Abstract
Gaze following allows individuals to detect the locus of attention of both conspecifics and other species. However, little is known about how this ability develops. We explored the emergence of bobwhite quail hatchlings’ ability to track human gaze by assessing their avoidance behavior in an open arena under five testing conditions: (1) a Direct Gaze condition, in which an experimenter looking down was positioned above one of two approach areas; (2) a Gaze Follow condition in which an experimenter, positioned equidistant between two approach areas, directed his/her gaze towards one of the areas; (3) a Masked Gaze Follow condition, in which the experimenter wore a mask during the Gaze Follow test; (4) a Deprived Face Experience condition, in which hatchlings were deprived of experience with human faces prior to the Gaze Follow test; and (5) a Control condition in which no experimenter was present during testing. Results revealed that hatchlings from the Direct Gaze condition preferred the non-gazed approach area at all ages tested. Hatchlings from the Gaze Follow condition preferred the non-gazed approach area at 48 and 72 h, but not at 24 h of age. In contrast, hatchlings from the Masked Gaze Follow, Deprived Face and Control conditions did not prefer either approach area at any age tested. These results indicate that experience with human faces plays a key role in the rapid emergence of gaze following behavior in bobwhite quail hatchlings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability to detect and follow gaze has been reported in many species of birds and mammals (e.g., Emery 2000; Flom et al. 2007; Moore and Dunham 1995). Gaze following allows for the detection of the locus of attention of both conspecifics and other species and likely provides important information about the location of potential prey or predators. Not surprisingly, gaze following has been demonstrated in a number of socially living mammals, including non-human primates, dogs, and goats (Emery 2000; Kaminski et al. 2001; Miklósi et al. 1998). Gaze following behavior has also been extensively studied in developmental psychology and a large body of research has documented the relevance and function of gaze following in human infants for the development and organization of social attention and social cognition (e.g., Flom et al. 2007; Johnson and Farroni 2003; Mundy et al. 1992; Tomasello 1995).
Despite the large body of work on gaze following in humans and nonhuman primates relatively little research has been conducted on birds. Gaze following abilities have, however, been reported to play a role in predator avoidance in several avian species. For example, Ristau (1991) conducted field experiments with piping and Wilson’s plovers and found that these birds adjust their injury-feigning displays based on a human intruder’s direction of gaze. Thus, if a human intruder walked and gazed towards the nest, these birds would place themselves in the intruder’s line of gaze and perform injury-feigning displays as if to distract the human intruder from the nest. A study conducted with bee-eaters likewise demonstrated that the frequency of entering their nest was significantly less when a human experimenter’s line of gaze was directed toward the nest. In contrast, if the experimenter positioned him or herself with their line of gaze to the nest occluded by a barrier, or their gaze directed away from the nest, the birds’ frequency of entering their nest increased (Watve et al. 2002).
The ability to discriminate different gaze directions has also been studied in common ravens. Bugnyar et al. (2004) showed that ravens can successfully track the gaze of a human experimenter to a specific location in a room. They can also position themselves in order to see what the human experimenter is seeing when their line of gaze is obstructed by a barrier. Schloegl et al. (2007) investigated the development of these abilities in ravens during the first 10 months following hatching. Young ravens responded to humans looking up soon after fledging, but did not track gaze behind a visual barrier (as seen in adult ravens) until 4 months later, suggesting the importance of experience with humans in the development of gaze following behavior.
