Abstract
The physico-chemical, polyphenols, antioxidant and antibacterial properties of berries and mixture of male and female leaves of Hippophae salicifolia were investigated. The mineral, vitamin C, sugar, total protein, and total tannin contents of the berries and the leaves were evaluated. Further, the extracts of berries and mixture of leaves samples obtained by successive solvent extraction were investigated for their polyphenols, antioxidant and antibacterial properties. Total phenolic content was highest in leaves-methanol extract (157.97 ± 2.09 mg GAE/g) followed by berries-aqueous extract (48.45 ± 1.94 mg GAE/g), while total flavonoid was predominant in leaves-acetone extract (75.64 ± 3.21 mg QE/g) and berries-methanol extract (28.93 ± 2.08 mg QE/g). Gallic acid, caffeic acid, and rutin were the major polyphenols confirmed by HPLC analysis. Berries-aqueous and leaves-methanol extracts showed excellent global antioxidant score. Best antibacterial activity was observed by methanol extracts against eight different strains. Overall, the leaves and berries of Hippophae salicifolia collected from Northeast India exhibited good antioxidant and antibacterial activity and can be utilized by food and pharmaceutical sectors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants are always considered as important natural resources because of their numerous benefits to serve mankind in the field of food, pharmaceutical, and cosmeceutical. The current trend describes the utilization of plants and their vegetative parts in developing various value-added products with low cost and sustainability. India holds four biodiversity hotspots, of which the Himalayas and Indo-Burma regions are in the Northeast part of India. The Himalayas which comprises Northeast India, Bhutan, and the central and eastern part of Nepal contains more than 10,000 species of flora and many of which are not yet adequately explored (Samant et al., 1998). Sea buckthorn (SBT) is among the various invasive species found in the Indian Himalayan regions of Himachal Pradesh, Ladakh, Uttaranchal, Sikkim, and Arunachal Pradesh. The Himalayas and Ladakh host approximately 30,000 ha and 12,000 ha of land under SBT cultivar, respectively (Stobdan et al., 2011). India produces three different species of SBT: H. rhamnoides, H. salicifolia, and H. tibetana.
The berries, leaves, and seeds, of SBT are widely used in juice, tea, oil, and for a wide variety of food, cosmeceutical, and pharmaceutical products that are available commercially. SBT has a long tradition of use in East Asia for its nutritional and medicinal value. Recently, SBT berries and leaves have acquired significant attention due to their high contents of minerals, sugars, vitamins, and bioactive compounds such as phenolics, flavonoids, tocopherols, carotenoids, phytosterols, unsaturated lipids, and volatile compounds that led to their utilization in nutraceuticals and cosmeceuticals products and natural food preservative agent (Upadhyay et al., 2010). SBT leaves contain higher nutrient values, polyphenols, and antioxidant properties than berries (Criste et al., 2020). The antioxidant activity is attributed to phenolic and flavonoid compounds which inhibit lipoxygenase, chelate metals, and scavenge free radicals (Michel et al., 2012; Olas et al., 2018). In addition, polyphenol compounds of SBT berries and leaves can also attribute to their antimicrobial properties. Further, SBT exerts wide-ranging activities including anti-inflammatory (Padwad et al., 2006), cytotoxic (Yang and Kallio, 2002), antiviral (Ursache et al., 2017), antiulcer (Xing et al., 2002), anti-atherogenic (Basu et al., 2007), cardioprotective and anticancer (Olas, 2016; Olas et al., 2018).
The nutritional and pro-health benefits of seeds, pulp, leaves, stems, and roots of Indian H. rhamnoides L. are well documented (Michel et al., 2012). However, due to the limited information about the geographical location, studies on H. salicifolia species from Northeast India is very limited. Regardless of nutritional and health benefits, SBT leaves remain as agricultural waste after the harvesting of berries. Even, the pomace and the seeds of the berries are thrown or being used as cattle feed after the juice extraction. SBT is a dioecious species and both of its male and female plants coexist in close proximity in the wild. Morphological identification between the plants is impossible except flowering to fruiting seasons. The leaves of plants of both types are reported to be a good source of bioactive compounds with slight differences in TPC, TFC, and antioxidant and antibacterial activity (Gupta and Kaul, 2017; Singh et al., 2014; Šne et al., 2013a; 2013b). In many literatures, the gender of the leaves was also not properly mentioned. There is no reporting of any coactivity of the mixture of male and female SBT leaves so far. Characterization of the mixture of leaves could help to effectively utilize SBT leaves without prior gender identification and comply with the demand of the increased research going on to explore the supplementary options for sustainable utilization of natural resources.
Therefore, this study is focused on investigating the phytochemical and nutritional composition of the 50–50% mixture of dry leaves and the whole berries of Hippophae salicifolia from Northeast India. Further, physicochemical analyses of mixed leaves and berries (CHNS, ash, protein, vitamin C, sugar, tannin, FTIR) were performed. Some important polyphenol compounds were extracted following successive solvent extraction and further identified by HPLC analysis. Additionally, the antioxidant potential and antibacterial activities of different extracts were evaluated. The global antioxidant score was calculated mathematically to choose the best antioxidant-rich extract. The nutritional and polyphenolic content and the antioxidant activity of extracts were compared with the previously reported data of the berries and either of individual plant leaves. To the best of our knowledge, this is the first comprehensive report on the compositional, polyphenol content, antioxidant and antibacterial activities of the berries and mixture of leaves of Hippophae salicifolia D. Don from Northeast India.
Materials and methods
Materials and chemicals
The berries and the male and female leaves of H. salicifolia D. Don were separately collected during the fruiting season from the state of Arunachal Pradesh, India. They were dried under shade, milled, and stored in a plastic bag. The leaves mixture was prepared with a 50:50 (% dry weight) mix of powdered male and female leaves. All chemicals, solvents, and reagents were of analytical grade procured from Merck India Pvt. Ltd., Sigma Aldrich, and Himedia. Cultures of Gram-positive bacteria: Staphylococcus aureus (MTCC 9886), Micrococcus luteus (MTCC 2848), Staphylococcus epidermidis (MTCC 9040), Bacillus subtilis (MTCC 1133); and Gram-negative bacteria: Escherichia coli (MTCC 1687), Enterobact eraerogenes (MTCC 8558), Klebsiella pneumonia (MTCC 4030), Pseudomonas aeruginosa (MTCC 8727) were purchased from Institute of Microbial Technology (Chandigarh, India).
Proximate and CHNS analysis
The moisture and total ash content were estimated according to A.O.A.C. (2012). Volatile matter and fixed carbon content were quantified by the method outlined by Piltz and Law (2007). The carbon (C), hydrogen (H), nitrogen (N), sulfur (S), and oxygen (O) analyses were carried out using a CHNSO analyzer (Make: Eurovector, Model: EA3000). The percentage of oxygen was determined by the difference.
Elemental composition
A finely powdered dry sample was mounted on a carbon tape and analyzed at different magnifications to determine the compositional elements using Field Emission Scanning Electron Microscope (FESEM) (Make: Zeiss, Model: Sigma 300).
Quantitative phytochemical analysis
The total soluble sugar and reducing sugar of whole SBT berries and the 50–50% mixed leaves were ascertained by following the protocol reported by Sadasivam and Manickam (2008). Total crude protein was determined by Lowry’s method (Lowry et al., 1951). Bovine serum albumin was considered as the reference to calculate the total protein content. Ascorbic acid was quantified according to the protocol elaborated by Arimboor et al., (2006) using HPLC (Shimadzu Corporation, Kyoto, Japan) equipped with C-18 column and PDA detector set at 246 nm. Total Tannin content was determined following the method of Polshettiwar et al. (2007) and was quantified in mg tannic acid equivalent (mg TAE) per gram (g).
