Abstract
Reactivity of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical in methanol, ethanol, isopropanol, isooctane, and ethyl acetate, was evaluated to assess the antioxidant capabilities in medium chain triacylglycerol. DPPH loss values were obtained over 30 min, with sampling every 5 min. Even the same concentration of antioxidants showed different DPPH reactivity depending on solvent. In methanol, 5 min was enough for α-tocopherol to react with DPPH, whereas BHT did not react with DPPH even after 30 min. Gallate series showed higher DPPH reactivity than TBHQ, sesamol, or BHA in methanol, while lower reactivity in isooctane. Antioxidants in ethanol and isopropanol reacted with DPPH less efficiently compared to those in methanol, the exception being sesamol. DPPH reactivity of gallate series in isooctane was lower than that of sesamol, TBHQ, and α-tocopherol. Combinatorial usage of methanol and isooctane for DPPH reactivity could provide reliable information on the antioxidant capacities of chemicals in edible oils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The beneficial effects of some edible oils on health are partially related to the presence of phenolic antioxidants, lipophilic tocopherols, and high percentage of unsaturated fatty acids (Wu et al., 2019). Phenolic compounds and tocopherols possess radical scavenging ability for lipid radicals, including alkyl, alkoxyl, and peroxyl radicals (Decker, 1998). Many assays based on conventional chemical reactions, spectrometry, and/or photochemiluminescence have been employed to determine total antioxidant capacities in edible oils (Christodouleas et al., 2011; Dhavamani et al., 2014; Laguerre et al., 2007; Lee et al., 2007; Song et al., 2016). Evaluation of antioxidant activities involves determination of primary, secondary, or tertiary oxidation products in bulk oils subjected to certain oxidation stresses. The oxidative stability index, Rancimat assay, Schaal oven test, and various model systems subjected to heated or photosensitized conditions are commonly used to evaluate the oxidative stability and antioxidant activities in bulk oils (Li et al., 2019; Song et al., 2016, 2017). However, the above-mentioned assays are time-consuming and results can be influenced by experimental conditions. Therefore, it is necessary to evaluate the actual antioxidant capacities of inherent or added chemicals in bulk oils using simple, quick, and reliable methods.
2,2-Diphenyl-1-picrylhydrazyl (DPPH), a stable free radical, is used in a basic in vitro screening method for evaluating the radical scavenging activity of natural or standard compounds (Elzaawely et al., 2005; Lee et al., 2007; Prescha, et al., 2014; Song et al., 2016; Tuberoso et al., 2007). Several reports on determining the antioxidant activities in bulk oils using DPPH have been reported in the literature. Tuberoso et al. (2007) adopted the DPPH dissolved system to evaluate the methanol-soluble and insoluble fractions of edible oils. Hydrophilic methanol extract was tested using methanolic DPPH, whereas lipophilic extract and oil was analyzed using DPPH dissolved in ethylacetate. Major antioxidant compounds in the lipophilic oil fraction were often derived from tocopherol (r = + 0.70) and polyunsaturated fatty acids (r = + 0.61). Phenolic compounds in edible oils can be extracted using methanol, and reduction in the DPPH absorbance of the extract can be attributed to the extracted phenolic compounds (Tuberoso et al., 2007). The protocol using methanol soluble or insoluble oil fractions is one of strategies to determine the total antioxidant activity in edible oils (Elzaawely et al., 2005; Prescha, et al., 2014). Pulgarin et al. (2010) developed a flow injection method based on the inhibition of the luminol chemiluminescence for evaluating the radical scavenging ability of oils in a microemulsion. However, reference results were obtained from the DPPH assay. Overall, the antioxidant activity in edible oils was evaluated in two ways, soluble or insoluble in methanol, representing hydrophilic or lipophilic compounds, respectively.
Prevc et al. (2013) tested different solvents, including ethyl acetate, methanol, mixture of methanol and isopropanol, and mixture of methanol and isopropanol, with acid–base pair tris(hydroxymethyl)aminomethane and acetic acid for DPPH to determine the antioxidant activity of vegetable oils. The authors used 0.1 g vegetable oil and 9.9 mL of DPPH in each solvent in a 15 mL centrifuge tube without including any phenolic compound extraction step (Prevc et al., 2013). DPPH dissolved in isooctane can detect changes in free radical scavenging antioxidants (FRSs) and radical scavenging compounds from oxidized lipids during lipid oxidation (Lee et al., 2007; Yeo et al., 2012; Song et al., 2016).