Few studies have explored the development of gaze detection in other avian species. However, several studies have indicated that human gaze directed toward domestic chickens is more effective at increasing the duration of tonic immobility than is an averted gaze, suggesting that chicks are able to discriminate between direct or averted gaze relatively soon after hatching (Gallup et al. 1971, 1972). Further, 3-day-old domestic chicks have been shown to take longer to move when placed in a novel environment if they are under the gaze of a human-like mask (Vallortigara and Zanforlin 1988). In a recent study of hemispheric lateralization, Rosa Salva et al. (2007) assessed 8-day-old domestic chicks’ gaze following abilities by recording their latency of approach to a surface containing food versus a clear surface (without food). During this procedure a human-like mask with adjustable eyes was positioned between the two surfaces and the eyes were directed toward the surface containing food. The authors reported differential latencies of responding depending on whether chicks received exposure to human faces during rearing. Chicks that received experience with human faces showed longer latencies to the averted gaze (i.e., the mask’s eyes were positioned away from the surface with food) and chicks naïve of exposure to human faces showed longer latencies of approach to the surface containing food when the mask’s gaze was directed towards the surface with food. This study provides additional support that chicks are sensitive to the direction of eye gaze and that this sensitivity is affected by experience with human faces during early development. However, it remains unclear when gaze following abilities emerge in avian hatchlings and what features of visual experience with human faces affects the emergence of gaze following abilities in the period following hatching.
In the present study, we explored the development of gaze following in Northern bobwhite quail (Colinus virginianus) hatchlings, a highly social and precocial avian species. We assessed 1, 2, and 3-day-old bobwhite quail hatchlings’ ability to track the gaze of a human experimenter in an open arena. We were particularly interested in whether bobwhite quail hatchlings’ ability to follow gaze would be present immediately following hatching or would emerge with increasing experience and exposure to humans during postnatal rearing. We hypothesized that early experience with faces would play a role in bobwhite quail hatchlings’ ability to follow human gaze.
Methods
Subjects
Subjects were incubator reared bobwhite quail hatchlings (C. virginianus). Fertilized unincubated eggs were received weekly from a commercial supplier and set in a BSS-160 Grumbach Incubator maintained at 75–80% relative humidity and 37.5°C. Following hatching, groups of 15–20 hatchlings were socially reared in large plastic tubs (25 cm wide × 15 cm high × 45 cm long) placed on shelves in a Nuaire Model NU-605-500 Animal Isolator (Plymouth, MN), which provided continuous filtered air. Ambient air temperature was maintained at approximately 30°C. Hatchlings also had continuous access to food and water except during the testing sessions. Hatchlings received exposure to various human caretakers several times a day during the changing of their food and water.
Apparatus
Postnatal behavioral tests took place in an arena 130 cm in diameter, surrounded by a circular wall 60 cm in height. The arena surface was painted flat black and the walls of the testing arena were insulated with a layer of foam to attenuate reverberation. The arena walls were covered with an opaque black curtain. A video camera mounted directly above the arena allowed for remote observation and data collection. Two semi-circular approach areas, each comprising approximately 5% of the total area of the testing arena and directly opposite to one another, were demarcated on a remote video monitor. The two approach areas each contained a 4 in. speaker mounted to the arena wall and hidden behind the black curtain (to allow for auditory stimulation). Both speakers were powered by separate RCA SA-155 integrated stereo amplifiers and the auditory stimuli were played by two Sony CDP-XE370 compact disk players. Ambient room temperature in the testing room was maintained between 29° and 32°C.
Procedure
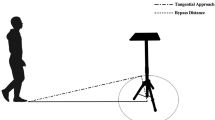
Bobwhite quail hatchlings were tested during one of five conditions: (1) a Direct Gaze condition, which presented a live human experimenter positioned directly above one of the two approach areas broadcasting identical bobwhite maternal assembly calls, looking down (Fig. 1a), (2) a Gaze Follow condition, which presented a human experimenter also looking down but positioned equidistant between the two approach areas broadcasting the identical bobwhite maternal calls. When hatchlings approached a pre-designated approach area, the experimenter directed his/her gaze toward that approach area (Fig. 1b), (3) a Masked Gaze Follow condition, which was identical to the Gaze Follow condition except that the human experimenter wore a mask throughout the testing procedure. The mask was designed such that the facial features of the individual wearing the mask were not visible to the hatchlings. It consisted of a baseball cap with several layers of a thin black mesh-like material positioned over the face that allowed one to see through the mask but did not allow the hatchlings to see the experimenter’s face (Fig. 2). Thus, hatchlings in this condition received ongoing experience with human faces during rearing but did not receive exposure to a human face during the testing trial (Fig. 3), (4) a Deprived Face Experience condition, in which hatchlings were deprived of all experience with human faces following hatching and were subsequently tested exactly as the hatchlings from the Gaze Follow condition described above. Experience with human faces was eliminated by having research assistants always wear the mask when interacting with this group of hatchlings, except during testing, and (5) a Control condition in which the human experimenter was not present during testing trials. This condition controlled for any naïve bias for either of the two approach areas in the testing arena. In the four conditions utilizing a human experimenter, the approach area containing the directed gaze was counter balanced across trials to control for any possible side biases. Three different experimenters participated as gazers and testers over the course of the study.
