Abstract
This study reports the various nutritional components of Selaginella tamariscina, which is traditionally used in folk or Chinese medicine. The iron nutrient content in S. tamariscina powder was 0.94 ± 0.06 mg/100 g powder, whereas selenium was present in a small amount, which showed strong antioxidant power. The total phenolic content of S. tamariscina powder was 8.65–11.61 mg gallic acid equivalents/g. S. tamariscina showed antioxidant activity in 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity. The ferric reducing antioxidant power of S. tamariscina powder was higher in the ethanol extract. Additionally, the ethanol extract demonstrated antimicrobial activity against Bacillus subtilis KCTC 2189. The level of high-density lipoprotein-cholesterol in the blood of ICR mice was significantly higher in the HF 20% + S. tamariscina 20% group than in the other groups (p < 0.05). The present study demonstrates that S. tamariscina, an abundantly existing plant, possesses antimicrobial, antioxidant, and anticytotoxic activities. S. tamariscina powder has potential as a functional food.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With an improvement in income levels and a changing diet, physiological benefits such as antioxidant, anticancer, and antifungal activities from plants have spurred great interest among both consumers and researchers, and the elucidation of these properties in plants has become an area of active research. Therefore, there has been increasing interest in studying the functional aspects of commercial vegetables (Lee et al., 2005).
Selaginella tamariscina [Selaginella tamariscina (P. Beauv.) Spring] is commonly called Kwonbaek due to its resemblance with a fist-like pine tree. In addition, it has been described as “hand,” “butcher hand,” “boson hand,” “fireweed,” and as a perennial pine (Chu et al., 2016). S. tamariscina is an evergreen perennial plant native to mountain rock walls and belongs to the order Selaginellales, the family Selaginellaceae, and the genus Selaginella. They reach a height of approximately 20 cm. They have spores that are egg-shaped triangles with serrated edges hang one by one at the end of their small twigs, and there have four rows of scaly leaves. S. tamariscina contains flavones, phenols, polysaccharides such as amino acids and trehalose, and small amounts of tannins, such as apigenin, amentoflavone, hinokiflavone, and isocryptomerin, which have also been reported as flavones (Jung et al., 2006; Lee et al., 2009; Shin and Kim, 1991). S. tamariscina is used in folk medicine to treat the side effects of mental instability, tumor prevention and healing, renal function enhancement, stones, asthma, bronchial disease, and radiation therapy. S. tamariscina is also used to treat bleeding and maintain hemostasis (Chu et al., 2016; Park and Rhee, 1994). It is also used to alleviate menstrual pain or bruising, improve blood circulation, and has been known to have a negative metabolic effect (Chu et al., 2016; Park and Rhee, 1994). In previous studies (Chu et al., 2016; Hsin et al., 2013; Kim et al., 2015; Nguyen et al., 2015; Yang et al., 2013), S. tamariscina was found to inhibit acetylcholinesterase and pharyngeal cancer cell activity; biflavonoids isolated from S. tamariscina extracts reported impact. It has also been reported to decrease the secretion of matrix metalloproteinase (MMP)-2 and MMP-9 in osteoblasts, as well decrease the expression of genes associated with the p38 and Akt signaling pathways (Chu et al., 2016; Hsin et al., 2013; Kim et al., 2015; Nguyen et al., 2015; Won et al., 2018; Yang et al., 2013). In addition, amentoflavone, an active ingredient of S. tamariscina, inhibits beta-amyloid formation in PC-12 neurons and inhibits antioxidant activity and apoptosis in hippocampal tissues (Chu et al., 2016; Sasaki et al., 2015; Zhang et al., 2015).
As pointed out in the study by Kim et al. (2019), S. tamariscina is actively used as a medicinal herb in Korea. A difference in the biological activity according to the processing of foams has been reported (Lee et al., 2006), but research on their biological activity following different processing conditions such as extraction, drying, and cooking for practical use as a food is lacking. Various studies on S. tamariscina have been conducted worldwide, primarily in China. S. tamariscina has been widely used in folk medicine since ancient times, but research has been conducted in the fields of oriental medicine and pharmacology. However, this plant has not been scientifically studied in the field of food and nutrition in Korea. Recently, as it is known as a health food through broadcast media, continuous research is required regarding its nutritional quality in Korea. Therefore, in this study, S. tamariscina was tested to determine its usefulness as a functional food through general component analysis, as well as through antibacterial, antioxidant, and cytotoxicity assays. In addition, its biochemical activity in mice was observed by adding S. tamariscina powder to their high-fat diet.
Materials and methods
Materials and reagents
Selaginella tamariscina was collected from rocks near Mt. Bulam between June and September 2017. Samples were freeze-dried (FDTL-4504, Operon, Gyeonggi-do, Korea) between −50 and −40 °C, and then used as samples while stored in a −20 °C freezer. Twenty grams of each ground material was added to 500 mL of deionized water (DIW) and ethanol, respectively, and then extracted twice using a heating mantle with a reflux condenser for 3 h. The extract was centrifuged after compression filtration (6000 rpm, 30 min, 4 °C), the supernatant was concentrated under vacuum at 45 °C, and then lyophilized (LYPH-LOCK 12, Labconco, Kansas City, Mo, USA) to obtain a powder sample. Dry materials (1 g of natural product) were sonicated with 10 mL of 80% methanol for 1 h to prepare a 100 mg/mL stock solution. The samples were then cooled to room temperature for 24 h. The extract was centrifuged at 8000 rpm for 10 min. The supernatants were collected at 4 °C prior to use within 24 h. Both 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were purchased from Fluka (Buchs, Switzerland). Folin-Ciocalteu reagent (FC reagent), potassium persulfate, 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Gallic acid, sodium carbonate, sodium acetate, acetic acid, and FeCl3·6H2O were obtained from Riedel-de Haën (Seelze, Germany). The other chemicals and solvents used in this study were of analytical grade (Han et al., 2019; Lee et al., 2017).