Although diverse solvents, including methanol, isopropyl alcohol, ethyl acetate, and isooctane, have been used for the DPPH assay (Prevc et al., 2013; Song et al., 2016; Tuberoso et al., 2007), the effects of solvent types on the reactivity of DPPH toward lipophilic or hydrophilic antioxidants in bulk oil are rare in the literature.
The objective of this study was to evaluate the DPPH method for evaluating the antioxidant capacity of natural and synthetic antioxidants and oxidation products in medium chain triacylglycerol (MCT) using methanol, ethanol, isooctane, isopropyl alcohol, and ethylacetate. Preliminary study showed that DPPH loss in MCT was relatively low, which implies MCT possesses low concentration of radical scavenging chemicals. Solvents were selected based on the previous reports on determining the antioxidant activity in edible oils using DPPH (Prevc et al., 2013; Song et al. 2016; Tuberoso et al., 2007).
Materials and methods
Materials
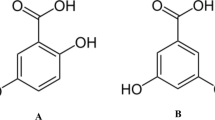
MCT was purchased from Dongbang Plastic Co. (Seoul, Korea) and used without any further refinement. Corn oil was purchased from a local grocery market (Suwon, Korea). DPPH, dimethyl sulfoxide (DMSO), silica gel (Davisil Grade 635, pore size 60 Å), tert-butylhydroquinone (TBHQ), α-tocopherol, sesamol, butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), methyl gallate (MG), propyl gallate (PG), and octyl gallate (OG) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Chemical structures of tested antioxidants are shown in Supplementary Fig. 1. Isopropyl alcohol and ethyl acetate were purchased from Daejung Chemical Co. (Seoul, Korea). Other reagent grade chemicals were purchased from Samchun Chemical Co. (Seoul, Korea).
Sample preparation
Calibration curve preparation for antioxidants in methanol and isooctane solvents
α-Tocopherol, sesamol, TBHQ, BHA, BHT, MG, PG, and OG were dissolved in 1.5 mL DMSO and vortex-mixed for 30 min. The mixtures of antioxidants and DMSO were diluted in methanol and isooctane to a final concentration of 0.5, 1.0, 1.5, and 2.0 mM.
Antioxidant preparation in each solvent
α-Tocopherol, sesamol, TBHQ, BHA, BHT, MG, PG, and OG were dissolved in 1.5 mL DMSO at a concentration of 1 mM and vortex-mixed for 30 min. The mixtures of antioxidants and DMSO were centrifuged at 12,225 g for 3 min to remove undissolved antioxidants, and then 1 mL of the supernatant was added to 20 mL of MCT. The oil samples were stirred for 2 h, and DMSO was removed from samples by nitrogen gas purging. Control sample was MCT with DMSO only.
Determination of antioxidant capacities in MCT using the DPPH assay
DPPH radicals were dissolved in each solvent, i.e., methanol, ethanol, isopropyl alcohol, isooctane, and ethyl acetate, at a final concentration of 0.1 mM. MCT samples containing antioxidants were mixed with each solvent at a final concentration of 40,000 ppm (w/v). For methanol and ethanol, the mixtures of MCT and solvent were vortex-mixed for 1 min, and centrifuged at 12,225 g for 3 min. Supernatant (0.25 mL) was reacted with 0.75 mL of 0.1 mM DPPH in methanol or ethanol.
For the assay of DPPH in isooctane, isopropyl alcohol, and ethyl acetate, 0.75 mL of 0.1 mM DPPH in isooctane, isopropyl alcohol, and ethyl acetate were mixed with 0.25 mL of each solvent containing 40,000 ppm oils (w/v), respectively.
The absorbance of the sample mixture was measured using a UV/VIS-spectrometer (Model Genesys 10uv, Thermo Fisher Scientific Inc., Waltham, MA, USA) after reacting in the dark for 5, 10, 15, 20, 25, or 30 min. The absorbance of DPPH dissolved in methanol, ethanol, isooctane, isopropyl alcohol, and ethyl acetate was determined at 517, 515, 509, 515 and 514 nm, respectively. Absorbance of DPPH was expressed as DPPH loss according to previous reports (Song et al., 2016, 2017).
