Abstract
Observational learning is the ability to learn through observing others’ behavior. The benefit of observational learning is apparent in that individuals can save time and energy without trial-and-error, thus enhance the chance of survival and reproduction. Cephalopods (octopus, squid, and cuttlefish) have the most sophisticated central nervous system among invertebrates, and it is conceivable that cephalopods can develop some forms of cognition. Although it has been suggested that octopuses have the capacity of observational learning, a previous study indicates that cuttlefish do not improve their predation tactics by observing conspecifics. Given that the danger avoidance is important for animals’ survival, we sought to reevaluate whether cuttlefish show some form of observational learning or observational conditioning under threatening conditions. Cuttlefish (Sepia pharaonis) were divided into three groups: the Experiencer group, the Observer group, and the Control group. In the training phase, a toy submarine was remotely controlled to expel the cuttlefish from its initially preferred place to establish the threat-place association in the Experiencer group. In the Observer group, the threat-place association was established by expelling a conspecific demonstrator at the observer’s initially preferred place while the observer watched the whole process from behind a transparent divider. In the Control group, the observer watched a conspecific and a static toy submarine without actual threat. In the testing phase, the choice of safe place in the absence of threat was used to probe the learning/conditioning of cuttlefish. In the Experiencer group, we found that animals chose the safe place more often than their initially preferred place after training, an indication of the association learning/conditioning. However, in the Observer group, only a subset of animals showed this threat-place association by observation, while the place preference was unchanged in the Control group. These results indicate that most cuttlefish did not learn by observing others, but individual differences exist, and some cuttlefish may have the potential of observational learning/conditioning within their cognitive capacities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals may learn to obtain food, to find mates, or to avoid attack by observing others, usually conspecifics (Galef and Laland 2005). Observational learning is one of the social learning techniques frequently seen in social animals. It occurs when observers change their behaviors after watching conspecifics perform a task or interact with the environment. The benefit of observational learning is apparent in that individuals can save time and energy without trial-and-error. Learning from their conspecifics may be more efficient and less dangerous and thus enhances their chance of survival and reproduction (Goodenough et al. 1993).
Numerous examples show that vertebrates are capable of observational learning (Galef and Laland 2005; Robert 1990). The most familiar species is human beings. Children may obtain information about what behaviors they should or should not be doing by watching their mothers rewarding or punishing their siblings. In other vertebrates, there are also examples of learning by observation to infer social rank (Grosenick et al. 2007), to get food resources (Lara et al. 2009), or to avoid danger (Mineka et al. 1984; Arai et al. 2007). However, whether invertebrates are also capable of observational learning remains controversial.
Cephalopods (octopus, squid, and cuttlefish) have the most sophisticated central nervous system among invertebrates (Hochner 2008), and it is conceivable that cephalopods can have some forms of cognition (Mather 2008). Although recent studies have demonstrated their cognitive abilities in various tasks, most of them rely on their asocial learning rather than social learning capabilities. For example, it was reported that cuttlefish show passive avoidance learning to inhibit their predatory behavior in the “prawn-in-the-tube” experiment, indicating a basic level of cognition (Agin et al. 2006). There are also studies suggesting that cephalopods may have higher levels of cognition, including spatial cognition (Karson et al. 2003; Alves et al. 2007; Jozet-Alves et al. 2008), conditional discrimination (Hvorecny et al. 2007), and even the ability to recognize individual humans (Anderson et al. 2010). Although it has been suggested that cephalopods may possess primary consciousness (Mather 2008), the observation that they do not understand that their reflected images are themselves not conspecifics when facing a mirror suggests that cephalopods do not have self-awareness (Palmer et al. 2006).
With complex brains and higher cognition, even though cephalopods are not social animals, they may have the capacity for observational learning. In octopus, it has been shown that an observer makes the same choice as a demonstrator when the observer is allowed to watch a conditioned conspecific choosing one of two colored balls before test (Fiorito and Scotto 1992). This result suggests that the untrained octopus can learn a two-choice task by observing others. However, some have argued that this study only shows some form of imitation rather than genuine observational learning (Biederman and Davey 1993). In cuttlefish, it was found that animals do not improve their predation tactics by observational learning (Boal et al. 2000). Although naive cuttlefish can prey on crabs without getting pinched after watching the experienced conspecifics capturing the crabs, they can achieve the same performance after merely being exposed to the crab odor before test. Thus, it appears that odor serves as a primer to evoke food arousal, and observation is not the key requirement for improving their predation tactics (Boal et al. 2000).