General composition analysis
The moisture content of the S. tamariscina powder was determined using the atmospheric pressure drying method (FS-620, Tokyo Seisakusho Co., LTD., Osaka, Japan), and the crude protein was analyzed via the micro-Kjeldahl method using a crude protein automatic analyzer (Kjeltec TM 2300, FOSS, Höganäs, Sweden). Ash content was measured using the direct painting method (KL-160, Toyo Seisakusho Co., LTD., Osaka, Japan), and crude fat content was measured using the Soxhlet method (SOX606, LABTECH, Seoul, Korea) (Choi et al., 2016; Lee et al., 2008).
Mineral content analysis
The content of certain minerals, including copper, zinc, iron, selenium, and manganese, were analyzed according to the method suggested by Kim et al. (2007). Sample pretreatment was carried out via dry decomposition and the resulting product was filtered to obtain a test solution of up to 100 mL with distilled water. The pretreated test solution was analyzed using an inductively coupled plasma spectrometer (ICP-AES, inductively coupled plasma-atomic emission spectrophotometer, Z6100, Hitachi, Tokyo, Japan) (Choi et al., 2016).
Preparation of bacterial cultures and antibacterial assay
All microorganisms were purchased from the Korean Collection for Type Culture (Daejeon, Korea). The purchased microorganisms were inoculated into a broth and incubated for 12 h at 30 °C and 37 °C. Stock cultures were maintained at −80 °C in a broth containing glycerol (20% v/v). The antimicrobial activity of the extract was determined using the disk diffusion method (Seul and Yang, 2017; Kim et al., 2016). Briefly, after 18 h of culture, a bacterial suspension in saline was diluted to a density of 1–2 × 108 CFU/mL (McFarland standard of 0.5). The bacterial suspension was gently distributed onto the surface of Mueller–Hinton agar (MHA) using sterile cotton-tipped swabs. Each sample extract (20 µL) was added to a paper disc (6 mm in diameter) that was placed on the MHA. A disk containing ceftazidime, gentamycin, cefotaxime, or chloramphenicol was used as a positive control, and the diameter of the inhibition zone (mm) was measured after incubation at 35 ± 1 °C for 16–20 h.
Total phenolic content (TPC) assay
TPC was determined with Folin-Ciocalteu reagent, using gallic acid as a standard. A 20 µL aliquot of a diluted sample (the stock solutions were further diluted with distilled water) was added to 100 μL of FC reagent and mixed well. After 5 min, 300 μL of 20% sodium carbonate solution was added, and the mixture was vortexed. The samples were incubated for 2 h at room temperature in the dark. The absorbance was measured at 765 nm using a UV–vis spectrophotometer (Ultrospec 3100 Pro, Amersham Bio., Cambridge, UK). The results are expressed as gallic acid equivalents (mg GAE/g dry weight [DW]). All experiments were performed in triplicate (Waterhouse, 2002).
Ferric reducing antioxidant power (FRAP) assay
The antioxidant capacity of S. tamariscina powder was estimated according to the procedure described by Pulido et al. (2000). The FRAP reagent included 25 mL of 300 mM acetate buffer (pH 3.6), 2.5 mL 10 mM TPTZ solution in 40 mM HCl, and 2.5 mL 20 mM FeCl3·6H2O. FRAP reagent (900 μL), prepared fresh and warmed to 37 °C, was added to 90 μL of distilled water and 30 μL of sample, as with the reagent blank. The final sample dilution in the reaction mixture was 1:34. The absorbance at 595 nm was measured every 15 s, and the reaction was monitored for 30 min. The results were corrected for dilution and were expressed as μmol Trolox/g DW of material. All measurements were performed in triplicate (Lee et al., 2017).