Testing
Hatchlings from all groups were tested individually at 24, 48, or 72 h following hatching. Testing consisted of a 5 min (300 s) simultaneous-choice test between two opposing approach areas broadcasting an identical unfamiliar bobwhite maternal call. This call was an individual variant of the species-typical bobwhite maternal assembly call, recorded in the field (Call A; Heaton et al. 1978). The bobwhite maternal call was used to increase attraction to the approach areas and to minimize freezing behavior that might occur during the testing procedure. The sound intensity of the maternal call broadcasted from each speaker was adjusted to peak at 65 dB, measured from the start position where each hatchling was introduced into the arena. Hatchlings were scored on both their latency of approach and the duration of time they spent in each of the two approach areas. Latency was defined as the amount of time (in seconds) that elapsed from the onset of the trial until the hatchling first entered an approach area. Duration was defined as the cumulative amount of time (in seconds) the hatchling remained in an approach area over the course of the testing trial. Each hatchling was tested only once. A Visual Basic computer program allowed for semi-automated collection of latency and duration of response to the test stimuli. Any hatchling that did not enter any approach area received a score of 300 s for latency (i.e., the length of the trial) and 0 s for duration and was considered a non-responder. These hatchlings were excluded from subsequent analyses.
Data analysis
The primary data of interest were measures of duration (in seconds) spent in the two approach areas during the test trials. Several analyses were performed on this interval data. First, individual preference scores (used in a number of prior studies of perceptual discrimination in bobwhite quail, see Lickliter and Hellewell 1992; Lickliter and Lewkowicz 1995 for examples) were determined. A “preference” was given if a hatchling spent at least twice as much time in one approach area as the other. A “no preference” for an approach area was given if a hatchling approached the two areas during a trial but did not spend at least twice as much time in one approach area as the other. These preference scores were evaluated by the Chi-Square Goodness-of-Fit Test to determine if the majority of subjects in a condition demonstrated a preference for the non-gazed approach area, the gazed approach area, or showed no preference during the simultaneous choice test.
Second, a proportion of total duration time (PTDT) was calculated from the time hatchlings spent in the non-gazed approach area relative to the total duration time spent in both non-gazed and gazed approach areas. A proportion of 0.50 reflects chance responding, whereas a proportion greater than 0.50 reflects a majority of time spent in the non-gazed approach area. A proportion less than 0.50 reflects a majority of time spent in the gazed approach area. One sample t tests were used to evaluate whether the mean PTDT spent in the non-gazed approach area was significantly greater than chance for a particular group. Additional between-groups comparisons of mean PTDT to the non-gazed area were evaluated with a one-way analysis of variance (ANOVA) and a post hoc multiple comparisons procedure (LSD). Statistical significance was set at alpha <0.05.