Given that danger avoidance is important for animals’ survival, the present study aimed to reevaluate whether cuttlefish show some form of observational learning or observational conditioning under threatening conditions. We probed the capacity of observational learning/conditioning by examining if cuttlefish can associate threat with a visual background by observing a conspecific experiencing the same threat. Increased safe place choice by the observer would suggest that cuttlefish have developed a goal-directed behavior by observing conspecifics. If cuttlefish merely copy the behavior of the demonstrator, they would not know which place is safe, and their place choices would be random.
Materials and methods
Subjects
Juvenile cuttlefish (Sepia pharaonis) were reared from eggs (collected from the by-catch of bottom trawls in southwestern Taiwan) at the National Museum of Marine Biology and Aquarium in Pingtung, Taiwan, and were transported to the National Tsing Hua University (NTHU) in Hsinchu at around 4 weeks old. All cuttlefish were maintained in the laboratory in two closed-circulation aquarium systems (700 L each; water temperature = 22 °C) at the NTHU. Animals were housed individually in plastic tanks (25.3 cm × 34 cm × 24 cm) inside the aquarium. They were fed with shrimp or fish at least once per day. The experiment started when the cuttlefish were 7 weeks post-hatching and about 2–3 cm in mantle length. In total, 34 cuttlefish were used in the present study: 10 were used in the Experiencer group, 14 were used in the Observer group, and 10 were used in the Control group (see below for group names). Among the 14 cuttlefish in the Observer group, 10 were assigned as the observers and 4 were the demonstrators. Similarly, among the 10 cuttlefish in the Control group, 7 were assigned as the observers and 3 were the demonstrators.
Experimental apparatus and procedure
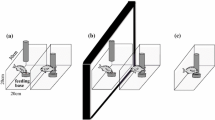
All experiments were conducted in an arena (56.5 cm × 38.5 cm × 24.5 cm) composed of an observation area and an experience/test area (made of an acrylic tank, 55 cm × 20 cm × 20 cm) separated by a transparent wall (Fig. 1a). The arena was filled with seawater to approximately 16 cm in depth, and the seawater was circulated between the two compartments. In the experience/test area, three transparent acrylic doors which could be removed manually from above (Fig. 1b) were used to separate two distinct background patterns (the middle divider) as well as two places for the toy submarine and the experimental subject (the left and right dividers). Three entrance holes (6.5 cm in diameter) were designed to allow cuttlefish to enter the experience/test area easily (Fig. 1b). A PVC pipe fitted at the middle hole was served as the entrance tube during the test phase, but was absent during the experience/observation phase (Fig. 1a). Two background patterns (white squares of size 4 or 1 cm on a black substrate) were used as distinct visual environments for studying threat-place association learning (Fig. 1b). The background patterns (laminated to be waterproof) were presented on the floor and wall outside the tank. During both training and testing phases, these two background patterns were randomly assigned to left and right sides of the experience/test area.
The experimental setup. a Top view. The experimental apparatus was composed of the observation area and the experience/test area. A PVC pipe located at the midline was served as the entrance tube during the test phase, but was absent during the experience/observation phase. The transparent sliding partitions were used to allow the toy submarines to enter the experience/test area. Black foam was used as a barrier to block other visual stimuli from the outside. b Side view. Different background patterns were placed on the wall and the bottom of each side in the experience/test area. During the experience/observation phase, the cuttlefish were put in the area of predetermined preferred side/background and disturbed by the toy submarine manipulated remotely by the experimenter at that site. During the test phase, toy submarines were placed at both sides to neutralize the presence of the threat
To provide a stable visual environment and minimize stress to the animals, all experimental trials were conducted inside a tent made of black plastic sheeting. Fluorescent light sources (Mitsubishi, FCL30EX-D/28 or Philips, TLE30W/54-765) were used to illuminate the arena. A digital video camera (SONY DCR-SR62 Handycam) was mounted above the arena to record the experimental procedures. A small window on the tent allowed opened for observation of the animals through the camera’s view finder, so the animals’ movements could be tracked from outside the chamber without disturbing them.
