Abstract
To examine the effects of early visual experience on preference for biological motion (BM), newly hatched chicks were exposed to a point-light animation (a visual stimulus composed of identical light points) depicting the following features of a hen: a walking hen (a BM stimulus), a rotating hen (a non-BM stimulus), a pendulum stimulus, a random motion stimulus and a stationary pattern. Chicks were then tested in a binary choice task, choosing between walking-hen and rotating-hen stimuli. Males exhibited a preference for BM if they had been trained with any animation except the stationary pattern stimulus, suggesting that the BM preference was not learned, but induced by motion stimuli. We found a significant positive correlation between the number of approaches in training and the preference in the test, but locomotion alone did not cause preference for BM. In contrast, females exhibited a particularly strong preference for walking-hen stimuli, but only when they had been trained with it. Furthermore, females (but not males) trained with random motion showed a preference for walking hen over walking cat (a biological motion animation depicting a cat), possibly suggesting that females are choosier than males. Chicks trained with a stationary pattern and untrained controls did not show a significant preference. The induction of BM preference is discussed in terms of possible ecological background of the sex differences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Johansson (1973) first reported that point-light animations can create a vivid and immediate percept of human locomotion if they are strategically placed on the joints of a walking human. This phenomenon is now widely used to study the perception of BM. Recently, a preference for BM has been reported in visually inexperienced animals, such as newly hatched domestic chicks (Vallortigara et al. 2005; Vallortigara and Regolin 2006). Similarly, in humans, 2-day-old newborn babies have been reported to show a BM preference in tests using the preference looking technique (Simion et al. 2008). Adult marmosets (particularly females) have been found to attend more to BM without specific training using the BM animation (Brown et al. 2010). These studies suggest that BM perception is based on an evolutionarily ancient mechanism, involving innately predisposed BM preferences in a variety of animals of different taxa.
BM discrimination is, however, subject to change by experience. Point-light animations can be discriminated by learning in a variety of non-human animals, including pigeons (Dittrich et al. 1998), cats (Blake 1993), rats (MacKinnon et al. 2010), baboons (Parron et al. 2007), and chimpanzees (Tomonaga 2001). These animal studies commonly report that many training trials were needed to reach the criteria, suggesting that the learned discrimination may be based on memorized elements of the animation. Furthermore, the human capability to discriminate motion in BM images can be enhanced without visual stimulation, because the ability to discriminate motion in BM animations is specifically enhanced by acquiring the novel motor patterns that correspond to the BM (Casile and Giese 2006).
These results raise a series of questions about how strongly BM perception relies on innate factors, how modifiable BM preference is, and whether locomotor activity can specifically enhance BM preference. Vallortigara et al. (2005) placed newly hatched chicks on a treadmill to walk for 30 min in complete darkness just before testing, because such motor activities are thought to be crucial for the predisposition to develop (Johnson et al. 1985; Johnson and Horn 1988). In the report by Vallortigara et al. (2005), the forced locomotor activity could have induced the BM preference for functional expression.
In addition, some evidence suggests that sex-related differences in BM perception may occur at a young age. Female chicks have been reported to lose sight of the mother hen less frequently than males (Workman and Andrew 1989). Furthermore, females stay longer near a familiar object than males, even at 3 days (Vallortigara 1992). Similar sex differences were found by Regolin et al. (2000), who reported that male chicks were neophilic compared to females, approaching novel animation that had not been used in training (Regolin et al. 2000).
To address these questions, we examined BM preference using a filial imprinting procedure with a particular focus on sex differences. During the imprinting period, chicks learn about visual features such as color and shape in a process of actively following the imprinting object (Matsushima et al. 2003 and Horn 2004 for reviews; also see Izawa et al. 2001). In the present study, male and female chicks were individually exposed to one of the five animations of different attributes without any pretreatment and were tested for their preference between point-light animations of a walking hen and a rigid rotating hen. In a second experiment, male and females chicks (both trained by using an animation composed of randomly moving point lights) were tested for their preference between animations of a walking hen and a walking cat.