Successive Soxhlet extraction
The dried sample (100 g) was successively extracted using n-hexane, chloroform, ethyl acetate, acetone, methanol, and distilled water until the solvent in the siphon of the Soxhlet apparatus is colorless. The solvent was evaporated using a vacuum rotary evaporator. Extracts were collected in amber-colored vials and stored in the fridge (4 °C) until analysis. The yield of extraction was calculated using Eq. (1).
Qualitative Phytochemical analysis
Phytochemical screening was carried out according to the method reported by Khandelwal (2008). The presence of carbohydrates, glycosides, proteins, amino acids, alkaloids, tannins, phenols, and flavonoids was qualitatively determined in the SBT berries and leaves extracts.
Fourier Transform Infrared Spectroscopy (FTIR)
Functional groups inherently present in the whole berries, mixtures of leaves, and each extract were determined using FTIR spectrometer (Shimadzu Corporation, Japan). A dry sample and KBr with a ratio of 1:100 were crushed and compressed into a thin pellet. Infrared spectra were recorded within wavenumbers between 4000 and 400 cm−1, at a resolution of 4 cm−1.
Total Phenolic Content (TPC)
Determination of TPC of the extracts was carried out using a method outlined by Sen et al. (2013). Briefly, 0.5 mL extract and 2.5 mL Folin-Ciocalteu reagent (10%) were thoroughly mixed. Then, 2 mL Na2CO3 (2% (w/v)) was poured into the mixture and vortexed. After 20 min of incubation in dark, absorbance was measured against blank at 765 nm. The calibration curve (R2 > 0.99) was plotted using 10–100 µg/mL gallic acid solution. TPC was expressed in mg gallic acid equivalent (mg GAE) per g of extract. The TPC per gram of dry berries and leaves (mg GAE/g DW) of each extract was calculated by multiplying the yield of extraction by TPC per gram of extract using Eq. (2).
Further, the grand total TPC obtained from the entire extraction process was obtained by adding the TPC per gram of dry sample in each extract.
Total flavonoid content (TFC)
TFC was analyzed using Sen et al. (2013) method. About 0.5 mL sample, 100 µL potassium acetate (1 M), and 100 µL aluminum nitrate (10% (w/v)) were added into a tube containing 4.3 mL ethanol and vigorously shaken. After 40 min incubation, absorbance was measured at 415 nm. Various concentrations of quercetin solution (10–100 µg/mL) were used to establish a calibration curve. TFC was expressed in mg quercetin equivalent (mg QE) per g of the extract. The TFC per gram of dry berries and leaves (mg QE/g DW), was calculated using Eq. (3).
The grand total TFC was computed by adding the TFC per gram of dry sample in each extract.
Identification of phenolic and flavonoid compounds
The HPLC equipped with UV–Vis detector (Shimadzu Corporation, Kyoto, Japan) and C18 column, 250 × 4.6 ID, was used to identify the phenolic and flavonoid compounds all extracts of the berries and leaves. The water:acetonitrile (63%:37%) with 1% phosphoric acid at 1 mL/min flow rate and water:methanol:acetonitrile in the ratio 45:40:15 with 1% glacial acetic acid at 0.5 mL/min flow rate was used as a mobile phase for the phenolic acids and flavonoids analysis, respectively. The detection wavelengths were 296 nm for gallic acid, caffeic acid, and ferulic acid, 310 nm for p-coumaric acid, and 368 nm for quercetin, myricetin, kaempferol, and rutin. The polyphenol standards were dissolved in methanol (HPLC grade) and filtered using a 0.45 µm filter (Axiva). 20 µL standards with various concentrations (10–50 µg/mL) were injected and a calibration curve was plotted. Similarly, 500 µg/mL of all extracts of SBT berries and leaves were injected for analysis. Polyphenols were quantified from each calibration plot (R2 ≥ 0.99) by comparing the retention time of the peaks of the sample with those of the known standards.
Antioxidant activity
The antioxidant activity of successive extracts was determined using DPPH and ABTS analysis. The antioxidant activity of extracts was calculated from the percentage scavenged according to Eq. (4). The plot of percentage scavenged versus extract concentration was used to calculate the IC50% value of each extract. IC50% is the extract concentration required to scavenge 50% of radicals in vitro. The DPPH and ABTS assays are briefly mentioned below.
where Acontrol = Absorbance of control, Asample = Absorbance of sample.
DPPH assay
The DPPH assay was performed using the method described by Sen et al. (2013). 1 mL DPPH reagent (0.1 mM) was added to various concentrations of 3 mL extract solution and vortexed. After incubated in dark for 30 min, absorbance was measured at 517 nm.
ABTS assay
The ABTS antioxidant assay was performed following Re et al. (1999). ABTS free radical was prepared by mixing 7 mM ABTS and 2.45 mM potassium persulfate solutions with a 1:1 ratio. After 12–16 h of incubation in dark, ABTS solution was diluted until the absorbance at 734 nm was about 0.7 ± 0.05. ABTS solution (2.5 mL) was thoroughly mixed with various concentrations of 0.5 mL extract and absorbance was measured after 20 min incubation. The IC50% values from both assays were compared with pure ascorbic acid.
Global antioxidant score (GAS)
T-score was used to calculate the GAS value. T-score is calculated by using Eq. (5):
where max and min represent the maximum and minimum values of variable X among the investigated extract, respectively (Leeuw et al., 2014). The variables assigned were TPC, TFC, and the percentage scavenged at 50 µg/mL extract concentration in DPPH and ABTS.
Antibacterial activity
Minimum inhibitory concentration (MIC)
MIC was estimated against eight bacterial strains using the procedure outlined by Sarker et al. (2007). Extracts were dissolved in 10% DMSO and sterilized with a 0.22 µm Millipore express filter. They were subjected to serial dilution (1000 µg/mL to 0.98 µg/mL). Then 50 µL of the sample, 30 µL of 3.3X broth media, and 10 µL of the bacterium were pipetted into the wells of 96 well plate in an aseptic condition. The positive control well contained 10% DMSO, media, and bacterial suspension, while the negative control well contained 10% DMSO and media. After incubating for 12–14 h with appropriate bacterium growth temperature, 10 µL resazurin indicator (1 mg/mL) was added to each well. The final concentration of bacterium in each well was 5 × 105 CFU/mL. The MIC of the extract was determined by checking the conversion of the color from blue to pink. The lowest concentration at which the blue color did not change to pink was the MIC of the extract.
Zone of inhibition (ZoI)
Agar well diffusion assay was done following the method of Bauer et al. (1966). 100 µL of bacterium culture (100 µL of 103 CFU/mL) was swabbed on the solidified agar surface. After the culture was absorbed, three holes were pierced into the agar surface. 50µL sterilized solutions (1 mg/mL) extracts of SBT berries and leaves were drained into the holes and kept in the laminar airflow for 45 min until the solution was well diffused into the solidified agar. The plates were incubated and ZoI was measured using a geometric ruler after 12–14 h of incubation.
Statistical analysis
All the experiments such as physicochemical analysis, phytochemical analysis, successive extraction, polyphenol content (TPC and TFC), and antioxidant and antibacterial activity (ZoI) were performed in duplicate of two individual experiments. All the data were reported as mean ± standard deviation. One-way ANOVA using MATLAB 2019a software was considered to check the significance level.