Calculation of log P of chemicals
Log P of chemicals was calculated using Molinspiration Cheminformatics (Molinspiration Cheminformatics 2019).
Statistical analysis
All data of DPPH loss were analyzed statistically by one-way analysis of variance (ANOVA) and Duncan’s multiple range test using SPSS software program version 19 (SPSS Inc., Chicago, IL, USA). A P value of less than 0.05 was considered statistically significant.
Results and discussion
DPPH reactivity of the tested compounds in the alcoholic solvent system
DPPH loss in methanol (a) and isooctane (b) for different concentrations of TBHQ, α-tocopherol, sesamol, BHT, BHA, MG, PG, and OG are shown in Fig. 1. Depending on the type of antioxidants, clear differences in the reactivity of DPPH were observed. Gallate series showed high DPPH reactivity, whereas BHT showed the lowest reactivity with DPPH in methanol solvent (Fig. 1A). In case of isooctane solvent, only α-tocopherol had concentration dependent DPPH reactivity (Fig. 1B).
The slopes and coefficient of determination for DPPH reactivity of lipophilic and hydrophilic antioxidants in methanol and isooctane are shown in Table 1. The highest slopes of antioxidants in methanol and in isooctane came from octyl gallate and α-tocopherol, respectively. All the gallate series had higher slopes than other series in methanol solvent, while only three antioxidants including α-tocopherol, BHA, and BHT had detectable DPPH reactivity in the isooctane system (Table 1). Slopes from gallate series in methanol were higher than those of α-tocopherol and BHT by 1.65 and 23.0 times, respectively. Only α-tocopherol showed high DPPH reactivity in both methanol and isooctane solvent systems.
DPPH reactivity of natural antioxidants with different solvents
The DPPH loss of MCT containing α-tocopherol and sesamol in diverse solvents are shown in Fig. 2. Although the same concentration of α-tocopherol and sesamol were dissolved in MCT, different reactivity of DPPH was observed depending on solvent types. The reactivity of DPPH with α-tocopherol showed the following increasing trend: isooctane, ethyl acetate, methanol, ethanol, and isopropanol (Fig. 2A). The highest and lowest values of DPPH loss were 0.965 µmol/g with isooctane and 0.596 µmol/g with isopropanol, respectively. In ethanol, the reaction was terminated at 5 min and there was no significant difference until 30 min. In ethyl acetate, there was no significant difference in the values from 20 min to 30 min, indicating that all reactions were completed within the first 20 min (Fig. 2A).
The reactivity of DPPH with sesamol was affected by the types of solvents and decreased in the following order: isooctane, ethanol, isopropanol, methanol, and ethyl acetate (Fig. 2B). The highest and lowest DPPH loss values were 1.860 µmol/g and 0.418 µmol/g with isooctane and ethyl acetate, respectively. The reactivity increased continuously up to 30 min in all solvents except for ethyl acetate. The reactivity of DPPH with sesamol in ethyl acetate did not significantly vary from 15 min to 30 min (p > 0.05).
DPPH reactivity of synthetic antioxidants with different solvents
Effects of diverse solvents on the DPPH loss of MCT containing TBHQ, BHA, and BHT are shown in Fig. 3. For TBHQ, the highest DPPH loss value was 1.788 µmol/g (with methanol), whereas the lowest DPPH loss value was 1.360 µmol/g (with isooctane). The reactivity of TBHQ with DPPH increased in the following order: methanol, ethanol, isopropanol, ethyl acetate, ethanol, and isooctane. In the case of isooctane, there was no significant difference in DPPH values from 5 min to 30 min, indicating that all reactions occurred within the first 5 min. Generally, the reactivity of TBHQ with DPPH in MCT was higher when the solvent was an alcohol (Fig. 3A).
In case of MCT containing BHA, the highest and lowest DPPH loss values were 0.931 µmol/g and 0.345 µmol/g with isooctane and ethyl acetate, respectively. The reactivity of BHA increased in the following order: isooctane, ethanol, methanol, isopropanol, and ethyl acetate. MCT containing isopropanol and ethyl acetate did not show any significant difference up to 30 min (p > 0.05) (Fig. 3B).