Materials and methods
Subjects
Newly hatched domestic chicks (109 males and 111 females) of the Leghorn (Julia) strain (Gallus domesticus) were used; the hatching day was denoted as day 1 of age. Fertilized eggs were supplied from a local hatchery (Hokuren Co., Iwamizawa, Japan) and incubated at 37–38 °C in darkness. Hatchlings were housed in another incubator kept in complete darkness. Experiments were performed between 09:00 and 18:00, but circadian cues (e.g., photoperiod) were not given. After hatching, chicks were housed individually and kept in complete darkness except during training and testing. A light-reflecting small plastic ball was attached to the head for offline analysis of the walking trajectories using Move-tr/2D software (Library, Tokyo, Japan). Chicks tested on day 1 or day 2 did not receive food or water. Chicks tested on day 5 were fed from day 2 with a mixture of baby food (3 ml) and powdered milk (1 ml) per day, which was supplied to the crop directly with a syringe, in a dark room. After the experiment, feathers and blood were sampled to determine sex, based on the CHD genes of sex chromosomes (Fridolfsson and Ellegren 1999). All experiments were conducted in accord with the guidelines and approval of the Committee on Animal Experiments of Hokkaido University. These guidelines are based on the national regulations for animal welfare in Japan (Law for Humane Treatment and Management of Animals; after partial amendment No. 68, 2005). After the experiments, chicks were killed using carbon dioxide according to the guidelines.
Apparatus
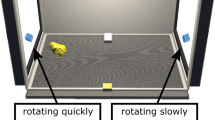
We used a dark chamber (37 × 20 × 40 cm) illuminated by infrared LEDs. The interior temperature was kept at 27–30 °C. The chamber was equipped with two LCD monitors (10.4″ (26.4 cm), 800 × 600 pixels, Logitec LCM-T102, Japan), one on each side (Fig. 1a). An electric shutter (liquid crystal film on transparent Plexiglass partition) was placed on each of the LCD screens to turn the visual stimulation on/off. Chicks’ behavior was recorded using an infrared CCD camera (250-k pixels with NTSC output, placed at the ceiling) and stored with a video recorder (DCR-SR60, Sony, Japan) for offline analysis.
Experimental apparatus and experimental procedures. a A dark chamber equipped with two LCD monitors. A shutter was placed over each LCD monitor to control visual stimulation. b In training trials (upper), two monitors were alternately turned on by shutters, so that the chick was exposed to the same training animation displayed on either of the monitors every 1 min. In test trials lasting 5 min each (lower), both monitors were turned on at the same time. We measured the preference between the two animations displayed simultaneously. c Schedule of training and test trials. Chicks were trained twice, with an interval of 1 h. At 30 min post-training, chicks received two test trials, with a 30-min interval
Animations
We used six types of point-light animations in training and testing. These animations (except the pendulum and stationary pattern) were identical to those used in a previous report (Vallortigara et al. 2005). All animations were constructed from identical yellow light points on a black background. W-hen and R-hen stimuli were constructed based on a video recording of a walking hen. The walking-hen stimulus (W-hen) consisted of 13 points, and the animation was constructed based on a real hen walking leftward. The rotating-hen stimulus (R-hen) consisted of a similar arrangement of 13 light points that mimicked a rigid hen rotating around its vertical axis (see below and Table 1 for further explanations). The pendulum stimulus consisted of 13 fixed but randomly arranged light points, periodically swinging around a center point like a pendulum. The random motion stimulus consisted of 13 randomly arranged light points that moved independently of each other. Speed of motion of the light points was roughly equated to those of the W-hen and R-hen animations. The stationary pattern was a single frame arbitrarily chosen from the random motion animation. The walking-cat stimulus (W-cat) was a 13 light-point animation based on a walking cat.
Initial pilot experiments suggested that the chicks trained with W-hen exhibited a preference toward W-hen at test. Untrained control chicks did not show such a preference. We therefore sought to determine the critical features of the W-hen stimulus using a systematic series of animations (Table 1). Both the W-hen and W-cat stimuli were characterized as BM. All stimuli except the random motion and stationary pattern stimuli were composed of repeated short video clips (1–3 s in duration); thus, these were denoted as periodic animation. All the videos were composed of identical light points. We assumed that the W-hen animation was the closest to real hens that chicks may encounter and that the other animations were further removed from the W-hen on the order of R-hen < pendulum < random motion < stationary pattern. Statistical analyses (see below) were conducted according to the systematic arrangement of the animations.