Results and discussion
The physicochemical properties of the whole berries and the 50–50% mixture of leaves of the male and female H. Salicifolia were investigated. The berries and the leaves of H. Salicifolia were successively extracted using hexane, chloroform, ethyl acetate, acetone, methanol, and water; and the yield of extraction, polyphenolic content, and the antioxidant and antibacterial activities of extracts were evaluated.
Proximate and CHNS analysis
The proximate and CHNS analysis of the whole berries and mixture of SBT leaves are presented in Table S1. Results of the study revealed slightly higher ash content in SBT leaves (3.85 ± 0.06%) than berries (3.23 ± 0.01%). The ash content of the sample used in the present study closely matches with the value reported by Attrey et al. (2012) for Indian H. rhamnoides. Leaves of Indian H. Salicifolia showed a significant difference in their ash content when compared with Poland H. rhamnoides variety (4.79–5.09%). Ash content in the berries was observed to be higher than the Romanian (2.83%) (Ion et al., 2019) and Indian (1.76%) (Bal et al., 2011) varieties of H. rhamnoides. In both samples (berries and leaves mixture), the portion of acid-insoluble ash was higher than water-soluble ash. Ash content of berries and leaves sample is under acceptable limits which suggest less physiological (plant tissue) and non-physiological (sand or soil) contamination.
The berries used in the present work exhibited 1.7-fold higher fixed carbon content than the leaves, while leaves had higher volatile matter. The volatile matters are mainly alcohols and esters of short-chain, branched, or n-fatty acids that affect the aroma and sensory characteristics of fruit and vegetables. These values were lower than the previously reported fixed carbon (20.48%) and volatile matter (77.55%) in Netherlands H. Rhamnoides foliage (Tiller et al., 2020). The CHNS analysis of berries and leaves revealed percentage of C (45.93 ± 0.35 and 47.10 ± 0.33%), H (5.88 ± 0.2 and 6.73 ± 0.12%) and low percentage of N (1.93 ± 0.11 and 3.75 ± 0.14%) and S (0.05 ± 0.02 and 0.08 ± 0.01%), respectively.
Elemental analysis
Elemental analysis of SBT berries and leaves by EDX analyzer showed 15 microelements that are necessary for human nutrition (Table S2). Potassium was the predominant element in both samples, while sodium and calcium were the second most abundant element in the berries and leaves, respectively. Other microelements such as calcium, silicon, magnesium, and phosphorus content of the leaves were substantially higher than berries. The composition of minerals like Potassium, Sodium, Magnesium, and some other elements of the berries were as stated for Chinese, Indian, and Romanian H. rhamnoides L. (Bal et al., 2011; Ion et al., 2019). However, compared to the reported literature, elements such as phosphorus, magnesium, calcium, zinc, iron, and silver showed significant variation. This variation could be attributed to the genetic difference and water holding capacity of the soil. Despite the well-documented SBT leaves and berries, no data is available in the literature on the mineral composition of SBT leaves.
Quantitative phytochemical analysis
The results of soluble and reducing sugar, protein, tannin, and vitamin C contents of SBT berries and leaves are presented in Table 1. Several authors have reported the sugar, protein, tannin, and vitamin C content in European and Chinese SBT (Hippophae rhamnoides L. species). Despite numerous benefits to nutritional values and biological activities, information on sugar content, protein, and tannin content in Indian H. salicifolia berries and leaves is rather limited. The total soluble sugar of leaves and berries was found to be 138.64 ± 3.3 and 120 ± 1.4 mg GE/g, respectively. The non-reducing sugar content was 30.51 ± 0.7 and 41.14 ± 2.3 mg GE/g for berries and leaves, respectively. Interestingly, the sugar content was found higher in leaves than in berries. A similar trend was also reported by Akšić et al. (2019) for different varieties of Blueberry and Strawberry, where the sugar content in leaves was higher than in fruits. They described that the ratio of sugar between fruits and leaves of a plant is dependent on the translocation of sugars from leaves to fruits which is strongly controlled by ecological factors such as light, CO2 concentration, temperature, etc. Another study also suggests that higher sugar content in plant tissue leads to higher production of secondary plant metabolites, specifically, phenolic and flavonoid compounds (Ibrahim et al., 2011). The present study also reveals that there is a higher concentration of TPC and TFC in SBT leaves than in the berries. The results of the present study could be supported by the above discussed two possible reasons. The results obtained in the present study are in the range of the total soluble sugar content (130 mg/g) of Chinese (Hussain et al., 2014) and reducing sugar content (9.7–59.1 mg/g) of Finnish (Tang and Tigerstedt, 2001) in the berries of H. rhamnoides. The content of protein is a good indicator of food value and the potential of dietary supplementation of SBT berries and leaves. The protein content of SBT leaves (102.86 ± 1.1 mg BSAE/g) was found to be higher than the berries (41.87 ± 1.6 mg BSAE/g). These values are similar to that reported in the berries of Romanian H. rhamnoides L. (3.99–45.35 mg/g) by Oprica et al. (2007). Similarly, the protein content of leaves in the present study is in accord with the reports by Kashif and Ullah (2013) and Stobdan et al. (2011).
Vitamin C is the main component of SBT berries with a 5 to 100-fold higher concentration than any other fruits or vegetables (Bal et al., 2011). The vitamin C content of berries and leaves was found to be 256.10 ± 1.3 and 106.47 ± 3.6 mg AAE/100 g, respectively (Table 1). Vitamin C content in the berries closely matched with the reported values for Canada ‘Terhi’ and ‘Tytti’ cultivars (200–300 mg/100 g) (Karhu, 2003), European (28–310 mg/100 g) (Yao et al., 1992), and Indian H. rhamnoides (168.3–509 mg/100 g) (Arimboor et al., 2006), but lower than Chinese Sinensis (200–2500 mg/100 g) (Bal et al., 2011). For leaves, Krejcarová et al. (2015) reported that the vitamin C content of Czech H. rhamnoides levels up to 370 mg/100 g which is higher than the present result. This variation in Vitamin C is mainly due to genotype, cultivar, and ripening stage and time of harvest. Ascorbic acid is an essential vitamin for the human body for its nourishment, oxidation scavenger, antibacterial, and other potent physiological actions.
Tannin-rich foods are considered to be of low nutritional value due to the adverse effect of tannins on protein digestibility. However, recent studies indicated that tannins are the leading contributors to antioxidant (Šne et al., 2013a; 2013b) and antimicrobial (Ncube et al., 2017) potency and provide positive health benefits. The total tannin content in the leaves (80.1 ± 2.63 mg TAE/g) was two-fold higher than berries (42 ± 0.65 mg TAE/g) as shown in Table 1. The total tannin content in the leaves was in agreement with the report of 80 mg/g by Krejcarová et al. (2015) and the range between 59 and 350 mg/g by Stobdan et al. (2011) for Indian H. rhamnoides. However, it was 11-fold higher than the values reported by Kuhkheil et al. (2017) for Iranian H. rhamnoides which ranges from 1.99 to 5.74 mg/g. The difference in tannin between the Hippophaė genus is mainly attributed to the difference in species, extraction method, and type of solvent, etc. (Heinäaho et al., 2006; Stobdan et al., 2011).