Results
The observed preference scores for all groups are shown in Table 1. Results revealed that hatchlings in the Direct Gaze group demonstrated a significant preference for the non-gazed approach area over the gazed approach area at 24, 48, and 72 h of age (χ² = 19.400 P < 0.001, df = 2; χ² = 20.571, P < 0.001, df = 1, and χ² = 27.793, P < 0.001, df = 2, respectively). Hatchlings from the Gaze Follow group also showed a significant preference for the non-gazed approach area at 48 h (χ² = 12.07, P < 0.001, df = 2) and 72 h of age (χ² = 38.60, P < 0.001, df = 2), but not at 24 h following hatching (χ² = 0.600; P = 0.741, df = 2). In contrast, hatchlings in the Masked Gaze Follow group did not show a significant preference for either approach area at 24, 48 and 72 h of age (χ² = 0.800, P = 0.670, df = 2, χ² = 3.800, P = 0.150, df = 2, and χ² = 1.800, P = 0.407, df = 2, respectively). Likewise, the Deprived Face Experience group did not show a significant preference for either approach area at 24, 48 and 72 h of age (χ² = 0.000; P = 1.000, df = 2, χ² = 1.357; P = 0.507, df = 2, and χ² = 0.500; P = 0.779, df = 2, respectively). Results for the Control group also did not show a significant preference for either approach area at 24 and 48 h of age (χ² = 4.200; P = 0.122, df = 2 and χ² = 5.600, P = 0.061, df = 2, respectively). However, the 72 h control group contained a significant number of hatchlings assigned to the “no preference” category (χ² = 7.200, P = 0.027, df = 2). Thus, the results of the Control group indicated that naïve hatchlings did not show a preference for either of the two approach areas at any age tested (Table 1).
In addition, parametric analyses were performed on all the experimental groups. One-sample t tests were performed on the mean PTDT spent in the non-gazed approach area against the chance value of 0.50 for the Direct Gaze group. Results revealed a significant PTDT to the non-gazed approach area at 24, 48, and 72 h of age (t 30 = 3.642, P = 0.001, t 28 = 7.617, P < 0.001, and t 28 = 3.829, P = 0.001, respectively). A one-way ANOVA compared the mean PTDT for the non-gazed area across all ages for the Direct Gaze group and was found to be non-significant at an alpha level of 0.05 (F 2,87 = 1.445, P = 0.242). These results demonstrate that bobwhite quail hatchlings as young as 1 day old actively avoid a human experimenter’s direct gaze. Hatchlings from the Gaze Follow group showed a significant PTDT to the non-gazed approach area at 48 h (t 28 = 2.470, P = 0.020) and 72 h (t 30 = 6.095, P < 0.001), but not at 24 h of age (t 30 = 0.37, P = 0.714). A one-way ANOVA compared the mean PTDT for the non-gazed area across all ages for the Gaze Follow group and was found to be statistically significant (F 2,88 = 5.439, P = 0.006). Post hoc (LSD) tests indicated that the mean PTDT for the non-gazed area of the 72 h group (M = 0.779, SD = 0.251) was significantly greater than the mean PTDT for the non-gazed area of the 24 h group (M = 0.522, SD = 0.321). However, the mean PTDT for the non-gazed area of the 48 h group (M = 0.655, SD = 0.331) did not differ significantly from the other groups of this condition. These results indicate that 2- and 3-day-old quail hatchlings are able to actively avoid a human experimenter’s line of gaze with the oldest (3-day-old) age group showing the strongest preference to the non-gazed approach area. However, this ability to track the direction of human gaze was not seen in 1-day-old hatchlings. In contrast, the Masked Gaze Follow group did not show a statistically significant PTDT for the non-gazed area at 24, 48, and 72 h of age (t 30 = −1.403, P = 0.171, t 30 = 1.605, P = 0.119, and t 30 = −0.493, P = 0.626, respectively). One-way ANOVAs for the Masked Gaze Follow and Deprived Face Experience groups were also non-significant at an alpha level of 0.05 (F 2,89 = 2.193, P = 0.118 and F 2,87 = 0.639, P = 0.530, respectively) (Table 2).
Discussion
In the present study we investigated the emergence of gaze following abilities in a precocial avian species, the bobwhite quail. Although previous work has demonstrated that birds are adept followers of human gaze, to our knowledge no studies have assessed this ability in 1 to 3-day-old avian hatchlings. Our results demonstrate the rapid emergence of gaze following behavior in bobwhite quail hatchlings. In addition, our results indicate that postnatal visual experience with human faces plays a critical role in this rapid emergence of gaze following behavior in the days following hatching.