Procedures
Chicks were individually trained and tested in the experimental chamber. Chicks received two training trials separated by a 1-h interval (Fig. 1c). During the interval, chicks were kept individually in the dark incubator. Each training trial lasted for 1 h, during which the same animation was displayed alternately on the right and left monitors every 1 min using shutters on each screen (Fig. 1a, b), that is, a total of 30 min for each monitor. In training, we counted the cumulative number of approaches to the monitor, measured as the number of times the subject crossed imaginary lines placed 6–7 cm from the shutter (dashed lines in Fig. 1b), while moving toward the monitor.
Thirty minutes after the second training trial, preference was examined in a binary choice test in two 5-min trials separated by a 30-min interval. In this test, each chick was carefully placed at the center of the chamber, in which two different animations were simultaneously and continuously displayed on both monitors; the side of the presentation was changed in the two test trials. We recorded the amount of “stay time” (i.e., the cumulative sum of the duration) for which the chick stayed in the area close to each screen (within the imaginary lines).
Experiment 1
Eight groups of chicks were compared; six groups were tested on day 2 and the other two groups on day 5. Untrained naïve control chicks were kept in complete darkness until testing. W-hen and R-hen stimuli were simultaneously presented at testing, and the difference in stay time (W-hen–R-hen) was used as the preference score.
Experiment 2
Four groups of chicks were compared; two groups were tested on day 2 and the other two groups on day 5. On each day, one group was trained with random motion, and the other group served as untrained naïve controls. W-hen and W-cat stimuli were simultaneously presented at testing, and the difference in stay time (W-hen–W-cat) was used as the preference score.
Statistical analysis
We used R (computer language developed for statistical computations, version 2.12.0) to construct a series of generalized linear models (GLMs), which were evaluated using Akaike information criteria (AICs). As the response variable (Y) denoting the preference, we analyzed the difference in stay time, that is, W-hen minus R-hen in experiment 1 and W-hen minus W-cat in experiment 2, respectively. The link function was assumed to be linear. In some models, the factor sex or the factors sex and age were analyzed, along with their interactions with the group factor. In addition, the correlation between the count of approaches in training and the preference in tests were analyzed using the Spearman rank correlation test at the significance level of p < 0.05.
Two types of formulation (types A and B) were made for the response variable (Y) in the data from day 2 of experiment 1; day-5 data were not included.
Thus, we examined two explanatory variables (group and sex). Group constitutes a variable that takes values 1 to 6, corresponding to the six experimental groups as follows: W-hen (group = 1), R-hen (=2), pendulum (=3), random motion (=4), stationary pattern (=5) and untrained control (= 6), respectively. Sex constitutes a categorical variable that is either male or female.
Here, we have one variable super_group that takes values 1 or 0, depending on how the six groups are further allocated to two super-groups. For example, an allocation [1,2,3 / 4,5,6] denotes a situation in which chicks of the first super-group (W-hen (1), R-hen (2), and pendulum (3); super_group = 1) behaved similarly, and chicks of the second super-group (random motion (4), stationary pattern (5), and untrained control (6); super_group = 0) also behaved similarly, but a difference occurred between the two super-groups. In order to investigate the interaction between training condition and sex, we allocated the groups to super-groups independently for males and females. We thus constructed five allocations ([1 / 2,3,4,5,6], [1,2 / 3,4,5,6], [1,2,3 / 4,5,6], [1,2,3,4 / 5,6], and [1,2,3,4,5 / 6]), therefore 25 (=5 × 5) allocations in total after considering all possible combinations for males and females. Note that 20 of these 25 allocations represented sex differences, and the other five did not. AICs were calculated for each allocation, so that we could exhaustively search the pattern of super-groups that most closely matched the observed preference.
Effects of day (age) were examined together with the factor sex in both experiments 1 and 2 by constructing the following models (type C). Data obtained in the random motion and untrained groups were included.
Here, we have three explanatory variables (sex, training, and age) together with two interaction terms (sex × training and sex × age). Sex denotes a categorical variable (either male or female), whereas training represents whether chicks were trained or not (random motion; training = 1, untrained; training = 0). Age represents whether chicks were tested at day 2 (age = 0) or day 5 (= 1).