Successive extraction
The successive Soxhlet extraction of SBT berries and leaves resulted in a total yield of 66.37% and 39.19%, respectively (Table S3). In both cases, methanol offered the highest yield because it dissolves a wide range of polar and non-polar compounds. On the other hand, acetone extract of berries and chloroform extract of leaves resulted in the lowest yield. The fat content in berries (14.54 ± 0.47%) was found 3-fold higher than leaves (3.93 ± 0.13%). These values are consistent with the result reported by Biel and Jaroszewska (2017) with H. rhamnoides variety from Poland and sinensis species from China (Yang and Kallio, 2001).
Qualitative phytochemical screening
The phytochemical screening results of SBT berries and leaves extracts are presented in Table S4. Carbohydrate, glycoside, protein, and amino acid were more noticeable in polar and moderately polar solvents. Alkaloid test was more appreciable in hexane and decline as the extraction progressed from non-polar to polar solvents and were not noticed in the aqueous extract of the berries and aqueous and methanol extracts of the leaves. It is confirmed from the qualitative analysis that all extracts contained tannin, phenolic, and flavonoid compounds. These compounds were more noticeable in acetone, methanol, and aqueous extracts followed by ethyl acetate, chloroform, and hexane extracts. A similar finding was reported by Ahmad and Ali (2013) in that many phytochemicals were presented in methanol extracts. Qualitative phytochemical screening of leaves extracts was better than berries extracts.
FTIR analysis
FTIR transmission plots of berries powder, leaves powder, and respective successive extractives are presented in Fig. 1. The characteristic absorption bands of the berries and leaves (Fig. 1A, B) at 3296 cm−1 correspond to the O–H stretching and H-bond that confirmed the presence of phenols and alcohols. The presence of aromatic rings of polyphenols and flavonoids in the berries, leaves, and successive extracts was confirmed with the peaks at 3436 cm−1 (OH), 1734–1743 cm−1 (O=C), and the in 3650–3584 cm−1 region which belongs to O–H stretching vibrational bands. The peaks at 2922 and 2853 cm−1for berries and leaves samples attributed to CH2 antisymmetric and symmetric stretching of an aliphatic chain of lipids, respectively. The sharp signal of these peaks for the berries is evidence of higher fat content. Moreover, these peaks reappeared in hexane and chloroform extracts of both berries and leaves (Fig. 1C, D). The spectra of extracts imparted the identical pattern of absorption bands with a minor difference revealing the presence of various functional groups including alkanes (2853), alkenes (2800–3000), aromatic rings (1365), and carbonyl (1734–1743) groups. Similar results of FTIR analysis have been reported for various cultivars of Romanian H. rhamnoides ssp. carpatica (Pop et al., 2014).
Total phenolic and flavonoid contents
The total phenolic (TPC) and flavonoid (TFC) contents of all the successive extracts are presented in Table 2. The TPC of berries extracts ranged from 11.99 ± 0.82 to 48.45 ± 1.94 mg GAE/g of extract. The aqueous extract had the highest concentration of TPC, followed by methanol, and the lowest in ethyl acetate extract. But in the case of the leaves, methanol extract (157.97 ± 0.86 mg GAE/g of extract) was found to contain the highest TPC followed by aqueous extract (144.63 ± 0.47 mg GAE/g) and least TPC in chloroform extract (15.18 ± 0.53 mg GAE/g). TFC of methanolic extract of berries was highest (28.93 ± 2.08 mg QE/g), followed by aqueous (24.81 ± 0.64 mg QE/g), and lowest in the ethyl acetate extract (9.73 ± 1.61 mg QE/g). On the other hand, TFC of leaves extracts ranged from 15.18 ± 0.53 to 157.97 ± 0.86 mg GAE/g of extract, being highest in acetone extract followed by methanol extract.
Computation of TPC/TFC per gram of sample (dry weight) in each extract was important to compare the amount of polyphenols extracted by a given solvent. The successive solvent extract gives the advantage to understand the TPC/TFC distribution cumulatively in each sample (berries or leaves). For instance; the concentration of TPC per gram of methanolic extracts of berries was lower (39.24 ± 1.3 mg GAE/g) than the concentration in aqueous extract (48.45 ± 1.94 mg GAE/g), but because of the higher extract yield, the TPC dry weight value of methanolic extract (8.68 mg GAE/g DW) was higher than the aqueous extract (3.97 mg GAE/g DW). The TFC yield in acetone and methanol extracts of the leaves also showed an identical phenomenon with TFC yield in methanol (10.58 mg QE/g DW) being higher than acetone (2 mg QE/g DW) extract. The grand total TPC achieved from the entire successive extraction of berries and leaves was 19.12 mg GAE/g of berries (DW) and 49.75 mg GAE/g of leaves (DW). Similarly, the TFC was found to be 13.58 mg QE/g of berries (DW), and 23.24 mg QE/g of leaves (DW), respectively. Overall, the extracts from leaves had higher TPC and TFC than berries. The results of TPC per g of extract of berries extracts were comparable with Romanian H. rhamnoides extract (10.12 to 18.66 mg GAE/g) (Criste et al., 2020). Noteworthy result is that the grand total TPC (19.12 mg GAE/g DW) in the present study was comparable with TPC values ranging from 20.78 to 34.60 mg GAE/g DW (crude methanolic extract) and 20.78 to 34.60 mg GAE/g DW (crude ethanol extract) for Iranian and Turkian H. rhamnoides, respectively (Ercisli et al., 2007; Kuhkheil et al., 2017). Likewise, TFC values for berries (13.58 mg QE/g DW) in this study were approximately similar to those reported values for other varieties of SBT (5.63–14.37 mg rutin equivalent/g (DW) (Pop et al., 2013) for Romanian and 3.54–8.54 mg/g for European H. rhamnoides variety. On the other hand, TFC per gram of extract of berries was found to be higher than that obtained by Criste et al. (2020) for Romanian H. rhamnoides cultivars (6.57–9.01 mg QE/g of extract).
Regarding leaves extract, Saikia and Handique (2013) reported that the crude methanolic extract of Indian H. salicifolia (from Sikkim-Himalayas region) had the highest concentration of TPC (98.7 mg GAE/g of extracts), followed by acetone, chloroform, and petroleum ether extracts. These values were lower than the TPC per gram of acetone, methanol, and aqueous extracts obtained from the current successive extraction. Criste et al. (2020) worked with four Romanian varieties of SBT leaves and observed TPC ranged from 41.6 to 48.12 mg GAE/g, emphasizes that the leaves had significantly higher TPC than berries (p < 0.05). According to Kumar et al. (2011), TPC in ethanol extracts of leaves of Indian H. rhamnoides ranging from 43.77 to 77.85 mg GAE/g DW, which is in agreement with the grand TPC (48.75 mg GAE/g DW) of the present study. In comparison, Upadhyay et al. (2010) analyzed aqueous and hydroalcoholic extracts of H. rhamnoides from North-West Himalayas and reported the TPC of 40.49 and 56.28 mg GAE/g DW, respectively.
The TFC of acetone, methanol, and aqueous extracts of H. salicifolia leaves was approximately 2-fold higher than the Romanian H. rhamnoides which contains 31.53–36.58 mg QE/g of extract. The grand total TFC (23.24 mg QE/g DW) of H. Salicifolia agreed with the previous report by Upadhyay et al. (2010). A large variation of TPC and TFC designates the composition of SBT is dependent on many parameters such as extraction method, time and temperature, type of solvent, harvest time, subspecies and cultivars, genotype, and geographic factors. Phenolic and flavonoid compounds are the most important bioactive compound which plays an important role as an antioxidant and antibacterial agent, with significant nutritional and health-promoting abilities (Pop et al., 2014). Hence, it could be concluded that acetone, methanol, and aqueous extracts of the berries and the leaves of H. salicifolia could be potent sources of phenolic compounds. In general, successive extracts (ethyl acetate, acetone, methanol, and aqueous extracts) of whole berries and the mixture of leaves in the present study showed a higher concentration of TPC and TFC than the previous studies on either male or female leaves individually (Gupta and Kaul, 2017; Singh et al., 2014; Šne et al., 2013a; 2013b).