The reactivity of BHT with DPPH was lower than other antioxidants, and the highest DPPH loss value was 0.182 µmol/g only (with isooctane; Fig. 3C). There were no significant differences in the DPPH loss of BHT with solvents (p > 0.05), except for isooctane from 10 to 30 min.
The DPPH loss for MCT containing MG, PG, and OG in diverse solvents are shown in Fig. 4. For MG, the highest and lowest DPPH loss values were 2.434 µmol/g and 0.245 µmol/g with methanol and isooctane, respectively. The reactivity of MG increased in the following order: methanol, ethanol, isopropanol, ethyl acetate, and isooctane (Fig. 4A). Similar trends were observed in PG and OG. The highest and the lowest DPPH loss values for PG were 2.344 µmol/g and 0.377 µmol/g, when the solvents were methanol and isooctane, respectively (Fig. 4B). For PG, the highest and lowest DPPH loss values were 2.929 µmol/g and 0.480 µmol/g with methanol and isooctane, respectively (Fig. 4C). The order of solvents for PG and OG were the same as for MG.
Relative DPPH reactivity of antioxidants with different solvents
The ratios of DPPH loss in different solvents to methanol containing TBHQ, α-tocopherol, sesamol, BHT, BHA, methyl gallate, propyl gallate, and octyl gallate at 5 min (a) and 30 min (b) are shown in Fig. 5. At 5 min reaction time, BHA, sesamol, α-tocopherol, and BHT showed higher DPPH radical scavenging capacities in isooctane than in methanol, whereas BHT and BHA in ethanol and ethyl acetate reacted with DPPH more efficiently than in the methanol system. Especially, sesamol exhibited a higher reactivity with DPPH in ethanol and isopropanol than in methanol (Fig. 5A).
After 30 min of reaction, BHT, BHA, sesamol, and α-tocopherol in isooctane had higher antioxidant capacities than in methanol (Fig. 5B). Sesamol in ethanol and isopropanol and BHT and α-tocopherol in ethyl acetate had higher DPPH reactivity values than those in methanol after 30 min reaction. Gallate series exhibited the highest and lowest reactivity with DPPH in methanol and isooctane, respectively.
Depending on the polarity of antioxidants and solvent types, different reactivity values were observed with DPPH in MCT. Antioxidants may react with DPPH radical by 4 different mechanisms including hydrogen atom transfer, proton-coupled electron-transfer, sequential proton-loss electron transfer, and electron-transfer proton-loss with solvent (Foti et al., 2008; Foti 2015). Depending on the solvents, DPPH can scavenge radicals by different mechanisms. In alcoholic solvents, electron transfer is the major pathway, whereas hydrogen atom transfer is the main mechanism for non-polar solvents (Foti et al., 2008; Foti, 2015).
The ability of solvents to form hydrogen bonds with antioxidant compounds could be critical for their DPPH reactivity. Isooctane, which has no hydrogen bond forming ability with gallate series, may not anchor gallate antioxidants and DPPH, and thus molecules of gallate series may have a lower chance to encounter DPPH in isooctane. However, in methanol, hydrogen bonds may form between the solvent, and thus molecules of gallate series and DPPH have a better chance to encounter each other.
Considering the structural characteristics and polarity of chemical compounds, the distinctive reactivity of DPPH could be explained. BHT, which has the lowest DPPH reactivity in all the tested solvents, contains a phenolic ring with two bulky hydrophobic groups at both ortho positions. BHA, which has a slightly higher DPPH reactivity, contains a phenolic ring with a bulky hydrophobic group at meta position, whereas TBHQ possess two hydroxyl groups at the para position and a bulky hydrophobic group at ortho position of the phenolic structure. Phenols with less crowd hydrophobic groups near hydroxyl group, such as BHA and TBHQ, may locate favorably compared to those with two hydrophobic groups like BHT (Supplementary Fig. 1). According to the report by Foti et al. (2008), the ortho H atoms on each of the phenol rings and o-nitro groups of the picryl ring of DPPH inhibit the access of phenolics to the nitrogen atom of DPPH. Two hydrophobic groups located near a hydroxyl group in BHT may hinder the approaches of protons to nitrogen atom sterically.