In the Direct Gaze group we assessed whether quail chicks would show an aversion to the proximity of human gaze. We assessed chicks’ preferences between an approach area with a maternal call and a human gazer positioned directly above it and an approach area with the maternal call alone. The results of this group demonstrated that 24, 48, and 72-h-old hatchlings avoid maintaining proximity to a human’s direct gaze, indicated by a significant preference for the approach area containing the maternal call without the experimenter’s direct gaze at all ages tested. This result indicates that an aversion to direct human gaze is present by 24 h following hatching in quail hatchlings.
The Gaze Follow group likewise assessed hatchlings’ avoidance of human gaze. However, this condition was designed to determine whether hatchlings can track a human’s line of gaze to direct their avoidance behavior. We tested whether avoidance of a particular approach area would be observed when the experimenter was positioned between both approach areas and their line of gaze was directed towards one of the two approach areas. The results of this group showed that 48 and 72-h-old, but not 24-h-old hatchlings showed a preference for the non-gazed approach area. These results suggest that bobwhite quail hatchlings are not only avoidant of a human’s direct gaze, they also avoid a human’s line of gaze from a distal location. The lack of aversion to gaze observed in 24-h-old chicks suggests that some feature(s) of postnatal experience play a role in the emergence of the ability to follow human gaze.
One question raised by the results of the Gaze Follow group was whether chicks were tracking the eye gaze of the experimenter or whether they were simply responding to the head movement and orientation of the human experimenter as he or she looked towards one of the two approach areas. Of course, it is possible that head movement and orientation is used in combination with the eyes for successful gaze direction detection. Previous comparative work with non-human primates has suggested that eyes alone are not sufficient to facilitate gaze following behaviors (e.g., Povinelli et al. 1999; Peignot and Anderson 1999); rather, both head and eye cues together are necessary for gaze following. The Masked Gaze Follow condition assessed the effectiveness of head movement and orientation in facilitating hatchlings’ gaze following. This testing procedure was identical to the Gaze Follow procedure, except the human experimenter wore a mask during the entire testing session. As previously described, this mask prevented the hatchlings from seeing the features of the experimenter’s face (including the eyes). If movement and orientation of the head (and not eye gaze) plays a key role in young hatchlings’ ability to track gaze, then the hatchlings from this group should have responded similar to the hatchlings in the Gaze Follow condition. However, hatchlings from the Masked Gaze Follow condition did not appear to track the experimenter’s line of gaze, showing no preference for either the gazed-at or non-gazed approach area during testing. These results indicate that head movement and orientation does not provide sufficient visual information for quail hatchlings to track the direction of human gaze. Additional research is needed to determine if both head movement and eyes combined are necessary for bobwhite quail hatchlings to successfully track human gaze, or if eye direction alone is sufficient.
Finally, we reasoned that if experience with human faces plays a role in the emergence of hatchlings’ gaze following behavior (as suggested by the results from the Gaze Follow group), then attenuating the amount of experience hatchlings received with human faces should disrupt or delay the emergence of their gaze following behavior. The Deprived Face Experience group explored this hypothesis. The results from this group revealed that hatchlings tested at 24, 48, or 72 h following hatching with the experimenter’s head orientation and eyes visible showed no preference for the non-gazed or gazed approach areas. Thus, rearing hatchlings without visual exposure to human faces disrupted their ability to track the direction of human gaze in the days following hatching. These results are the first to provide evidence that visual experience with human faces plays an integral role in hatchlings’ ability to track human gaze and are in line with previous findings that suggest that exposure to human caretakers sensitizes young birds’ ability to follow directed human gaze (Schloegl et al. 2007).