Experimental designs
The timeline of the experiments is shown in Fig. 2. To determine if each cuttlefish has initial side (left/right) and size (large/small) preferences, all animals went through the initial preference test. The training phase followed immediately to allow cuttlefish associate the threat-place against their initially preferred side/background. The testing phase was then used to probe their threat-place association. To examine if the association learning could be strengthened by repetition, cuttlefish from both the Experiencer and Observer groups went through the training and testing phases twice during the experiments (Fig. 2). The timeline in different groups varied slightly depending on conditions.
Timeline of the experimental design. Cuttlefish in a the Experiencer group, b the Observer group, and c the Control group all started the experiments with 12 trials of the initial preference test. Training phases were immediately followed to allow cuttlefish associate the threat-place against their initial preferred side/background (see Methods for details). Subsequent test phases were used to probe the strength of this threat-place association in these groups of cuttlefish. Two consecutive training and test phases were used to examine whether the threat-place association learning could be strengthened by repetition, except in the Control group where only one training phase was used
Initial preference test
Cuttlefish were carefully transferred to the entrance tube (Fig. 1a), and temporarily restrained in the PVC pipe by blocking the front and rear doors to deprive them of all visual stimuli. After removing the front door, the animal was allowed to enter the experience/test area freely, and the cuttlefish’s response was video recorded for 5 min. The initial place preference of each cuttlefish was determined over 12 trials (see below for the criteria of initial preference).
Training phase: the Experiencer group
Cuttlefish were transferred to the experience area through the entrance hole on their preferred place (determined by the initial preference test) and were temporarily restrained in this space by transparent partitions (Fig. 1b). The entrance hole was then blocked by a plastic sheet to prevent escape. After 5 min of acclimation, the partitions in the middle and in the cuttlefish’s preferred side were lifted, and the toy submarine on the cuttlefish’s preferred side was remotely controlled to expel and disturb the animal on that area. The disturbing action continued until the animal moved to and stayed on its non-preferred side, or for 3 min of active disturbance. The animal was then moved back to its home tank. Two liters of fresh seawater were exchanged in the arena before the next trial. Training of each animal was carried out once a day for six successive days.
Training phase: the Observer group
Similarly, the demonstrator cuttlefish was transferred to the experience area through the entrance hole on the observer’s preferred side (determined by the initial preference test) and was temporarily restrained in this space by transparent partitions (Fig. 1b). Subsequently, the observer cuttlefish was transferred to the observation area (Fig. 1a). After 5 min of acclimation for both the demonstrator and the observer, the partitions in the middle and on the demonstrator’s side were lifted, and the toy submarine was remotely controlled to expel and disturb the demonstrator on the observer’s preferred side, while the observer cuttlefish watched the entire process from behind a transparent wall. The disturbing action continued until the demonstrator moved to and stayed on the observer’s non-preferred side, or for 3 min of active disturbance. The observer was then moved back to its home tank before the demonstrator was removed. Two liters of fresh seawater were exchanged in the arena before the next trial. Training of each observer was carried out once a day for six successive days, and 4 demonstrators were randomly assigned in the training phase.
Training phase: the Control group
As in the training protocol for cuttlefish in the Observer group, the demonstrator was transferred to the observer’s preferred place, and the observer cuttlefish in this group also watched the entire process from behind a transparent wall, except that the demonstrator was freely moving around for 3 min and the toy submarines were present on both sides but inactive. Two liters of fresh seawater were exchanged in the arena before the next trial. Training for each observer was carried out once a day for six successive days, and 3 demonstrators were randomly assigned in the training phase.
Testing phase
The preferred place test was conducted for all cuttlefish groups. Each animal was tested 12 times after training. The procedure of the test was similar to the one used in the initial preference test. The only difference is that there were two toy submarines in the tank, each separated by a transparent partition on one side (Fig. 1b), to evoke memory of the threat, while no toy submarine was present in the initial preference test.