ABTS radical scavenging activity and DPPH radical scavenging activity
The ABTS radical cation decolorization assay described by Lee et al. (2017) was performed, with slight modifications. An ABTS•+ solution was prepared by reacting 7 mM aqueous ABTS solution with 140 mM (2.45 mM final concentration) potassium persulfate (K2S2O8). After storage in the dark for 12–16 h, the radical cation solution was further diluted in phosphate buffered saline (PBS, pH 7.4) to an absorbance of 0.7 (±0.02) at 734 nm and equilibrated at 30 °C. PBS was used as a blank solution. Diluted samples (10 μL) were mixed with 1 mL of the ABTS•+solution and the decrease in the absorbance was measured after 15 min. Measurements were performed in triplicate compared with the blank solution. The ABTS radical cation scavenging capacity was calculated using the following equation:
The DPPH assay was performed according to the method described by Thaipong et al. (2006). The stock solution was prepared by dissolving 24 mg DPPH in 100 mL methanol and then stored at −20 °C until use. The working solution was obtained by mixing 10 mL stock solution with 45 mL methanol to obtain an absorbance of 1.1 ± 0.02 units at 515 nm using a spectrophotometer. Diluted samples (50 μL) were added to 2 mL of DPPH solution and mixed. The absorbance of the remaining DPPH was determined after 30 min at 515 nm. The blank sample contained methanol. The percentage of DPPH radical scavenging capacity was calculated using the following equation:
EC50 values were calculated from data using a mathematical method based on the principle of the right-angled triangle: EC50 = D−[(A-50% max response)·(D–C)]/(A−B), where A is the immediate higher response of 50% of the maximum response, B is the immediate lower response of 50% of the maximum response, D = log concentration corresponding to the A response, and C = log concentration corresponding to the B response. The Trolox equivalent antioxidant capacity (TEAC) values were expressed as μmol Trolox/g DW of material. All measurements were performed in triplicate.
Cell viability assay
Ethyl alcohol (Sigma-Aldrich, 459844, St. Louis, MO, USA) was added to the S. tamariscina powder sample to create a 100 mg/mL solution. MRC5 cells, a human-derived normal lung cell line, were purchased from ATCC (American Type Culture Collection) and were cultured in minimum essential medium (MEM) in a 5% CO2 incubator at 37 °C. After seeding and culturing MRC5 cells (1 × 104 cells/well) in a 96-well plate, twofold serial dilutions were performed at concentrations of 12.5, 25, 50, 100, 200, and 400 μg/mL, and cultured for 24 h. Cell viability was measured using an MTT assay kit (Abcam, ab211091, Cambridge, UK), and the absorbance at 590 nm was measured using a microplate reader (Synergy H1 Hybrid Multi-Mode Microplate Reader, BioTek, Winooski, VT, USA).
Experimental animals and breeding conditions
Male Institute of Cancer Research (ICR) mice (6–7 weeks old) were purchased from Hallim Experimental Animal Co., Ltd., and were used per experimental group. The mice were acclimatized with a commercially available solid diet (PicoLab® Rodent Diet) for 1 week, then completely randomly grouped according to their weight, and reared while supplying water and food ad libitum for 60 days. During the experiment, the breeding conditions were maintained at a room temperature of 20 ± 2 °C and humidity of 40–60%, and the contrast was adjusted every 11 ± 1 h. The experiment was conducted from October 11, 2017, to December 30, 2017. All animal experiments were approved by the Committee of Laboratory Animals in accordance with the institutional guidelines of Sahmyook University, Republic of Korea (SYUIACUC 2017-017).
Animal feed composition
The feed composition was based on the weight ratio, and the feed of the control group consisted of 60% carbohydrates (starch + sucrose + glucose + fructose + lactose), 21% proteins, 13% lipids (beef oil), 1% vitamins, 3% minerals, and 2% fiber. The feed of the high-fat diet group consisted of 53% carbohydrates (starch + sucrose + glucose + fructose + lactose), 21% proteins, 20% lipids (beef oil), 1% vitamins, 3% minerals, and 2% fiber. The feed composition of the experimental group with S. tamariscina powder added to the high-fat diet consisted of 33% carbohydrates (starch + sucrose + glucose + fructose + lactose), 20% S. tamariscina powder, 21% proteins, 20% lipids (beef oil), 1% vitamins, 3% minerals, and 2% fiber.
Blood collection
Experimental animals were fasted for 12 h on the last day of rearing, anesthetized with CO2 gas, their thoracic cavities surgically opened, and blood was collected from their hearts. Each blood sample was placed in a refrigerator at 4 °C for approximately 1 h. Serum was separated via centrifugation at 3000 rpm for 15 min at 5 °C. The separated serum was stored in a −70 °C freezer until use, where an aliquot of 100 μL was placed into a microfuge tube and used in subsequent experiments.