HPLC analysis
The identification and quantification of different phenolic and flavonoid compounds present in SBT leaves and berries extracts are presented in Table 3. While, the HPLC chromatograms of the standards and extracts of berries and leaves are given in Fig. S1 (A-C) and Fig. S2 (A-C), respectively. In this study, different polyphenols identified in berries and leaves were gallic acid, caffeic acid, ferulic acid, p-coumaric acid, myricetin, quercetin, kaempferol, and rutin. These compounds were identified mostly in methanolic, acetone, aqueous, and ethyl acetate extracts and were found below the detection limit in hexane and chloroform extracts.
The concentration of polyphenolic compounds found in leaves extracts was higher than berries extracts. Gallic acid was the most abundant analyte in the leaves (50.93 ± 0.33 to 144.09 ± 0.64 mg/g) and berries (6.25 ± 0.16 to 30.18 ± 0.78 mg/g) extracts. It was the only compound detected in the highest concentration in the aqueous extract of berries compared to other phenolic compounds. The gallic acid content of Indian H. salicifolia leaves in the current study was in agreement with the ethyl acetate fraction of H. rhamnoides (Kumar et al., 2013). However, it was found to be less than Indian (West Himalaya) H. rhamnoides leaf extracts (314.09 mg/g). The content of caffeic acid in berries extracts was considerably lower ranged from (0.88 ± 0.02 mg/g (ethyl acetate)) to 1.18 ± 0.01 mg/g (methanol)), while it was the second major phenolic compound in leaves ranging from 9.73 ± 0.01 mg/g (ethyl acetate) to 64.47 ± 0.56 mg/g (methanol). The p-coumaric acid content of leaves samples in the present study was comparatively higher than reported (0.032 mg/g of extract) by Criste et al. (2020).
Rutin was a predominant dietary flavonoid found in both berries and leaves extract. The rutin content in berries ranged from 0.45 mg/g (acetone) to 1.48 ± 0.02 mg/g (methanol) and in leaves, 3.04 ± 0.01 mg/g (ethyl acetate) to 9.5 ± 1.4 mg/g (aqueous) which was higher than other flavonoid aglycones (myricetin, quercetin, and kaempferol). The rutin content in berries and leaves extracts was in the range as previously reported in other varieties of rhamnoides from different countries (Cho et al., 2017; Dong et al., 2017; Fatima et al., 2015; Kumar et al., 2013; Sharma et al., 2008). Besides, Criste et al. (2020) reported that the leaves and berries of four different Romanian H. rhamnoides cultivars as a rich source of phenolic compounds, particularly quercetin and hydrocinnamic acid derivatives. Quercetin was found to be in the range of 0.01 mg/g (methanol) to 0.15 ± 0.001 mg/g (ethyl acetate) in berries and 0.04 mg/g (aqueous) to 2.62 ± 0.01 mg/g (ethyl acetate) in leaves extracts. But, kaempferol was detected in acetone, ethyl acetate, and aqueous extract of berries. The values of quercetin and kaempferol contents of berries and leaves extracts were similar to those reported for Romanian, Korean, Chinese, and Indian H. rhamnoides (Cho et al., 2017; Dong et al., 2017; Fatima et al., 2015; Kumar et al., 2013; Sharma et al., 2008). Another flavonoid compound, myricetin was found only in berries ranged from 0.01 ± 0.003 to 0.08 ± 0.01 mg/g. Studies by Fatima et al. (2015) and Sharma et al. (2008) on SBT berries from Canada and India reported the myricetin content from 0.036 to 0.17 mg/g FW, and 0.0173 mg/g DW, respectively. Kumar et al. (2013) demonstrated that SBT leaves extracts are rich in quercetin-3-glucoside, quercetin-3-galactoside, quercetin, rutin, and kaempferol, which matched closely with SBT H. salicifolia variety in the current study.
In vitro antioxidant activity
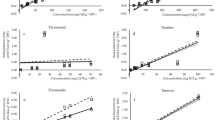
The antioxidant potency of SBT berries and mixture of leaves extracts were evaluated by comparing the 50% inhibition concentration (IC50%) of the extracts with ascorbic acid (AA) in DPPH and ABTS assays (Fig. 2A, B). All the extracts revealed significant antioxidant activity. Among all the extracts, berries-hexane extracts, leaves-hexane, and leaves-chloroform extracts had shown the lowest antioxidant activity. This is because of the presence of lower scavenging molecules compared to extracts obtained from polar and moderately polar solvents. The acetone, methanol, and aqueous extracts showed greater antioxidant activity for both assays. For berries, aqueous extract had highest antioxidant capacity with IC50% of 16.6 ± 0.48 µg/mL, followed by 22.35 ± 1.1 µg/mL (methanol) and 33.72 ± 2.3 µg/mL (acetone) in DPPH assay. The highest antioxidant activity of aqueous extracts of berries could mainly be due to its highest content of TPC and gallic acid. A similar trend of antioxidant activity for successive solvent extracts of SBT leaves (hexane (87 mg TE/g) and ethyl acetate (161 mg TE/g) and water (275 mg TE/g)) was also observed by Michel et al. (2012). Among the leaves extracts, acetone (2.42 ± 0.1 µg/mL), methanol (2.65.78 ± 0.2 µg/mL), and aqueous (3.68 µg/mL) extracts depicted the highest scavenging activity in DPPH assay. Moreover, acetone (9.48 ± 0.13 µg/mL) and methanol (8.78 ± 0.11 µg/mL) extracts of leaves has shown exceptionally stronger antioxidant capacity than pure ascorbic acid (10.35 ± 0.1 µg/mL) in ABTS assay. The activity of aqueous extract (10.48 ± 0.23 µg/mL) of leaves was also found to be almost equivalent to ascorbic acid. The in vitro antioxidant activity of the mixture of leaves in the current study were found to be better than the reported values for individual male and female leaves of SBT (Gupta and Kaul, 2017; Singh et al., 2014; Šne et al., 2013a; 2013b).
The antioxidant activity of extracts of (A) berries extracts, (B) leaves extracts in DPPH and ABTS assays, (C) global antioxidant score for berries extracts, (D) global antioxidant score (GAS) for leaves extracts. AA, ascorbic acid; HX, hexane; CL, chloroform; EA, ethyl acetate; AC, acetone; MT, methanol; AQ, aqueous
The results obtained in the current work suggest that the antioxidant capacity of extracts is more likely due to concentrations of phenolic and flavonoid contents of the extracts. A similar trend was described in other studies published earlier which also found a strong correlation between phenolic contents of extracts and antioxidant capacity evaluated by different assays (Guo et al., 2017; Sen et al., 2013). The antioxidant potency of phenolic and flavonoid compounds is mainly influenced by the extra OH group (Rice-Evans et al., 1996), thus the presence of more OH groups on the aromatic ring of polyphenol favors to the lower IC50% results in DPPH and ABTS assays for acetone, methanol, and water extracts in the current study.