Criteria for determining the initial place preference
Behavioral responses were examined by playback of the recorded videos using iMovie (version 8.0.6) on a Macintosh computer. For the 5 min of the initial preference test, the time spent on each side (left or right) or on each background pattern (large or small squares) was measured. It is known that cuttlefish have a side-turning preference (Karson et al. 2003; Hvorecny et al. 2007; Alves et al. 2007) and a background pattern size preference (Lee et al. 2012), both of which may dominate their place preferences. Thus, the initially preferred place of the cuttlefish in the present study was assigned to the side or the background pattern where the animal spent most of time in all 12 trials.
Data analysis
As in the measurement in the initial place preference test, the time spent of cuttlefish on the safe side in the post-training test was determined from the recorded video for all 12 trials. The difference in the time cuttlefish spent on the safe side between pre- and post-training or between 1st and 2nd post-training trials were examined using one-sample t test for all three animal groups. Note that the safe side for pre-training animals is the opposite side of their initially preferred place. To evaluate the time spent by individual cuttlefish on the safe side between pre- and post-training or between 1st and 2nd post-training trials for the Experiencer and Observer groups, independent t test was used on the data from each animal. Statistics were conducted using SPSS.
Results
Association learning/conditioning in the Experiencer group
The mean time spent on the safe side for pre-training (naïve), 1st post-training, and 2nd post-training cuttlefish increased steadily in the Experiencer group (Fig. 3a). One-sample t tests comparing the difference of time spent on the safe side between pre- and post-trainings and between 1st and 2nd post-trainings showed that cuttlefish stayed significantly longer on the safe side after the second training than during the initial preference test (t(9) = 2.91, P = 0.017), an indication of association learning/conditioning in the Experiencer group.
Only the Experiencer group shows a significant threat-place association after training. Average time spent on the safe side for pre-training (naïve), 1st post-training, and 2nd post-training cuttlefish increased steadily in a the Experiencer group and b the Observer group. However, one-sample t tests showed that only the Experiencer group had a significant time difference between pre-training and 2nd post-training (P = 0.017). Although the Observer group also showed increased time spent on the safe side from pre-training to 2nd post-training, the difference was not statistically significant (P = 0.057). c In the Control group, the time difference between pre- and post-trainings was not significant (P = 0.823). *P < 0.05. Error bars indicate SEM
Although the Experiencer group showed a significant threat-place association after two training sessions, individual difference existed in their performances. While most animals increased their time spent on the safe side after training, the independent t test showed that only Animals #1, #2, and #8 in the Experiencer group (No1, t(22) = 2.48, P = 0.021 and t(22) = 2.72, P = 0.013; No2, t(22) = 4.16, P = 0.001 and t(22) = 4.19, P = 0.001; No8, t(22) = 2.28, P = 0.033) had a significant time difference between pre- and post-trainings or between 1st and 2nd trainings (Fig. 4a). However, after Holm-Bonferroni correction for multiple comparisons, only Animals #1 and #2 had a significant time difference after training.
Only a subset of cuttlefish in both the Experiencer and Observer groups show a significant threat-place association after training. Average time spent on the safe side for individual cuttlefish of pre-training (naïve), 1st post-training, and 2nd post-training in a the Experiencer group and b the Observer group. While most animals increased their time spent on the safe side after training, independent t tests showed that only Animals #1, #2, and #8 in the Experiencer group (No1, P = 0.021/0.013; No2, P = 0.001/0.001; No8, P = 0.033), and Animals #1 and #7 in the Observer group (No1, P = 0.015/0.001; No7, P = 0.040) had a significant time difference between pre- and post-trainings or between 1st and 2nd trainings. However, after Holm-Bonferroni correction for multiple comparisons, only Animals #1 and #2 in the Experiencer group and Animal #1 in the Observer group had a significant time difference after training. *P < 0.05. Error bars indicate SEM
Observational learning/conditioning in the Observer group?