References
Bugnyar T, Stowe M, Heinrich B (2004) Ravens, Corvus corax, follow gaze direction of humans around obstacles. Proc R Soc B 271:1331–1336
Emery NJ (2000) The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci Behav Rev 24:581–604
Flom R, Lee K, Muir D (2007) Gaze-following: its development and significance. Erlbaum, Mahwah
Gallup GG, Nash RF, Ellison AL (1971) Tonic immobility as a reaction to predation: artificial eyes as a fear stimulus for chickens. Psychon Sci 23:79–80
Gallup GG, Cummings WH, Nash RF (1972) The experimenter as an independent variable in studies of animal hypnosis in chickens (Gallus gallus). Anim Behav 20:166–169
Heaton MB, Miller DB, Goodwin DG (1978) Species-specific auditory discrimination in bobwhite quail neonates. Dev Psychobiol 11:13–21
Johnson MH, Farroni T (2003) Perceiving and acting on the eyes: the development and neural basis of eye gaze perception. In: Pascalis O, Slater A (eds) The development of face processing in infants and early childhood. Nova Science, Hauppauge, pp 155–167
Kaminski J, Bräuer J, Call J, Tomasello M (2001) Goats follow the visual gaze of conspecifics. Adv Ethol 36:174–180
Lickliter R, Hellewell T (1992) Contextual determinants of auditory learning in bobwhite quail embryos and hatchlings. Dev Psychobiol 25:1–24
Lickliter R, Lewkowicz DJ (1995) Intersensory experience and early perceptual development: attenuated prenatal sensory stimulation affects postnatal auditory and visual responsiveness in bobwhite quail chicks. Dev Psychol 31:609–618
Miklósi A, Polgárdi R, Topál J, Csányi V (1998) Use of experimenter-given cues in dogs. Anim Cogn 1:113–121
Moore C, Dunham PJ (1995) Joint attention: its origins and role in development. Erlbaum, Hillsdale
Mundy P, Kasari C, Sigman M (1992) Joint attention, affective sharing, and intersubjectivity. Infant Behav Dev 15:377–381
Peignot P, Anderson JR (1999) Use of experimenter-given manual and facial cues by gorillas (Gorilla gorilla) in an object-choice task. J Comp Psychol 113:253–260
Povinelli DJ, Bierschwale DT, Reaux JE, Cech CG (1999) Comprehension of seeing as a referential act in young children, but not juvenile chimpanzees. Br J Dev Psychol 17:37–60
Ristau CA (1991) Aspects of the cognitive ethology of an injury-feigning bird, the piping plover. In: Ristau CA (ed) Cognitive ethology: the minds of other animals. Erlbaum, Hillsdale, pp 91–126
Rosa Salva O, Regolin L, Vallortigara G (2007) Chicks discriminate gaze with their right hemisphere. Behav Brain Res 177:15–21
Schloegl C, Kotrschal K, Bugnyar T (2007) Gaze following in common ravens: ontogeny and habituation. Anim Behav 74:769–778
Tomasello M (1995) Joint attention and social cognition. In: Moore C, Dunham P (eds) Joint attention: its origins and role in development. Erlbaum, Hillsdale, pp 103–130
Vallortigara G, Zanforlin M (1988) Open-field behavior of young chicks (Gallus gallus): antipredatory responses, social reinstatement motivation, and gender effects. Anim Learn Behav 16:359–362
Watve M, Thakar J, Kale A, Puntambekar S, Shaikh I, Vaze K, Jog M, Paranjape S (2002) Bee-eaters (Merops orientalis) respond to what a predator can see. Anim Cogn 5:253–259
Acknowledgments
This research was supported by grants NICHD RO1 HD048423 and NIGMS R25 GM61347. Data was collected at the Developmental Psychobiology Lab at Florida International University. Animal care and use complied with both institutional and federal (NIH) guidelines, as well as the ethical standards for the treatment of animals endorsed by the American Psychological Association and the International Society for Developmental Psychobiology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaime, M., Lopez, J.P. & Lickliter, R. Bobwhite quail (Colinus virginianus) hatchlings track the direction of human gaze. Anim Cogn 12, 559–565 (2009). https://doi.org/10.1007/s10071-009-0214-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-009-0214-3