In addition, the hydrophilic characteristics of phenolic structures may play a role in DPPH reactivity in alcohol solvents. Gallate series, which possess an ester group having two oxygen atoms, had high DPPH reactivity in alcohol and low in isooctane solvents. Hydrophilic ester groups in the para position of the gallate series may prefer the methanol environment, which accelerates the reaction with DPPH. However, the hydrophobic groups of the gallate series may not harmonize with isooctane, and have low DPPH reactivity and limited accessibility toward the nitrogen radical.
Log P value—the partition coefficient of a compound between the organic and aqueous phase—and was tested to explain the different reactivity of antioxidants with DPPH. The log P value of DPPH is 5.09, which is characteristic of relative hydrophobic properties. The log P values of DPPH, methanol, and isooctane were 5.09, -0.32, and 3.67, respectively. BHT, which has a log P value of 5.43, had the lowest DPPH reactivity irrespective of the solvent type. α-Tocopherol, which has the highest log P value (9.04), showed lower DPPH reactivity than TBHQ (log P value of 3.08) and sesamol (log P value of 1.35) in alcohol and isooctane. The log P values of methyl, propyl, and octyl gallates were 0.85, 0.73, and 4.31, respectively. However, the reactivity of gallate series with DPPH in methanol was not significantly different. Therefore, the log P value alone may not explain the reactivity of antioxidants with DPPH.
Three points for explaining the difference in DPPH reactivity are suggested here, bulky hydrophilic position of the phenolic group of antioxidants, polarity of the solvents surrounding DPPH, and hydrogen bond formation between antioxidants and solvent. The bulky hydrophilic moiety of phenols should be located away from hydroxyl group, irrespective of the polarity of the solvents, which is the most important condition to obtain high DPPH radical scavenging ability. The polarity of solvents affects the solubility of phenolics having hydrophilic groups. Solvent type may govern the kinetic rates of DPPH reactivity. Most antioxidant compounds did not reach the equilibrium condition after 30 min. Gallate series in isopropyl alcohol reached the equilibrium state after 5 min, whereas those in methanol did not, which implies that the more non-polar properties of alcoholic solvents may stabilize the hydrophilic antioxidants and help achieve complete reactions. All tested antioxidants except sesamol and BHT reacted with DPPH in isopropanol for the 10 min reaction time (Fig. 1), whereas α-tocopherol only reacted with DPPH in methanol and showed a fast reaction pattern (Fig. 1). However, all compounds except sesamol in methanol did not exhibit any significant difference in DPPH loss at both 25 and 30 min (p > 0.05), which implies that a reaction duration of 30 min might be long enough to determine the radical scavenging activity of the compounds.
Although the reliability of antioxidant activity measurement using DPPH assays is questionable and influenced by many factors (Foti 2015), the DPPH method is still useful for screening antioxidant activity in bulk oil. Song et al. (2016) confirmed that DPPH in methanol could reflect the content of free radical scavenging compound (FRS) in edible oils, whereas that in isooctane could react with both FRS and total polar materials (TPM), which are typical oxidation products in lipid oxidation. The authors suggested a new parameter or ‘Antioxidant-prooxidant balance (APB) value’, which was determined using the ratio of DPPH loss in methanol and DPPH loss in isooctane. Due to the pro-oxidant nature of TPM (Choi et al. 2017), any comparison between DPPH loss in methanol and isooctane should be carefully evaluated. In combination with methanol and isooctane solvents for DPPH, the balance between antioxidants and pro-oxidative oxidation products can be determined, which can predict the oxidative stability in edible oils and processed foods prepared with edible oils.
In conclusion, the DPPH reactivity of the lipophilic and hydrophilic antioxidants in MCT was affected by the type of solvent used for dissolving DPPH. The free radical scavenging activity of antioxidants should be tested using DPPH in both methanol and isooctane. The average value of two assays could reflect the true antioxidant capacities, as some compounds including members of the gallate series showed completely different results depending on the type of solvent. However, high free radical scavenging against DPPH radical does not guarantee effective scavenging for the alkoxy radicals of lipids. The DPPH assay is one of the tools for the indirect screening for lipid radicals. True effectiveness of chemicals should be evaluated in target foods or biological products.