Results
Experiment 1: BM preference
Following training with animation, day-2 males preferred W-hen to R-hen in test, irrespective of whether they had been exposed to W-hen or other stimulus types (R-hen, pendulum and random motion) (Fig. 2). On the other hand, among females, only day-2 chicks that had been trained with W-hen stimuli showed a preference for W-hen. In the following, we will show two lines of statistical computation for these conclusions.
Experiment 1: walking hen versus rotating hen. Induced preference for biological motion in newly hatched males (experiment 1). Difference in stay time (walking hen (W-hen) minus rotating hen (R-hen), sec, mean ± SEM) in test trials are shown for each group of chicks. Open and filled columns indicate data obtained in day-2 and day-5 chicks, respectively. Groups differed in the point-light animations used in the training trials: walking hen (W-hen), rotating hen (R-hen), pendulum, random motion (random m.), stationary pattern (stationary). Data were compared together with those obtained in untrained control chicks (untrained). For statistics, see text and Tables 2, 3 and 4
The effects of visual experiences were supported by the GLM analysis based on the type A formulation (Table 2). The full model (composed of group and sex) gave rise to the smallest AIC (1,237.8). In contrast, the partial models (composed only of group or sex) exhibited large AICs, and the AIC for the null model (sex and group variables not included) was even larger (1,257.2). We therefore examined whether the effects of visual experiences differed between males and females in the following analysis using the type B formulation.
Differences between males and females were confirmed (Table 3). Four allocations were chosen for the smallest AICs and compared with the five allocations that did not assume sex differences, that is, the allocations with identical super-grouping between males and females. The combination of super-groups ([1,2,3,4 / 5,6] for males and [1 / 2,3,4,5,6] for females) exhibited substantially smaller AIC (1,222.3) than the second (1,232.9), the third (1,235.2 and the fourth models (1,235.9). On the other hand, all five models without sex differences gave rise to larger AICs (1,241.9–1,254.2), and the AIC of the null model (no super-grouping included) was largest (1,257.2).
In males (but not in females), individuals with higher approach scores during training exhibited a stronger preference for BM. In Fig. 3, data obtained in four groups (W-hen, R-hen, pendulum, and random motion) are plotted against the number of approaches (open circles). A Spearman rank test revealed a significant correlation among males (r = 0.45, t = 2.626, 0.01 < p < 0.05, n = 29) but not among females (r = −0.11, t = 0.635, 0.05 < p, n = 34). Stationary pattern group data are shown as gray disks (Fig. 3). It should be noted that the number of approaches in this group (ranging from 13 to 130 in seven males and from 36 to 109 in seven females) overlapped with those in the other four groups, although the preference of these chicks was distributed around 0 s, as in the untrained chicks (data not plotted). We therefore conclude that the locomotor activity involved in training alone does not cause the BM preference we observed in testing.
Influences of approach count in training on biological motion preference (experiment 1). Difference in stay time (y axis) was plotted against the number of approaches to the monitors in training trials (x axis). Symbols represent individual chicks. Open circles indicate chicks in the “W-hen,” “R-hen,” “pendulum” and “random motion” groups. Gray circles indicate chicks in the group “stationary” (stationary pattern); note that these chicks also showed a considerable number of approaches in training, but failed to show distinct preferences in testing. Day-2 data were merged, and day-5 data and untrained data (control at both ages) were not included
The effects of age were examined in groups trained with random motion. A GLM analysis based on type C formulation revealed a clear contribution of age, but interaction terms with sex were not included among the best three models with the smallest AIC (Table 4).
Experiment 2: Hen preference
When trained with random motion, females (but not males) preferred the W-hen to the W-cat stimuli in both ages (days 2 and 5, Fig. 4). No preference was found in trained males or naïve chicks of either sex. GLM analysis based on the type C formulation (Table 5) revealed a clear contribution of the interaction term (sex × training), but age was not included among the best 3 models with the smallest AIC.