Analysis of blood lipid concentration
Serum cholesterol content was measured using the o-phthaldehyde method(Rudel and Morris, 1973). Samples were aliquoted at 0.1 mL, 0.3 mL of 33% KOH solution and 3.0 mL of 95% ethanol were added, the solution was mixed well and was heated in a water bath at 60 °C for 15 min and then cooled. To this, 5.0 mL of nucleic acid was added and mixed, 3.0 mL of distilled water was added, and then mixed well for 1 min, and the layers were separated, and 1.0 mL of the nucleic acid layer was separated. The nucleic acid layer was concentrated and dried with nitrogen, and 2.0 mL of o-phthaldehyde reagent was added, mixing well. After 10 min, 1.0 mL of concentrated sulfuric acid was added as a color developing reagent, and the solution was mixed well. After the addition of sulfuric acid, a spectrophotometer (Human Corporation, Seoul, Korea) was used to measure the absorbance of the solution at 550 nm, and the cholesterol content was quantified according to a standard calibration curve. High-density lipoprotein (HDL)-cholesterol (HDL-C 555, Eiken Co., Tokyo, Japan) and low density lipoprotein (LDL)-cholesterol (BLF, Eiken Co., Tokyo, Japan) kit reagents were used according to the corresponding manufacturer’s instructions. To measure the HDL-cholesterol content, 0.3 mL was placed in a test tube, and 0.3 mL of precipitation reagent was added, mixed well, and incubated at room temperature for 10 min, followed by centrifugation at 700 × g for 10 min. Then, 50 μL of the supernatant, 50 μL of the prepared standard solution (100 mg/dL), and 3.0 mL of the HDL coloring reagent were added to 50 μL of distilled water as a blank, mixed well, and heated in a water bath at 37 °C for 5 min. The HDL-cholesterol content was quantified by measuring the absorbance at 555 nm using a blank as a control. To measure the LDL-cholesterol content, 0.1 mL of serum, 0.1 mL of standard serum, and 4.0 mL each of BLF kit reagents I and II, respectively, were added to a test tube, and mixed well for 5 s, then incubated at room temperature (25 ± 3 °C) for 25 min. Within 10 min of standing, the LDL-cholesterol content was quantified by measuring the absorbance at 650 nm using a spectrophotometer with distilled water as a control. Serum triglyceride levels were analyzed using a TG kit reagent (Sigma chemical Co., St. Louis, MO, USA). Ten microliters of serum, 10 μL of standard solution (300 mg/dL), and 1.0 mL of TG kit reagent were added to 10 μL of deionized water as a blank, mixed well, and then reacted in a water bath at 37 °C for 5 min, using the blank as a control. The TG content was quantified by measuring the absorbance at 540 nm using a spectrophotometer.
Biochemical analysis of liver function
Alkaline phosphtase (ALP) concentrations were measured using a commercial kit (Asan Pharmaceutical Co., Ltd., Seoul, Korea), and serum alanine transminase (ALT) and aspartate transminase (AST) concentrations were measured using the Reitman and Frankel method (1957). serum lactate dehydrogenase (LDH) was measured using cytotoxicity assay kit (Thermo Scientific, Rockford, IL, USA).
Statistical analysis
All data were analyzed using the SPSS package version 18.0 (Statistical Package for Social Science, SPSS Inc., Chicago, IL, USA) program to calculate the means and standard deviations. All samples used in the experiment were repeated three times. Comparison of mean values was performed using the t-test and one-way ANOVA, and significance analysis (p < 0.05) of the differences between means was performed using Duncan's multiple test method. Correlations between the antioxidant activities of S. tamariscina powder were analyzed using Pearson’s correlation analysis (p < 0.01).
Results and discussion
General composition analysis
The results of the general composition analysis of S. tamariscina powder are presented in Table 1. The freeze-dried S. tamariscina powder contained 2.61 ± 0.39% water, 17.39 ± 0.38% protein, 1.23 ± 0.06% fat, and 7.67 ± 0.54% ash. In a previous study (Sheo and Lee, 1989), ferns belonging to the same category contained 2.7% water, 19.8% crude protein, 2.9% crude fat, 7.9% ash, and 31.7% dietary fiber. The results of general composition analysis of S. tamariscina powder in this study showed similar content to ferns. Also, The Japanese royal fern was reported to contain 90.6 g water, 2.4 g protein, 0.1 g fat, and 0.7 g of ash (RDA, 2006).
Mineral content analysis
The mineral content of S. tamariscina powder is shown in Table 1. Per 100 g, the freeze-dried S. tamariscina powder contained 0.94 ± 0.06 mg of iron, 0.53 ± 0.02 mg of manganese, 0.44 ± 0.01 mg of zinc, and 0.26 ± 0.01 mg of copper. In addition, there was a very small amount of the strong antioxidant selenium (0.01 ± 0.001 mg). In particular, when the iron content was compared with P. aquilinum var. latiusculum (2.5 g) (Sheo and Lee, 1989) and Japanese royal fern (1.7 g) (RDA, 2006), the S. tamariscina powder in this study showed a low level.
Antibacterial activity assays
The antimicrobial activity of S. tamariscina powder is shown in Table 2. DIW extracts of S. tamariscina powder did not show antibacterial activity against Bacillus subtilis KCTC 2189, S. aureus KCTC 3881, P. aeruginosa KCTC 2004, E. aerogenes KCTC 2190 or E. coli KCTC 1682. However, the ethanol extract of S. tamariscina powder showed antibacterial activity against B. subtilis KCTC 2189. Therefore, S. tamariscina powder ethanol extract, which showed antimicrobial activity against gram-positive bacteria, could be effectively used as an antimicrobial agent. A previous study (Lee et al., 2009) reported that isocryptomerin isolated from S. tamariscina showed strong antibacterial activity against gram-positive and gram-negative bacterial strains, including Staphylococcus aureus. In particular, S. tamariscina powder has been reported to have synergistic effects when combined with cefotaxime. These data suggest the potential of S. tamariscina as a therapeutic compound against infectious diseases. Amentoflavone is a biflavonoid compound with antioxidant, anticancer, antibacterial, antiviral, anti-inflammatory, and sunscreen properties that can be isolated from S. tamariscina (Oh et al., 2013). Amentoflavone extracted from S. tamariscina showed strong antifungal activity against several pathogenic strains and could be used as a major compound in the development of antifungals; additionally, it has a weak hemolytic effect on human red blood cells (Jung et al., 2006).