In most cases, leaves extracts had shown better antioxidant capacity than the berries extracts and these results are analogous to the previous reports by (Criste et al., 2020). Sharma et al. (2008) estimated the Trolox equivalent antioxidant capacity (TEAC) of ethanol extract of berries and leaves of Indian H. rhamnoides L. using DPPH and ABTS assay and observed to vary in the range of 8.33 and 31.37 mg/g and 37.16 and 39.55 mg/g, respectively. As discussed above, it is very difficult to identify the best antioxidant extract based on the TPC, TFC, or antioxidant activity. Thus, the global antioxidant score (GAS) was calculated and the results are presented in Fig. 2C, D. The results of GAS were derived by integrating the raw data of TPC, TFC (Table 2), and the percentage scavenged at 50 µg/mL extract concentration in DPPH, and ABTS assay (Table S5). The GAS results were presented on a scale of 0 to 4 from worst to best antioxidant-containing extract. From GAS calculation, it was observed that aqueous extracts of berries had a maximum overall GAS value of 3.71, followed by methanol and acetone extracts. Whereas, among leaves extract, methanol extract exhibited a maximum GAS score of 3.93. These GAS values give a clear idea on understanding the best overall antioxidant extract and this mathematical calculation is now a day been popular in many food and beverage industries (Purohit et al., 2021a; 2021b). For instance, in the case of berries extract, TFC value of the berries-methanol extract was higher than its aqueous counterpart, whereas in DPPH assay the results were in reverse order. GAS analysis computed the overall antioxidant parameters (as explained above) and presented an overall best extract (aqueous in this case, despite TFC of methanol is higher).
Antibacterial activity
SBT leaves and berries extracts were subjected to minimum inhibitory concentration (MIC) and zone of inhibition (ZoI) study to examine the antibacterial activity and the results obtained are presented in Table 4 and Table S6, respectively. The bacterial strains used in this study are reported to be foodborne pathogen which causes serious food poisoning or infections in human (Jeong et al., 2010; Purohit et al., 2021a; 2021b). All extracts (except hexane and chloroform extracts of berries) showed a noticeable antibacterial property. Acetone and methanol extracts of the berries showed strong antibacterial potency against all bacterial strains at 125 µg/mL MIC, followed by aqueous, and ethyl acetate extract. Despite higher TPC and antioxidant capacity, the aqueous extracts of whole berries (250–500 µg/mL) and leaves (125–500 µg/mL) exhibited lower antibacterial activity than methanol and acetone extracts. A similar trend was observed by Babaeekhou and Ghane (2020) during their study on successive extraction of Z. Officinale rhizome with n-hexane, ethyl acetate, methanol, and distilled water. Criste et al. (2020), found the MIC of 12.5–25 g/mL with berries extracts and 6.2–25 g/mL with leaves extracts against S. aureus.
The methanol extracts of leaves exhibited maximum antibacterial activity with the lowest MIC of 31.3 µg/mL for S. aureus and B. subtilis. Similarly, acetone and ethyl acetate extracts showed MIC of 62.5 µg/mL for S. aureus, M. luteus, E. coli, P. aeruginosa, and S. aureus, M. luteus, respectively. The MIC of ethyl acetate, acetone, and methanol extracts of leaves in the current was lower than the crude (methanol, acetone, chloroform, and petroleum ether) leaf extracts of Indian H. salicifolia D. Don reported by Saikia and Handique (2013) in the range from 250 to 500 µg/mL against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Enterobacter aerogenes, Klebsiella pneumonia, and Pseudomonas aeruginosa.
The ZoI analysis gave a clear idea about the antibacterial nature of various extracts from the leaves and berries of SBT. All the berries extracts (except hexane and chloroform) showed the highest ZoI against B. Subtilis strain (aqueous extract = 11.5 ± 2.1 mm) followed by S. Epidermidis and M. luteus. On the contrary, leaves extracts of SBT were effective for all four strains of gram-positive bacteria. Similarly, the leaves extracts (except chloroform extract) showed remarkable inhibition for gram-negative bacteria. Most of the leaves extracts were effective against the tough E. coli (methanol extract = 14 mm) which makes the SBT leaves extract a strong antibacterial agent. Minimum to zero inhibition by berries extracts could be because of the poor penetration capacity of these extracts into the hard peptidoglycan layer of the gram-negative bacteria. The antibacterial activity of leaves extracts against S. aureus and E. coli was in the range of those obtained by Kumar et al. (2013). Upadhyay et al. (2010) compared the antibacterial properties of SBT leaf extracts and found that hydroalcoholic extract (19 mm) has better activity than aqueous extract (14 mm) with higher ZoI (19 mm) for B. cereus.
The difference in antibacterial activity of extracts is attributed to the content of bioactive compounds such as vitamins, tannins, and polyphenol compounds, especially quercetin derivatives, gallic acid, caffeic acid, and ferulic acid. It was also noticed that the antibacterial effect of phenolic compounds was more prominent than flavonoids (El-chaghaby et al., 2014). The inhibitory characteristics of phenols are related to the site(s) and the number of conjugated hydroxyl groups in such a way that more hydroxylation results in more toxicity to microorganisms (Cowan, 1999). Successive solvent extracts of berries and leaves of H. salicifolia were more effective against Gram-positive bacteria than Gram-negative. This renders Gram-negative bacteria provides hydrophilic molecules deposited in the outer membrane that objects to the leakage of hydrophobic materials into the cytoplasm.
In conclusion, the berries and the leaves of H. salicifolia grown in North-East India showed significant dietary and bioactive properties, with the leaves mainly found to be more nutritious and biologically active than the berries. Vitamin C was found to be the predominant phytonutrient found in the berries, while the total soluble sugar content of the leaves was the highest. Nevertheless, SBT leaves have not yet been significantly recognized as a food and food supplement, attempts have to be done to introduce the scope and procedure of Novel Food Regulation to use SBT leaves as food and food supplement. The berries and leaves extract provided good polyphenolic compounds such as gallic acid, caffeic acid, ferulic acid, p-coumaric acid, quercetin, myricetin, kaempferol, and rutin, with significant oxidation and food-borne bacterial inhibition activities, meaningfully higher activities with leaves extract. Further, this study compares the less known H. salicifolia of North-East India with the most popular H. rhamnoides variety in India as well as other cultivars present in the world. Our result indicated that successive extracts (ethyl acetate, acetone, methanol, and aqueous extracts) of whole berries and the mixture of leaves in the current study had a higher concentration of TPC and TFC with higher antioxidant activity than the previous reports for H. rhamnoides variety on either male or female leaves individually. Overall, the present research demonstrates the importance of H. salicifolia berries and leaves as an option to be utilized as bioactive ingredients, dietary supplements, food preservatives, and pharmaceuticals.