Experiment 2: walking hen versus walking cat. Induced preference for hen-like animation in females (experiment 2). Differences in stay time (W-hen minus W-cat) are shown as mean ± SEM. Open and filled columns indicate data obtained in day-2 and day-5 chicks, respectively. Data obtained from chicks trained with random motion were compared with untrained control data. See Table 5 for statistics
Discussion
BM preference is biologically predisposed and not learned
The present results revealed two major findings: (1) BM preference has an innate basis, but (2) it can be induced for functional expression in the early post-hatch period, particularly in males. In experiment 1, males trained with any animation stimulus preferred the W-hen to the R-hen stimuli at testing (Fig. 2). It should also to be noted that males trained with the pendulum or random animations preferred the W-hen stimulus, even though they had never seen it before. The results of the present study thus differ from previous reports, in which animals had the opportunity to memorize elements of the point-light animations.
A preference for BM could occur via specific learning through the chick’s own locomotion, as has been reported in humans (Casile and Giese 2006). However, this is not plausible in the present case, because the males trained with random motion walked a considerable distance but did not show a preference for W-hen stimuli (Fig. 3). However, no clear preference was found in the untrained chicks, in contrast to the previous report by Vallortigara et al. (2005). The discrepancy may be ascribed to the much smaller sample sizes of the present study, different genetic backgrounds of the subject chicks, or the pretreatment of the chicks tested in the previous report (Vallortigara et al. 2005). Further studies using domestic chicks of different strains and studies using different species of Galliformes are needed.
Possible involvement of induced BM preference in imprinting
The current results suggest that imprinting is a complex phenomenon involving multiple processes, in which innate preference and memory formation interact. In the present study, chicks that actively moved between the two opposing monitors tended to show a stronger preference for BM (Fig. 3, males). Such an activity dependence has been documented in imprinting since Hess (1958, 1959) reported “the law of effort,” which states that the further a chick runs, the more intensively it is imprinted.
Furthermore, the BM preference appeared only in day-2 chicks and was not found in day-5 chicks (Fig. 2; Table 4), similarly to the sensitive period in imprinting (Hess 1958, 1959; Bateson 1979). This finding suggests that, in nature, the induced BM preference may help chicks learn the visual features of their mother hen more effectively, as Vallortigara et al. (2005) previously discussed.
Ecological accounts of sex differences
The sex differences in BM preference found in this study may be caused by differences in reproductive strategies. In mate choice, females are generally choosier than males, and choices are often based on motion perception. For example, in wild red jungle fowls (Gallus gallus, the ancestor of domestic chickens), females choose males based on their external traits (e.g., morphology of cockscomb) and courtship displays (Zuk et al. 1995).
The notion that females are choosier is also supported by the results of experiment 2, in which females preferred the W-hen to the W-cat stimulus at both days 2 and 5. Since both W-hen and W-cat are BM animations, females would be expected to exhibit a preference based on more specific attributes than those examined in experiment 1. Future studies should examine whether a similar sex difference also occurs in sexually mature females and males.
Alternatively, the sex difference may be explained in terms of the different uses of space by males and females. In the case of domestic chickens, a dominant male maintains and patrols a large territory where a number of females reside (McBride et al. 1969). These ecological contexts are in accord with the notion that male chickens are likely to seek novelty, to establish a large territory. The sex difference found in experiment 1 might thus be explained by a difference in novelty-seeking behavior. However, this cannot account for the sex difference found in experiment 2.
A third alternative explanation for the sex differences we observed is the influence of genetic differences in domesticated chickens. The strain of chickens used in the present study was bred specifically so that the flight feathers of female chicks grow faster than those of males, as a cue for determining the sex of hatchlings. A series of sex-linked alleles are reported to influence various other traits such as growth rate, sexual maturity, and the rate of survival (Dunnington et al. 1986; Tamura et al. 1987). It is therefore critically important to examine whether similar sex differences also occur in other birds of the order Galliformes such as quails, which have been less selectively bred than domesticated chickens.