TPC assay
The TPC of S. tamariscina powder is shown in Table 3, which ranged from 8.65 to 11.61. The TPC of the DIW extract was 8.65 ± 0.61 GAE mg/g extract, whereas it was 11.61 ± 0.30 GAE mg/g in the ethanol extract, which was significantly higher (p < 0.05). It has been reported a higher total phenolic compound content is associated with higher antioxidant activity (Lee et al., 2018). In a previous study (Jang et al., 2014; Kim et al., 2019), phenolic compounds were reported to have excellent anticancer, anti-inflammatory, antioxidant, and antibacterial effects. In particular, they have also been shown to inhibit the accumulation of various lipids. Kim et al. (2019) reported the yield of S. tamariscina as 10.3%, with a TPC as 60.29 GAE mg/g and a total flavonoid content of 14.90 quercetin equivalent (QE) mg/g. Lee et al. (2006) reported that the TPC of S. tamariscina was 99.3 GAE mg/g and its total flavonoid content was 37.2 QE mg/g. As pointed out in the study by Kim et al. (2019), there may be differences in the reported values depending on the cultivation environment of the sample or due to differences in the methods of measuring the total flavonoid and phenolic content. Shin et al. (2016) reported that the TPC of H. plumaeforme, T. kanedae, and L. juniperoideum was 96.53, 59.20 and 119.87 mg GAE/g, respectively, and the TPC of S. tamariscina was similar to that of mosses and ferns. In a study by Lee et al. (2005), a total polyphenol content of 130.22, and 120.69 μg/mg Cirsium nipponicum (Maxim.) Makino leaf and Athyrium acutipinnulum Kodama ex Nakai leaf extracts, respectively, were reported. Achyranthes japonica (Miq.) Nakai root and C. nipponicum (Maxim.) Makino seeds and stems reported very low levels of total polyphenol content, at 16.74, 29.20, and 27.27 μg/mg, respectively. There were differences in TPC depending on the plant variety and test method used. Berries are known to have a high TPC. The TPC content of berries was 9.03 GAE mg/g for blueberry, 5.34 GAE mg/g for raspberry (Jeong et al., 2008), 73.66 GAE mg/g for maquiberry, 59.26 GAE mg/g for aronia, and 43.70 GAE mg/g for blackcurrant (Chung, 2016). A comparison of the TPC of S. tamariscina powder with berries showed that the phenolic content of S. tamariscina powder was even higher than that of certain berries.
FRAP assay
Table 3 shows the reducing power of the DIW and ethanol extracts of S. tamariscina powder as determined via FRAP. The total FRAP of S. tamariscina powder was found to be 139.14–161.08 TEAC μmol/g. The FRAP of the S. tamariscina powder was higher in the ethanol extract (161.08 ± 0.93 TEAC μmol/g) than in the DIW extract (139.14 ± 1.73 TEAC μmol/g) (p < 0.05). Lee et al. (2018), the FRAP value of cherry was 86.94 TEAC μmol/g, and the value of S. tamariscina powder in this study showed a higher values. Therefore, in this study, the FRAP value of S. tamariscina powder showed a similar tendency in antioxidant effect when compared to cherry or berries (Lee et al., 2018).
ABTS radical radical scavenging activity and DPPH radical scavenging activity
The ABTS and DPPH radical scavenging activities of S. tamariscina powder are shown in Table 3. The ABTS radical scavenging activity of S. tamariscina powder was 187.05–261.19 TEAC μmol/g, with an EC50 value of 3.88–5.41 mg/mL. The DPPH radical scavenging activity of S. tamariscina powder was 52.36–65.29 TEAC μmol/g, with an EC50 value of 12.11–15.10 mg/mL. Therefore, the ABTS radical cation decolorization activity and DPPH radical scavenging activity of S. tamariscina powder were significantly higher in the ethanol extract than in the DIW extract (p < 0.05). In a previous study (Lee et al., 2005), the ABTS radical scavenging activity RC50 values of Athyrium acutipinnulum Kodama ex Nakai leaf and root extracts were 40.93 and 35.39%, respectively, whereas Solidago virgaurea subsp (gigantea (Nakai) Kitam) root extract was reported to have an ABTS radical scavenging activity RC50 value of 29.08 μg/mL, indicating high scavenging activity. In the study by Lee et al. (2005), DPPH radical scavenging activity showed the highest RC50 values of 13.02 and 14.91 μg/mL in Athyrium acutipinnulum Kodama ex Nakai leaf and Solidago virgaurea subsp, respectively. It was reported that their DPPH radical scavenging activity was not significantly different compared to that of BHA at 5.25 μg/mL. In a previous study (Shin et al., 2016), H. plumaeforme, T. kanedae, and L. juniperoideum that underwent hydrothermal extraction method based on a tenfold dilution, was found to have an ABTS radical scavenging activity of 2587.33, 1637.00, and 1997.00 μmol Trolox/mg, respectively. DPPH measurements of H. plumaeforme, T. kanedae, and L. juniperoideum reported values of 298.78, 89.89 and 284.33 μmol Trolox/mg, respectively (Shin et al., 2016). The DIW extract of cherry as presented by Lee et al. (2018) showed high levels of ABTS radical scavenging activity when compared to S. tamariscina at 117.00 TEAC μmol/g. The DPPH radical scavenging activity of S. tamariscina was found to be as high as that of maquiberry (78.17 TEAC μmol/g) (Lee et al., 2018). Previous studies (Adnan et al., 2021; Kang et al., 1996) reported that DPPH radical scavenging activity and ABTS radical scavenging activity were higher as phenolic compounds increased, and this study clearly demonstrared the antioxidant effect of S. tamariscina.