References
AOAC International, AOAC International. Official methods of analysis of AOAC. Association of Official Analytical Chemists International, 19th ed. AOAC International, Arlington, TX (2012)
Ahmad B, Ali J. Physiochemical, minerals, phytochemical contents, antimicrobial activities evaluation and fourier transform infrared (FTIR) analysis of Hippophae rhamnoides L. leaves extracts. African Journal of Pharmacy and Pharmacology. 7: 375-388 (2013)
Akšić MF, Tosti T, Sredojević M, Milivojević J, Meland M, Natić M. Comparison of sugar profile between leaves and fruits of blueberry and strawberry cultivars grown in organic and integrated production system. Plants. 8: 205 (2019)
Arimboor R, Venugopalan VV, Sarinkumar K, Arumughan C, Sawhney RC. Integrated processing of fresh Indian sea buckthorn (Hippophae rhamnoides) berries and chemical evaluation of products. Science of Food and Agriculture. 86: 2345-2353 (2006)
Arimboor R, Kumar KS, Arumughan C. Simultaneous estimation of phenolic acids in sea buckthorn (Hippophaë rhamnoides) using RP-HPLC with DAD. Journal of Pharmaceutical and Biomedical Analysis. 47(1): 31–38 (2008)
Attrey DP, Singh AK, Katyal J, Naved T. Pharmacognostical Characterization and Preliminary Phytochemical Investigation of Seabuckthorn (Hippophae rhamnoides L.) Leaves. Indo Global Journal of Pharmaceutical Sciences. 2: 108-113 (2012)
Babaeekhou L, Ghane M. Antimicrobial activity of ginger on cariogenic bacteria: molecular networking and molecular docking analyses. Journal of Biomolecular Structure and Dynamics 39: 2164-2175 (2020)
Bal LM, Meda V, Naik SN, Satya S. Sea buckthorn berries: a potential source of valuable nutrients for nutraceuticals and cosmoceuticals. Food Research International. 44: 1718-1727 (2011)
Basu M, Prasad R, Jayamurthy P, Pal K, Arumughan C, Sawhney RC. Anti-atherogenic effects of seabuckthorn (Hippophaea rhamnoides) seed oil. Phytomedicine. 14: 770-777 (2007)
Bauer A, Kirby W, Sherris J. Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology. 43, 493-496 (1966)
Biel W, Jaroszewska, A. The nutritional value of leaves of selected berry species. Scientia Agricola. 74: 405-410 (2017)
Cho CH, Jang H, Lee M, Kang H, Heo HJ, Kim D-O. Sea buckthorn (Hippophae rhamnoides L.) leaf extracts protect neuronal PC-12 cells from oxidative stress. Journal of Microbiology and Biotechnology. 27, 1257-1265 (2017)
Cowan MM. Plant products as antimicrobial agents. American Society for Microbiology. 8: 564-582 (1999)
Criste A, Urcan AC, Bunea A, Furtuna FRP, Olah NK, Madden RH, Corcionivoschi N. Phytochemical composition and biological activity of berries and leaves from four Romanian Sea Buckthorn (Hippophae Rhamnoides L.) varieties. Molecules. 25(5), 1-21 (2020)
Dong R, Su J, Nian H, Shen H, Zhai X, Xin H, Qin L, Han T. Chemical fingerprint and quantitative analysis of flavonoids for quality control of Sea buckthorn leaves by HPLC and UHPLC-ESI-QTOF-MS. Journal of Functional Foods. 37, 513-522 (2017)
El-chaghaby GA, Ahmad AF, Ramis ES. Evaluation of the antioxidant and antibacterial properties of various solvents extracts of Annona squamosa L. leaves. Arabian Journal of Chemistry 7: 227-233 (2014)
Ercisli S, Orhan E, Ozdemir O, Sengul M. The genotypic effects on the chemical composition and antioxidant activity of sea buckthorn (Hippophae rhamnoides L.) berries grown in Turkey. Scientia Horticulturae. 115: 27-33 (2007)
Fatima T, Kesari V, Watt I, Wishart D, Todd JF, Schroeder WR, Paliyath G, Krishna P. Metabolite profiling and expression analysis of flavonoid, Vitamin C and tocopherol biosynthesis genes in the antioxidant-rich sea buckthorn (Hippophae rhamnoides L.). Phytochemistry. 118: 181-191 (2015)
Guo R, Guo X, Li T, Fu X, Liu RH. Comparative assessment of phytochemical profiles, antioxidant and antiproliferative activities of Sea buckthorn (Hippophaë rhamnoides L.) berries. Food Chemistry. 221: 997-1003 (2017)
Gupta D, Kaul V. Antioxidant activity vis-a-vis phenolic content in leaves of Seabuckthorn from Kargil District (J and K, India): a preliminary study. National Academy Science Letters. 40: 53-56 (2017)
Heinäaho M, Pusenius J, Julkunen-Tiitto R. Effects of different organic farming methods on the concentration of phenolic compounds in sea buckthorn leaves. Journal of Agricultural and Food Chemistry. 54: 7678-7685 (2006)
Hussain MM, Ali S, Awan S, Hussain MM, Hussain I. Analysis of minerals and vitamins in sea buckthorn (Hippophae rhamnoids) pulp collected from Ghizer and Skardu districts of Gilgit-Baltistan. International Journal of Biosciences. 6655: 144-152 (2014)
Ibrahim MH, Jaafar HZE, Rahmat A, Rahman ZA. The relationship between phenolics and flavonoids production with total non structural carbohydrate and photosynthetic rate in Labisia pumila Benth. under high CO2 and nitrogen fertilization. Molecules. 16: 162-174 (2011)
Ion VA, Parvulescu OC, Velcea D, Popa O, Ahmadi M. Physico-chemical parameters and antioxidant activity of Romanian Sea Buckthorn Berries. Revista de Chimie 70: 4187-4192 (2019)
Jeong JH, Lee JW, Kim KS, Kim J-S, Han SN, Yu CY, Lee JK, Kwon YS, Kim MJ. Antioxidant and antimicrobial activities of extracts from a medicinal, Sea Buckthorn. J Journal of Korean Society Applied Biological Chemistry. 53: 33-38 (2010)
Karhu S. New sea buckthorn cultivars Terhi, Tytti and Tarmo, pp. 83-84. In T Hovi, S Karhu, M-M Linna, T Suojala (eds) Harvest—horticultural research results 2000-2002 (2003)
Kashif M, Ullah S. Chemical composition and minerals analysis of Hippophae rhamnoides, Azadirachta indica, Punica granatu and Ocimum sanctum Leaves. World Journal of Dairy and Food Sciences. 8: 67-73 (2013)
Khandelwal KR. Practical pharmacognosy (24th ed.). Pragati Books Pvt. Ltd (2008)
Krejcarová J, Straková E, Suchý P, Herzig I, Karásková K. Sea buckthorn (Hippophae rhamnoides L.) as a potential source of nutraceutics and its therapeutic possibilities—A review. Acta Veterinaria Brno. 84: 257-268 (2015)
Kuhkheil A, Badi HN, Mehrafarin A, Abdossi V. Chemical constituents of sea buckthorn (Hippophae rhamnoides L.) fruit in populations of central Alborz Mountains in Iran. Research Journal of Pharmacognosy 4(3): 1-12 (2017)
Kumar MSY, Dutta R, Prasad D, Misra K. Subcritical water extraction of antioxidant compounds from Seabuckthorn (Hippophae rhamnoides) leaves for the comparative evaluation of antioxidant activity. Food Chemistry. 127: 1309-1316 (2011)
Kumar MSY, Tirpude RJ, Maheshwari DT, Bansal A, Misra K. Antioxidant and antimicrobial properties of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves in vitro. Food Chemistry. 141: 3443-3450 (2013)
Leeuw R, Kevers C, Pincemail J, Defraigne JO, Dommes J. Antioxidant capacity and phenolic composition of red wines from various grape varieties: specificity of Pinot Noir. Journal of Food Composition and Analysis. 36: 40-50 (2014)
Lowry HO, Rosebrough JN, Farr LA, Randall LR. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 193: 265-275 (1951)