References
Bateson P (1979) How do sensitive periods arise and what are they for? Anim Behav 27:470–486
Blake R (1993) Cats perceive biological motion. Psychol Sci 4:54–57
Brown J, Kaplan G, Rogers LJ, Vallortigara G (2010) Perception of biological motion in common marmosets (Callithrix Jacchus): by females only. Anim Cogn 13:555–564
Casile A, Giese MA (2006) Nonvisual motor training influences biological motion perception. Curr Biol 16:69–74
Dittrich WH, Lea SEG, Barrett J, Gurr PR (1998) Categorization of natural movements by pigeons: visual concept discrimination and biological motion. J Exp Anal Behav 70:281–299
Dunnington EA, Siegel PB, Gross WB (1986) Sex-linked feathering alleles (K, k+) in chickens of diverse genetic backgrounds. Avian Pathol 15:139–148
Fridolfsson A, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116–121
Hess EH (1958) “Imprinting” in animals. Sci Am 198:81–90
Hess EH (1959) Imprinting. Science 130:133–141
Horn G (2004) Pathways of the past: the imprint of memory. Nat Rev Neurosci 5:108–120
Izawa E, Yanagihara S, Atsumi T, Matsushima T (2001) The role of basal ganglia in reinforcement learning and imprinting in domestic chicks. NeuroReport 12:1743–1747
Johansson G (1973) Visual perception of biological motion and a model for its analysis. Percept Psychophys 14:201–211
Johnson MH, Horn G (1988) Development of filial preferences in dark reared chicks. Anim Behav 36:675–683
Johnson MH, Bolhuis JJ, Horn G (1985) Interaction between acquired preferences and developing predispositions during imprinting. Anim Behav 33:1000–1006
MacKinnon LM, Troje NF, Dringenberg HC (2010) Do rats (Rattus norvegicus) perceive biological motion? Exp Brain Res 205:571–576
Matsushima T, Izawa E, Aoki N, Yanagihara S (2003) The mind through chick eyes: memory, cognition and anticipation. Zool Sci 20:395–408
McBride G, Parer IP, Foenander F (1969) The social organization and behavior of the feral domestic fowl. Anim Behav Monogr 2:127–181
Parron C, Deruelle C, Fagot J (2007) Processing of biological motion point-light displays by baboons (Papio papio). J Exper Psychol Anim Behav Proc 33:381–391
Regolin L, Tommasi L, Vallortigara G (2000) Visual perception of biological motion in newly hatched chicks as revealed by an imprinting procedure. Anim Cogn 3:53–60
Simion F, Regolin L, Bulf H (2008) A predisposition for biological motion in the newborn baby. PNAS 105:809–813
Tamura C, Takahashi T, Tanaka M (1987) Effects of late feathering gene on growth, laying performance and egg qualities. Bull Takikawa Anim Husb Exp Stn 23:29–34 (in Japanese)
Tomonaga M (2001) Visual search for biological motion patterns in chimpanzees (Pan troglodytes). Psychologia 44:46–59
Vallortigara G (1992) Affiliation and aggression as related to gender in domestic chicks (Gallus gallus). J Comp Psychol 106:53–57
Vallortigara G, Regolin L (2006) Gravity bias in the interpretation of biological motion by inexperienced chicks. Curr Biol 16:R279–R280
Vallortigara G, Regolin L, Marconato F (2005) Visually inexperienced chicks exhibit spontaneous preference for biological motion patterns. PLoS Biol 3:1312–1316
Workman L, Andrew RJ (1989) Simultaneous changes in behavior and in lateralization during the development of male and female domestic chicks. Anim Behav 38:596–605
Zuk M, Popma SL, Johnsen TS (1995) Male courtship displays, ornaments and female mate choice in captive red jungle fowl. Behaviour 132:821–836
Acknowledgments
This study was supported by grant-in-aid for scientific research to T. M. from the Japanese Society for Promotion of Sciences (#22570070) and from the Japanese Ministry for Education, Culture, Sports, Science and Technology (#22120502) for Innovative Area on the “Study of the neural dynamics for understanding communication in terms of complex hetero systems.” Helpful comments on the manuscript by Dr. Masayo Soma and valuable suggestions on the statistical computations by Dr. Ai Kawamori are highly appreciated. We also wish to acknowledge constructive comments and helpful suggestions by the anonymous referees.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miura, M., Matsushima, T. Preference for biological motion in domestic chicks: sex-dependent effect of early visual experience. Anim Cogn 15, 871–879 (2012). https://doi.org/10.1007/s10071-012-0514-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-012-0514-x