Correlation coefficients among antioxidant activities with S. tamariscina.
In the case of ethanol extraction, TPC was positively correlated with ABTS radical scavenging activity (r = 0.961), DPPH radical scavenging activity (r = 0.927), and FRAP (r = 0.768) (p < 0.01) (Table 4). In this study, TPC and antioxidant activity showed a stronger positive correlation in the ethanol extracts than in the DIW extracts.
Cell viability assay
The cell viability assay results of using S. tamariscina powder is presented in Table 5. It was found that the cell viability upon treatment at each concentration of S. tamariscina powder was the highest at 98.62 ± 0.56% in the 12.5 μg/mL treatment group, and it was maintained at approximately 96–97% in the groups treated with 25 μg/mL or more of the extract. The results of the MTT assay confirmed that the survival rate of MRC5 cells decreased with an increase in the treatment concentration of S. tamariscina powder. Lee et al. (2000a, 2000b) reported that when fibroblast NIH3T3 cells were treated with different concentrations of cadmium, cell viability decreased according to the concentration of Eoseongcho extract added, with an MTT50 of 33.04 μM. Treatment with Houttuynia cordata Thunb. extract was also reported to increase cell number and have a cell regeneration effect on fibroblasts.
Animal blood lipid concentration
The blood lipid concentrations of the rats are shown in Table 6. The total cholesterol concentration in the blood was the lowest in the control group at 152.67 ± 7.72 mg/dL (range, 152.67–213.00 in all groups). However, in the HF 20% + S. tamariscina 20% group, the total cholesterol content (213.00 ± 3.21 mg/dL) was higher than that of the other groups (p < 0.05). In particular, HDL-cholesterol levels were significantly higher in the HF 20% + S. tamariscina 20% group (150.00 ± 10.53 mg/dL) than in the other groups (p < 0.05). It was reported that the normal blood total cholesterol in mice was 2–20 mg/dL, and that the amount of LDL-cholesterol in the blood was observed to be 10.47–82.7 mg/dL, showing a large difference depending on the measurer (Sheo and Sheo, 2002). In addition, in this study, HDL-cholesterol levels were significantly higher when S. tamariscina was administered in addition to a high-fat diet. HDL-cholesterol is a beneficial lipoprotein that reduces the risk of developing cardiovascular diseases. It has been reported that increasing the proportion of HDL-cholesterol in total cholesterol has the potential to not only suppress dyslipidemia but also prevent various types of atherosclerosis, including coronary atherosclerosis (Park et al., 2009). Research is being conducted to lower blood cholesterol and triglyceride levels using various natural extracts, and since this study one of these researches, more comprehensive follow-up studies on the antioxidant and lipid profile improvement effect of S. tamariscina are needed in the future.
Biochemical analysis of liver function
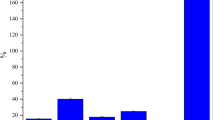
The results of biochemical blood analysis in rats are presented in Table 6. Observed ALP levels were in the range of 41.00 to 53.67 U/L and all three groups were within the normal range of 40 to 250 U/L. AST level was 149.00 ± 16.00 U/L in the HF 20% group, but decreased to 126.00 ± 18.01 U/L upon the addition of S. tamariscina powder in the HF 20% + S. tamariscina 20% group (p < 0.05). Blood LDH was significantly higher in the HF 20% + S. tamariscina 20% group (967.00 ± 125.92 U/L) than in the other two groups (p < 0.05). AST levels in the mice were determined to be 88.00–149.00 U/L, which were higher than the normal range of 7–40 U/L. Previous studies (Choi et al., 2016) have reported that AST plays a central role in supplying amino groups in vivo and is also involved in gluconeogenesis. In addition, it has also been reported that when the level of AST increases, it reflects cellular degeneration and the necrosis of distributed organs, and is widely used as a potent indicator of liver and heart diseases (Choi et al., 2016). In this study, ALT levels were within the normal range of 4–43 U/L in all groups. Abnormalities in blood ALT levels were reported to be associated with metabolic syndrome in a recent study (Kim, 2009). The normal level of LDH in human blood is 250–350 IU/L. In this study, LDH levels were out of the normal range in HF 20% + S. tamariscina 20% group compared to the normal range in humans. LDH is an enzyme that acts when the sugar in the body is decomposed and converted into energy; it has been reported that when cells are destroyed, blood LDH levels increase (Choi et al., 2016). In addition, blood LDH is a useful parameter for the screening of malignant tumors, liver diseases, heart diseases, and blood diseases.
In summary, the ABTS radical scavenging activity, DPPH radical scavenging activity, and FRAP reducing activity of S. tamariscina powder were higher in the ethanol extract compared to the DIW extract. The ethanol extract showed antibacterial activity against B. subtilis KCTC 2189. In addition, S. tamariscina powder increased levels of blood HDL-cholesterol, a “good cholesterol”. Therefore, S. tamariscina powder extract was confirmed as a functional food material. To further utilize S. tamariscina as a functional food material, it is necessary to determine the optimal extraction method for enhancing the functionality of S. tamariscina powder and to develop it into various foods.