Average time spent on the safe side for pre-training (naïve), 1st post-training, and 2nd post-training cuttlefish also increased steadily in the Observer group (Fig. 3b). However, one-sample t tests comparing the difference in time spent on the safe side between pre- and post-training and between 1st and 2nd post-training trials showed that cuttlefish did not stay significantly longer on the safe side even after the second training (t(9) = 2.18, P = 0.057), though the P value is close to the statistical significance. This result gives no evidence that animals in the Observer group learned the threat-place association after two training sessions. In addition, simply presenting a conspecific and a static toy submarine without actual threat during the training session did not affect the observer cuttlefish’s place choices in the Control group (Fig. 3c; t(6) = −0.23, P = 0.823).
Although the Observer group as a whole did not show a significant threat-place association after two training sessions, individual difference did exist in their performances. The independent t test for assessing if individual animals increased their time spent on the safe side after training found that Animals #1 and #7 (No1, t(22) = 2.65, P = 0.015 and t(22) = 4.06, P = 0.001; No7, t(22) = 2.19, P = 0.040) had a significant time difference between pre- and post-trainings or between 1st and 2nd trainings (Fig. 4b). However, after Holm-Bonferroni correction for multiple comparisons, only Animal #1 had a significant time difference after training. This result indicates that a subset of cuttlefish in the Observer group did learn the threat-place association after training.
Discussion
The stability of the initial preference throughout the experiment
Our experiments started off when cuttlefish were 7 weeks old (2–3 cm ML) and ended when the animals were around the age of 12–15 weeks (4–5 cm ML). Inevitably, the body size of cuttlefish increased as the experiment progressed, and thus, the ratio of the background pattern size to their body size decreased accordingly. It is possible that a cuttlefish whose initial preference was small white squares would change its preference as it grew larger. If this is the case, then our finding that cuttlefish could apparently learn by association between threat and place might have a different explanation: cuttlefish may have simply chosen a similar ratio of the background pattern size to their body size in the test phase. However, the fact that some cuttlefish that initially preferred large white squares also behaved against their initial preferences in the test sessions (i.e., preferred small squares) excludes this possibility.
Alternatively, one might suspect that cuttlefish’s initial preference could change with age, and thus, the choice change after training was due to their ontogenetic innate preference change rather than learning the threat-place association. Although the most direct way of verifying this alternative is to monitor the cuttlefish preference throughout the experiments, the training procedure implemented in the present study might confound learning with the initial preference, thus rendering such verification intractable. Furthermore, it is known that 7–15 weeks post-hatching are a period of dynamic neurological growth and development in cuttlefish (Dickel et al. 2000; Poirier et al. 2005; Lee et al. 2010), so the initial preference might change in the course of the experiment. Indeed, in a separate study, we examined the size preference of cuttlefish at different ages and found that on average, cuttlefish reared in an enriched environment tend to prefer an object size similar to their body size at week 4, but changed this preference to smaller objects at week 12 (Lee et al. 2012). However, the fact that some cuttlefish that initially preferred small white squares also behaved against their initial preferences in the test sessions (i.e., preferred large squares) excludes this possibility.
Individual difference exists in association and observational learning/conditioning
In cephalopods, previous studies indicate that several behaviors show individual differences. For example, individual cuttlefish have different side-turning preferences and different strategies in spatial learning (Alves et al. 2007). It has also been shown that not all octopuses demonstrate conditional discrimination, and even for those that do display this learning ability, the time for finding the open burrow differs among individuals (Hvorecny et al. 2007). In addition, it has been reported that individual gloomy octopuses show either shy or bold episodic personality in a video playback experiment (Pronk et al. 2010). Similarly, dumpling squids also show individually different shy/bold behaviors throughout development (Sinn et al. 2008). In the present study, we found that a subset of cuttlefish could associate threat with a background pattern by their own self-experience or by observing others after the initial training, but some animals did not show any progress even after receiving a second training session. These results suggest that individual difference among cuttlefish may explain the variability found in both association and observational learning/conditioning in our experiments. Furthermore, by comparing the number of animals choosing the safe place in 1st post-training and 2nd post-training, it is apparent that more cuttlefish chose the safe place after the second training session, which suggests that some individuals may improve their learning if they receive further training.
Criteria for observational learning
We use the term “observational learning” in a broad sense, that is, learning and making progress through observing of, or interacting with, others without trial-and-error learning.