References
Choi HS, Kim MJ, Lee JH. Effects of polar and non-polar compounds from oxidized oils on oxidative stability in corn oil. The European Journal of Lipid Science and Technology 1: 1700312 (2017)
Christodouleas D, Papadopoulos K, Calokerinos AC. determination of total antioxidant activity of edible oils as well as their aqueous and organic extracts by chemiluminescence. Food Analytical Methods 4: 475-484 (2011)
Decker EA. Antioxidant mechanisms. pp. 397–401. In: Food lipids. Akoh K, Min DB (eds). Marcel Dekker, New York, NY, USA (1998)
Dhavamani S, Chandra Rao Y, Lokesh BR. Total antioxidant activity of selected vegetable oils and their influence on total antioxidant values in vivo: A photochemiluminescence based analysis. Food Chemistry 164: 551-555 (2014)
Elzaawely AA, Xuan TD, Tawata S. Antioxidant and antibacterial activities of Rumex japonicus HOUTT aerial parts. Biological and Pharmaceutical Bulletin 28: 2225–2230 (2005)
Foti MC. Use and abuse of the DPPH• radical. Journal of Agricultural and Food Chemistry 63: 8765-8776 (2015).
Foti MC, Daquino C, Mackie ID, DiLabio GA, Ingold KU. Reaction of phenols with the 2,2-diphenyl-1-picrylhydrazyl radical. kinetics and dft calculations applied to determine ArO-H bond dissociation enthalpies and reaction mechanism. The Journal of Organic Chemistry 73: 9270-9282 (2008)
Laguerre M, Lecomte J, Villeneuve P. Evaluation of the ability of antioxidants to counteract lipid oxidation: existing methods, new trends and challenges. Progress in Lipid Research 46: 244-282 (2007)
Lee JM, Chung H, Chang PS, Lee JH. Development of a method predicting the oxidative stability of edible oils using 2,2-diphenyl-1-picrylhydrazyl (DPPH). Food Chemistry 103: 662-669 (2007)
Li X, Li Y, Liu R, Zhao C, Jin Q, Wang X. Oxidation degree of soybean oil at induction time point under Rancimat test condition: Theoretical derivation and experimental observation. Food Research International 120: 756-762 (2019)
Molinspiration Cheminformatics. Calculation of Molecular Properties and Bioactivity Score. https://www.molinspiration.com/cgi-bin/properties. Accessed August. 27, 2020.
Prescha A, Grajzer M, Dedyk M, Grajeta H. The antioxidant activity and oxidative stability of cold-pressed oils. The Journal of the American Oil Chemists’ Society 91: 1291–1301 (2014)
Prevc T, Šegatin N, Ulrih NP, Cigić B. DPPH assay of vegetable oils and model antioxidants in protic and aprotic solvents. Talanta 109: 13-19 (2013).
Pulgarin JA, Bermejo LFG, Duran AC. Evaluation of the antioxidant activity of vegetable oils based on luminol chemiluminescence in a microemulsion. The European Journal of Lipid Science and Technology 112: 1294-1301 (2010)
Song JH, Jang EY, Kim MJ, Kim YJ, Lee JH. Development of a spectroscopic method to determine the content of free radical scavenging compounds and oxidation products in thermally oxidised oils. International Journal of Food Science and Technology 51: 2424-2432 (2016)
Song JH, Kim MJ, Kim YJ, Lee JH. Monitoring changes in acid value, total polar material, and antioxidant capacity of oils used for frying chicken. Food Chemistry 220: 306-312 (2017)
Tuberoso CIG, Kowalczyk A, Sarritzu E, Cabras P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chemistry 103: 1494–1501 (2007)
Yeo JD, Jeong MK, Lee JH. Correlation between changes of free radical compounds and DPPH absorbance during thermal oxidation. Food Science and Biotechnology 21: 199-203 (2012)
Wu G, Chang C, Hong C, Zhang H, Huang J, Jin Q, Wang X. Phenolic compounds as stabilizers of oils and antioxidative mechanisms under frying conditions: A comprehensive review. Trends in Food Science and Technology 92: 33-45 (2019)
Acknowledgements
This research was supported by a grant (NRF-2020R1A2C2006600) from the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology, Republic of Korea and High Value-added Food Technology Development Program through the iPET(Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, and Forestry, 119029-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
La, J., Kim, MJ. & Lee, J. Evaluation of solvent effects on the DPPH reactivity for determining the antioxidant activity in oil matrix. Food Sci Biotechnol 30, 367–375 (2021). https://doi.org/10.1007/s10068-020-00874-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-020-00874-9