References
Adnan M, Siddiqui AJ, Jamal A, Hamadou WS, Awadelkareem AM, Sachidanandan M, Patel M. Evidence-based medicinal potential and possible role of Selaginella in the prevention of modern chronic diseases: ethnopharmacological and ethnobotanical perspective. Records of Natural Products. 1–26 (2021)
Choi KS, Kim YH, Shin KO. Effect of mulberry extract on the lipid profile and liver function in mice fed a high fat diet. The Korean Journal of Food and Nutrition. 29: 411–419 (2016)
Chu SJ, Heo JS, Sohn KH. Selaginella tamariscina extract improves scopolamine-induced learning and memory dificits in rats. Korea Journal of Pharmacognosy. 47: 319–326 (2016)
Chung HJ. Comparision of bioactive constituents and biological activities of aronia, blackcurrant, and maquiberry. Journal of the Korean Society of Food Science and Nutrition. 45: 1122–1129 (2016)
Han KS, Jung TH, Shin KO. Studies on the general analysis and antioxidant component analysis of Chenopodium album var. centrorubrum and biochemical analysis of blood of mice administered C. album. Korean Journal of Food Science and Technology. 51: 492–498 (2019)
Hsin CH, Wu BC, Chuang CY, Yang SF, Hsieh YH, Ho HY, Lin HP, Chen MK, Lin, CW. Selaginella tamariscina extract suppresses TPA-induced invasion and metastasis through inhibition of MMP-9 in human nasopharyngeal carcinoma HONE-1 cells. BMC Complementary & Alternative Medicine. 13: 234 (2013)
Jang M, Park H, Hong E, Kim GH. Comparison of the antibacterial activity of domestic Cirsium japonicum collected from different regions. Korean Journal of Food and Cookery Science. 30: 278–283 (2014)
Jeong CH, Choi SG, Heo HJ. Analysis of nutritional compositions and antioxidative activities of Korean commercial blueberry and raspberry. Journal of the Korean Society of Food Science and Nutrition. 37: 1375–1381 (2008)
Jung HJ, Sung WS, Yeo SH, Kim HS, Lee IS, Woo ER, Lee DG. Antifungal effect of amentoflavone derived from Selaginella tamariscina. Archives of Pharmacal Research. 29: 746–751 (2006)
Kang YH, Park YK, Lee GD. The nitrite scavenging and electron donating ability of phenolic compounds. Korean Journal of Food Science and Technology. 28: 624–30 (1996)
Kim JH. Relationship between elevated serum alanine aminotransferase concentration and metabolic syndrome in Korean adults. Korean Journal of Nutrition. 42:732–739 (2009)
Kim HR, Lee JH, Kim YS, Kim KM. Chemical characteristics and enzyme activities of Icheon Ge-Geol radish, Ganwha turnip, and Korean radish. Journal of Food Science and Technology. 39: 255–259 (2007)
Kim JH, Cho CW, Tai BH, Yang SY, Choi GS, Kang JS, Kim H. Soluble epoxide hydrolase inhibitory activity of selaginellin derivatives from Selatinella tamariscina. Molecules. 20: 21405–21414 (2015)
Kim GH, Lee SY, Lee AR. The effect of Selaginella tamariscina on inhibition of pancreatic lipase and lipid accumulation. The Korean Journal of Food and Nutrition. 32: 27–32 (2019)
Kim YS, Shim HM, Kim KY. Antimicrobial effect of Caesalpinia sappan L. extract on foodborne bacteria. Journal of the Korean Society of Food Science and Nutrition. 45: 1026–1034 (2016)
Lee SO, Lee HJ, Yu MH, Im HG, Lee IS. Total polyphenol contents and antioxidant activities of methanol extracts from vegetables produced in Ullung Island. Korean Journal of Food Science and Technology. 37: 233–240 (2005)
Lee BC, Sim GS, Kim JH, Kim JH, Pyo HB. Effect of the processed Selaginella tamariscina on antioxidation and inhibition of matrix metalloproteinase. Journal of Society Cosmetic Scientists Korea. 32: 69–74 (2006)
Lee KS, Kwon YJ, Lee KY. Analysis of chemical composition, vitamin, mineral and antioxidative effect of the lotus leaf. Journal of the Korean Society of Food Science and Nutrition. 37: 1622–1626 (2008)
Lee J, Choi Y, Woo ER, Lee DG. Antibacterial and synergistic activity of isocryptomerin isolated from Selaginella tamariscina. Journal of Microbiology and Biotechnology. 19: 204–207 (2009)
Lee KW, Kim YH, Shin KO. In vitro antioxidant activities and antimicrobial activity of lotus (leaf, stem, and seed pod) extracts. The Korean Journal of Food and Nutrition. 30: 771–779 (2017)
Lee KW, Je HJ, Jung TH, Lee YL, Choi JH, Hwang HJ, Shin KO. Comparison of components and antioxidant activity of cherry, aronia, and maquiberry. The Korean Journal of Food and Nutrition. 31: 729–736 (2018)
Lee JH, You IS, Kim JS, Lee KN, Chung WY, Han DS, Baek SH. The inhibitory effects of Houttuynia cordata Thunb against cadmium induced cytotoxicity (II): The 40th Annual convention of Pharmaceutical society of Korea & international symposium. pp. 162 (2000a)