Michel T, Destandau E, Floch GL, Lucchesi ME, Elfakir C. Antimicrobial, antioxidant and phytochemical investigations of sea buckthorn (Hippophaë rhamnoides L.) leaf, stem, root and seed. Food Chemistry. 131: 754-760 (2012)
Ncube NS, Afolayan AJ, Okoh AI. Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. African Journal of Biotechnology. 7: 1797-1806 (2017)
Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food and Chemical Toxicology. 97, 199-204 (2016)
Olas B, Skalski B, Ulanowska K. The anticancer activity of sea buckthorn [Elaeagnus rhamnoides (L.) A. Nelson]. Frontiers in Pharmacology. 9, 1-8 (2018)
Oprica L, Olteanu Z, Zamfirache MM, Truta E, Surdu S, Rati IV, Manzu C, Milian G, Rosu C. The content of soluble proteins in Hyppophae rhamnoides ssp. carpathica varieties harvested from different regions of Romania, pp. 73-79. In: 3rd international Sea Bucthorn Association Conference (2007)
Padwad Y, Ganju L, Jain M, Chanda S, Karan D, Banerjee PK, Sawhney RC. Effect of leaf extract of Seabuckthorn on lipopolysaccharide induced inflammatory response in murine macrophages. International Immunopharmacology. 6: 46-52 (2006)
Piltz J, Law D. IFIA-laboratory methods manual. Australian Fodder Industry Association Inc, Balwyn (2007)
Polshettiwar SA, Ganjiwale RO, Wadher SJ, Yeole PG. Spectrophotometric estimation of Total tannin in some ayurvedic eye drop. Ind J Pharm Sci. 69: 574-576 (2007)
Pop RM, Buzoianu AD, Rati IV, Socaciu C. Untargeted metabolomics for sea buckthorn (Hippophae Rhamnoides ssp. carpatica) berries and leaves: Fourier transform infrared spectroscopy as a rapid approach for evaluation and discrimination. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 42: 545-550 (2014)
Pop RM, Socaciu C, Pintea A, Buzoianu AD, Sanders MG, Gruppen H, Vincken J-P. UHPLC/PDA-ESI/MS analysis of the main berry and leaf flavonol glycosides from different Carpathian Hippophaë rhamnoides L. varieties. Phytochemical Analysis. 24: 484-492 (2013)
Purohit S, Barik CR, Kalita D, Sahoo L, Goud VV. Exploration of nutritional, antioxidant and antibacterial properties of unutilized rind and seed of passion fruit from Northeast India. Journal of Food Measurement and Characterization. 15: 3153-3167 (2021)
Purohit S, Kalita D, Barik CR, Sahoo L, Goud VV. Evaluation of thermophysical, biochemical and antibacterial properties of unconventional vegetable oil from Northeast India. Materials Science for Energy Technologies. 4: 81-91 (2021)
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 26: 1231-1237 (1999)
Rice-Evans CA, Miller NJ, Paganga G. Structure- antioxidant activity relationships of flavonoids and phenolic acid. Free Radical Biology and Medicine. 20: 933-956 (1996)
Sadasivam S, Manickam A. Biochemical methods, New Delhi, New Age. 3rd ed. International Publishers. New Age International (P) Limited (2008)
Saikia M, Handique PJ. Antioxidant and antibacterial activity of leaf, bark, pulp and seed extracts of seabuckthorn (Hippophae salicifolia D. Don) of Sikkim Himalayas. Journal of Medicinal Plants Research. 7: 1330-1338 (2013)
Samant SS, Dhar U, Palni LMS. Medicinal plants of Indian Himalaya: diversity, distribution and potential value. G. B. Pant Inst. of Himalayan Environment and Development, Almora (1998)
Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 42: 321-324 (2007)
Sen S, De B, Devanna N, Chakraborty R. Total phenolic, total flavonoid content, and antioxidant capacity of the leaves of Meyna spinosa Roxb., an Indian medicinal plant. Chinese Journal of Natural Medicines. 11: 149-157 (2013)
Sharma UK, Sharma K, Sharma N, Sharma A, Singh HP, Sinha AK Microwave-assisted efficient extraction of different parts of Hippophae rhamnoides for the comparative evaluation of antioxidant activity and quantification of its phenolic constituents by reverse-phase high-performance liquid chromatography (RP-HPLC). Journal of Agricultural and Food Chemistry. 56: 374-379 (2008)
Singh AK, Deep P, Dubey S, Attrey DP, Naved T. Comparative studies on antioxidant activity, total phenol content and high performance thin layer chromatography analysis of seabuckthorn (Hippophae rhamnoides l) leaves. Pharmacognosy Journal. 6: 5-8 (2014)
Šne E, Galoburda R, Segliņa D. Sea buckthorn vegetative parts—a good source of bioactive compounds. Proceedings of the Latvian Academy of Sciences, Section B: Natural, Exact, and Applied Sciences. 67: 101-108 (2013)
Šne E, Segliņa D, Galoburda R, Krasnova I. Content of phenolic compounds in various sea buckthorn parts. Proceedings of the Latvian Academy of Sciences, Section B: Natural, Exact, and Applied Sciences. 67: 411-415(2013)
Stobdan T, Yadav A, Mishra GP, Chaurasia OP, Srivastava RB. Seabuckthorn: The Super Plant. Defence Institute of High Altitude Research, Defence Research and Development Organisation (2011)
Tang X, Tigerstedt PMA. Variation of physical and chemical characters within an elite sea buckthorn (Hippophae rhamnoides L.) breeding population. Scientia Horticulturae 88: 203-214 (2001)
Tiller MB, Oswald BP, Schuijn M. Preliminary flammability assessment of Sea Buckthorn from the Netherlands compared to known flammable shrubs of the southern and western. Journal of Forest Research. 9(3), 1-6 (2020)
Upadhyay NK, Kumar MSY, Gupta A. Antioxidant, cytoprotective and antibacterial effects of Sea buckthorn (Hippophae rhamnoides L.) leaves. Food and Chemical Toxicology. 48: 3443-3448 (2010)
Ursache F-M, Ghinea IO, Turturica M, Aprodu I, Râpeanu G, Stanciuc N. Phytochemicals content and antioxidant properties of sea buckthorn (Hippophae rhamnoides L.) as affected by heat treatment—quantitative spectroscopic and kinetic approaches. Food Chemistry. 233: 442-449 (2017)
Xing J, Yang B, Dong Y, Wang B, Wang J, Kallio HP. Effects of sea buckthorn (Hippophaë rhamnoides L.) seed and pulp oils on experimental models of gastric ulcer in rats. Fitoterapia. 73: 644-650 (2002)
Yang B, Kallio H. Composition and physiological effects of sea buckthorn (Hippophaë) lipids. Trends in Food Science and Technology. 13: 160-167 (2002)
Yang B, Kallio HP. Fatty acid composition of lipids in sea buckthorn (Hippophaë rhamnoides L.) berries of different origins. Journal of Agricultural and Food Chemistry. 49: 1939-1947 (2001)
Yao Y, Tigerstedt APM, Joy P. Variation of vitamin C concentration and character correlation between and within Natural Sea Buckthorn (Hippophae rhamnoides L.) populations. Acta Agriculturae Scandinavica. 42: 12-17 (1992)
Acknowledgements
Mr. Abebe Moges would like to acknowledge sponsorship from Haramaya University under the Ethiopian Ministry of Science and Higher Education, Govt of Ethiopia. The authors gratefully acknowledge to Department of Biotechnology (DBT) (grant no. BT/311/NE/TBP/2012), Government of India, for their fund and support to conduct this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moges, A., Barik, C.R., Purohit, S. et al. Dietary and bioactive properties of the berries and leaves from the underutilized Hippophae salicifolia D. Don grown in Northeast India. Food Sci Biotechnol 30, 1555–1569 (2021). https://doi.org/10.1007/s10068-021-00988-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-021-00988-8