comp Biochem Physiol Part A Physiol 42:85–95
Porter D, Neuringer A (1984) Music discriminations by pigeons. J Exp Psychol Anim Behav Process 10:138
Powter DM, Gladstone W (2009) Habitat-mediated use of space by juvenile and mating adult Port Jackson sharks, Heterodontus portusjacksoni, in Eastern Australia. Pac Sci 63:1–14
R Core Team (2016). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Radford C, Stanley J, Simpson S, Jeffs A (2011) Juvenile coral reef fish use sound to locate habitats. Coral Reefs 30:295–305
Remage-Healey L, Nowacek DP, Bass AH (2006) Dolphin foraging sounds suppress calling and elevate stress hormone levels in a prey species, the Gulf toadfish. J Exp Biol 209:4444–4451
Richardson WJ, Greene CR Jr, Malme CI, Thomson DH (2013) Marine mammals and noise. Academic Press, San Diego
Ryan LA, Hart NS, Collin SP, Hemmi JM (2016) Visual resolution and contrast sensitivity in two benthic sharks. J Exp Biol 219:3971–3980
Scheipl F, Greven S, Küchenhoff H (2008) Size and power of tests for a zero random effect variance or polynomial regression in additive and linear mixed models. Comput Stat Data Anal 52:3283–3299
Schielzeth H, Nakagawa S (2013) rptR: repeatability for Gaussian and non-Gaussian data. R package version 0.6. 405/r52
Schluessel V (2015) Who would have thought that ‘Jaws’ also has brains? Cognitive functions in elasmobranchs. Anim Cogn 18:19–37
Shettleworth SJ (2010) Cognition, evolution, and behavior. Oxford University Press, Oxford
Sih A, Del Giudice M (2012) Linking behavioural syndromes and cognition: a behavioural ecology perspective. Philos Trans R Soc B Biol Sci 367:2762–2772
Simpson SD, Meekan M, Montgomery J, McCauley R, Jeffs A (2005) Homeward Sound. Science 308:221–221
Simpson SD, Meekan MG, Larsen NJ, McCauley RD, Jeffs A (2010) Behavioral plasticity in larval reef fish: orientation is influenced by recent acoustic experiences. Behav Ecol 21:1098–1105
Slabbekoorn H, Bouton N, van Opzeeland I, Coers A, ten Cate C, Popper AN (2010) A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol Evol 25:419–427
Spedicato GA, Kang TS, Yalamanchi SB, Yadav D (2016) The markovchain package: a package for easily handling Discrete Markov Chains in R. https://cran.r-project.org/web/packages/markovchain/markovchain.pdf. Accessed 6 Nov 2017
Trompf L, Brown C (2014) Personality affects learning and trade-offs between private and social information in guppies, Poecilia reticulata. Anim Behav 88:99–106
Tyack P (1998). Acoustic communication under the sea. Hopp SL, Owren MJ, Evans CS (eds) Animal acoustic communication. Springer, New York, pp 163–220
Watanabe S, Sato K (1999) Discriminative stimulus properties of music in Java sparrows. Behav Proc 47:53–57
White S, Wagner T, Gowan C, Braithwaite V (2016) Can personality predict individual differences in brook trout spatial learning ability? Behav Process 414:220–228
Wilson M, Acolas M-L, Bégout M-L, Madsen PT, Wahlberg M (2008) Allis shad (Alosa alosa) exhibit an intensity-graded behavioral response when exposed to ultrasound. J Acoust Soc Am 124:EL243-EL247
Zion B, Barki A (2012) Ranching fish using acoustic conditioning: has it reached a dead end? Aquaculture 344–349:3–11
Zion B, Karplus I, Barki A (2010) Generalization and discrimination of positive and negative acoustic stimuli in the common carp (Cyprinus carpio). Behav Process 83:306–310
Zuur A, Ieno E, Walker N, Saveliev A, Smith G (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
This research was funded by the Department of Biological Sciences at Macquarie University, and CVP was supported by an Endeavour Postgraduate (PhD) Scholarship. We thank the members and interns of the Fish Lab and SIMS for husbandry assistance, Jack Gruber for help running the experiment, Drew Allen for advice on statistical analysis, and Vera Schlüessel and two anonymous reviewers for their insightful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This work was approved by the Macquarie University Animal Ethics Committee under ARA 2014-003.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vila Pouca, C., Brown, C. Food approach conditioning and discrimination learning using sound cues in benthic sharks. Anim Cogn 21, 481–492 (2018). https://doi.org/10.1007/s10071-018-1183-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-018-1183-1