Fiorito and Scotto (1992) specified that observational learning should be significantly faster than the learning obtained by classical conditioning. In other words, learning is facilitated by observing others performing the task. They reported that octopus was able to learn a task by observing the behavior of a conspecific for a short period of time, indicating the observational learning in this species. However, some critics have suggested that this kind of learning behavior should be classified as “observational conditioning” rather than observational learning (Heyes 1994).
Observational conditioning is regarded as a kind of stimulus–stimulus (S–S) learning (Heyes 1994). The observer is exposed to the relationship between the stimulus (S1) and the unconditioned response which acted as an unconditioned stimulus (S2) represented by the demonstrator in responding to the stimulus (S1) at t 1. When the observer experienced the same stimulus (S1) at t 2, it would subsequently make the same response (called the matching response) to the stimulus as the demonstrator did. For example, rhesus monkeys have been shown to acquire fear responses through observational conditioning. Laboratory-reared rhesus monkeys do not show any fear to the presence of a snake initially. However, they display fear in response to snake stimuli (S1) after observing their wild conspecifics behave fearfully (S2) in the presence of a snake (S1) (Mineka et al. 1984).
In contrast, observational learning is regarded as a subset of response-reinforcer (R-S) learning (Heyes 1994). The demonstrator exposes the observer to a relationship between a response and a reinforcer at t 1. At t 2, the observer exposed to the relationship would lead to a behavior change of the observer. It has been demonstrated that observational learning occurs in rats using a bidirectional control (Heyes and Dawson 1990). This design minimizes the effects of local enhancement and distinguishes reinforcer-stimulus learning from stimulus-reinforcer learning by examining the response in the acquisition test, reversal of left–right discrimination, and response extinction test. In the study of Heyes and Dawson, rats were allowed to observe their conspecifics pushing a joystick to the left or to the right for food reward. They found that rats that observed left-pushing made more left response during the acquisition test, and rats that observed the demonstrator pushing in the direction that had been reinforced before would take a longer time to reach the reversal criteria in the reversal test. Furthermore, these rats made more responses in the extinction test than those that observed the demonstrator pushing in the direction opposite to that previously reinforced. These findings suggest that rats have learned the reinforce-stimulus relationship, indicating observational learning.
If one takes the strict definition of observational learning described above, our data might be viewed as a kind of stimulus–stimulus learning, in which the relationship of the agitated response (S2) of the demonstrator cuttlefish and the threat stimulus (S1) was provided to the observer during training. Consequently, the learning behavior of the observer cuttlefish would be considered as a combination of observational conditioning and associative learning, rather than observational learning.
References
Agin V, Chichery R, Dickel L, Chichery MP (2006) The “prawn-in-the-tube” procedure in the cuttlefish: habituation or passive avoidance learning? Learn Mem 13(1):97–101. doi:10.1101/lm.90106
Alves C, Chichery R, Boal J, Dickel L (2007) Orientation in the cuttlefish Sepia officinalis: response versus place learning. Anim Cogn 10(1):29–36
Anderson RC, Mather JA, Monette MQ, Zimsen SRM (2010) Octopuses (Enteroctopus dofleini) recognize individual humans. J Appl Anim Welf Sci 13(3):261–272
Arai T, Tominaga O, Seikai T, Masuda R (2007) Observational learning improves predator avoidance in hatchery-reared Japanese flounder Paralichthys olivaceus juveniles. J Sea Res 58(1):59–64. doi:10.1016/J.Seares.2007.01.004
Biederman GB, Davey VA (1993) Social learning in invertebrates. Science 259(5101):1627–1628. doi:10.1126/science.259.5101.1627