Lee JH, You IS, Kim JS, Lee KN, Chung WY, Han DS, Baek SH. The inhibitory effects of Houttuynia cordata Thunb against cadmium induced cytotoxicity (II). Yakkak Hoeji. 44: 432–439 (2000b)
Nguyen PH, Ji DJ, Han YR, Choi JS, Rhyu DY, Min BS, Woo, MH. Selaginellin and biflavonoids as protein tyrosine phosphatase 1B inhibitors from Selaginella tamariscina and their glucose uptake stimulatory effects. Bioorganic & Medicinal Chemistry. 23: 3730–3737 (2015)
Oh JE, Rho HS, Yang Y, Yoon JY, Lee J, Hong YD, Kim HC, Choi SS, Kim TW, Shin SS, Cho JY. Extracellular signal-regulated kinase is a direct target of the anti-inflammatory compound amentoflavone derived from Torreya nucifera. Mediators of Inflammation. 2013: 1–11 (2013)
Park SH, Rhee IJ. Effect of Selaginella tamariscina on U937 cytotoxicity. Journal of the Korean Society of Food and Nutrition. 23: 799–804 (1994)
Park GJ, Lee HW, Park BR, Park SJ, Kim JD. Effects of Puerariae Flos on antioxidative activities and lipid levels in hyperlipidemic Sprague-Dawley rats. Journal of the Korean Society of Food Science and Nutrition. 38: 846–851 (2009)
Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. Journal of Agricultural and Food Chemistry. 48: 3396–3402 (2000)
RDA [Rural Development Administration]. Food composition table I. 7 revision. National Rural Resources Development Institute, RDA. Korea. pp. 130–137 (2006)
Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. American Journal of Clinical Pathology. 28: 56–63 (1957)
Rudel LL, Morris MD. Determination of cholesterol using o-phthalaldehyde. Journal of Lipid Research. 14: 364–366 (1973)
Sasaki H, Kitoh Y, Tsukada M, Miki K, Koyama K, Juliawaty LD, Hakim EH, Takahashi K, Kinoshita K. Inhibitory activities of biflavonoids against amyloid-β peptide 42 cytotoxicity in PC-12 cells. Bioorganic & Medicinal Chemistry Letters. 25: 2831–2833 (2015)
Seul YS, Yang EJ. Optimization of extraction conditions and investigation of stability of Solanum nigrum extract with anti-yeast activity. Journal of the Korean Society of Food Science and Nutrition. 46: 1523–1530 (2017)
Sheo HJ, Lee MY. A toxicological study of young fronds of Bracken fern (Pteridium aquilinum var latiusculum) collected in Kwang Ju area. Journal of the Korean Society of Food Science and Nutrition. 18: 255–264 (1989)
Sheo HJ, Sheo YS. Adverse effects of the megadose perilla oil on the rats metabolism. Journal of the Korean Society of Food Science and Nutrition. 31:277–283 (2002)
Shin DI, Kim JW. Flavonoid constituents of Selaginella tamariscina. Korea Journal of Pharmacognosy. 22: 207–210 (1991)
Shin KO, Choi KS, Kim YH. In vitro antioxidative activity of moss extract, and effect of moss on serum lipid level of mice fed with high-fat diet. Tropical Journal of Pharmaceutical Research. 15: 1215–1224 (2016)
Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne DH. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. Journal of Food Composition and Analysis. 19: 669–675 (2006)
Waterhouse AL. Current Protocols in Food Analytical Chemistry. pp.I1.1.1–I1.1.8 (2002)
Won AN, Kim SA, Ahn JY, Han JH, Kim CH, Lee JH, Kim DI. HO-1 induction by Selaginella tamariscina extract inhibits inflammatory response in lipopolysaccharide-stimulated RAW 264.7 macrophages. Evidence-Based Complementary and Alternative Medicine. 2018: 1–10 (2018)
Yang JS, Lin CW, Hsieh YS, Cheng HL, Leu KH, Yang SF, Lu KS. Selaginella tamariscina(Beauv.) processes antimetastatic effects on human osteosarcoma cells by decreasing MMP-2 and MMP-9 secretions via p38 and Akt signaling pathways. Food and Chemical Toxicology. 59: 801–807 (2013)
Zhang Z, Sun T, Niu JG, He ZQ, Liu Y, Wang F. Amentoflavone protects hippocampal neurons: antiinflammatory, antioxidative and anti apoptotic effects. Neural Regeneration Research. 14: 969–978 (2015)
Acknowledgements
This paper was supported by the Fund of the Sahmyook University in 2020 (RI12020018).
Author information
Authors and Affiliations
Contributions
Hyo-Jeong Hwang: Data analysis. Jeong-Yeon Kim: Sample analysis. Kyung-Ok Shin: Conceptualization, Writing –original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hwang, HJ., Kim, JY. & Shin, KO. A study on the nutritional and biochemical analysis of Selaginella tamariscina powder. Food Sci Biotechnol 30, 1445–1454 (2021). https://doi.org/10.1007/s10068-021-00991-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-021-00991-z