Boal JG, Wittenberg KM, Hanlon RT (2000) Observational learning does not explain improvement in predation tactics by cuttlefish (Mollusca: Cephalopoda). Behav Process 52(2–3):141–153
Dickel L, Boal JG, Budelmann BU (2000) The effect of early experience on learning and memory in cuttlefish. Dev Psychobiol 36(2):101–110. doi:10.1002/(SICI)1098-2302(200003)36:2<101:AID-DEV2>3.0.CO;2-L
Fiorito G, Scotto P (1992) Observational learning in Octopus vulgaris. Science 256(5056):545–547. doi:10.1126/science.256.5056.545
Galef BG, Laland KN (2005) Social learning in animals: empirical studies and theoretical models. Bioscience 55(6):489–499. doi:10.1641/0006-3568(2005)055[0489:SLIAES]2.0.CO;2
Goodenough J, McGuire B, Wallace RA (1993) Perspectives on animal behavior. Wiley, New York
Grosenick L, Clement TS, Fernald RD (2007) Fish can infer social rank by observation alone. Nature 445(7126):429–432
Heyes CM (1994) Social learning in animals: categories and mechanisms. Biol Rev 69(2):207–231
Heyes CM, Dawson GR (1990) A demonstration of observational learning in rats using a bidirectional control. Q J Exp Psychol B 42(1):59–71
Hochner B (2008) Octopuses. Curr Biol 18(19):R897–R898. doi:10.1016/j.cub.2008.07.057
Hvorecny L, Grudowski J, Blakeslee C, Simmons T, Roy P, Brooks J, Hanner R, Beigel M, Karson M, Nichols R, Holm J, Boal J (2007) Octopuses (Octopus bimaculoides) and cuttlefishes (Sepia pharaonis, S. officinalis) can conditionally discriminate. Anim Cogn 10(4):449–459
Jozet-Alves C, Moderan J, Dickel L (2008) Sex differences in spatial cognition in an invertebrate: the cuttlefish. Proc Biol Sci 275(1646):2049–2054. doi:10.1098/rspb.2008.0501
Karson MA, Jean GB, Hanlon RT (2003) Experimental evidence for spatial learning in cuttlefish (Sepia officinalis). J Comp Psychol 117(2):149–155
Lara C, Gonzalez JM, Hudson R (2009) Observational learning in the white-eared hummingbird (Hylocharis leucotis): experimental evidence. Ethology 115(9):872–878. doi:10.1111/J.1439-0310.2009.01668.X
Lee YH, Yan HY, Chiao CC (2010) Visual contrast modulates maturation of camouflage body patterning in cuttlefish (Sepia pharaonis). J Comp Psychol 124(3):261–270. doi:10.1037/a0019461
Lee YH, Yan HY, Chiao CC (2012) Effects of early visual experience on the background preference in juvenile cuttlefish Sepia pharaonis. Biol Lett 8:740–743
Mather JA (2008) Cephalopod consciousness: behavioural evidence. Conscious Cogn 17(1):37–48
Mineka S, Davidson M, Cook M, Keir R (1984) Observational conditioning of snake fear in rhesus monkeys. J Abnorm Psychol 93(4):355–372
Palmer ME, Calvé MR, Adamo SA (2006) Response of female cuttlefish Sepia officinalis (Cephalopoda) to mirrors and conspecifics: evidence for signaling in female cuttlefish. Anim Cogn 9(2):151–155. doi:10.1007/s10071-005-0009-0
Poirier R, Chichery R, Dickel L (2005) Early experience and postembryonic maturation of body patterns in cuttlefish (Sepia officinalis). J Comp Psychol 119(2):230–237. doi:10.1037/0735-7036.119.2.230
Pronk R, Wilson DR, Harcourt R (2010) Video playback demonstrates episodic personality in the gloomy octopus. J Exp Biol 213:1035–1041
Robert M (1990) Observational learning in fish, birds, and mammals: a classified bibliography spanning over 100 years of research. Psych Record 40:289–311
Sinn DL, Gosling SD, Moltschaniwskyj NA (2008) Development of shy/bold behaviour in squid: context-specific phenotypes associated with developmental plasticity. Anim Behav 75(2):433–442
Acknowledgments
We thank Drs. Chung-Cheng Lu and En-Cheng Yang for discussion of this project, and Yi-Hsin Lee and Chih-Chiang Lee for assistance of animal care. We are also grateful to Dr. Chih-Wei Chang and Mr. Tse-Ming Hsiao of the National Museum of Marine Biology and Aquarium for maintaining cuttlefish eggs and hatchlings. This work was supported by the National Science Council of Taiwan (NSC-98-2628-B-007-001-MY3 to CCC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, KL., Chiao, CC. Can cuttlefish learn by observing others?. Anim Cogn 16, 313–320 (2013). https://doi.org/10.1007/s10071-012-0573-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-012-0573-z