Abstract
The effects of parthenolide (PL), a sesquiterpene lactone obtained from feverfew plant, on lipid accumulation and signaling pathway in adipocytes were investigated. PL significantly inhibited lipid accumulation and adipogenic factors during adipogenesis. In particular, PL exerted its inhibitory effects in early adipogenic stage by regulating the early adipogenic factors. In addition, PL regulated the expression of adipokines; leptin, retinol binding protein, and resistin mRNAs were downregulated, whereas adiponectin gene expression was increased. Furthermore, PL significantly reduced intracellular reactive oxygen species (ROS) production during adipogenesis. This PL-mediated regulation of ROS production was associated with the regulation of nuclear factor erythroid 2-related factor (Nrf2)-kelch-like ECH-associated protein 1 (Keap1) pathway. PL effectively increased the abundance of Nrf2 and its target proteins, heme oxygenase-1 (HO-1) and NADPH dehydrogenase 1 (NQO1), by promoting the nuclear translocation of Nrf2, indicating that PL-mediated anti-adipogenic effects are associated with the Nrf2/Keap1 pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parthenolide (PL) is a phytochemical found in the feverfew plant (Tanacetum parthenium), which has been used for a long time as a medicinal plant (Pareek et al., 2011). The feverfew plant has been traditionally used to treat various diseases including arthritis, asthma, constipation, abdominal pain, dermatitis, toothache, fever, headache, and inflammatory diseases (Pareek et al., 2011). In particular, the fresh or dried leaves of this plant are consumed for the beneficial effects (Pareek et al., 2011). Feverfew has been known to contain over 30 different sesquiterpene lactones including germacranolides, guaianolides, and eudesmanolides (Pareek et al., 2011). PL is the most abundant sesquiterpene lactone present as an active chemical in feverfew (Pareek et al., 2011). Feverfew and PL have been commercially used as ingredients for different functional health supplements (Nelson et al., 2002). Although PL has been reported to have biological activities on cancer and inflammation (Feltenstein et al., 2004), the effects of PL on lipid accumulation in adipocytes has never been evaluated.
Obesity is defined as an excess accumulation of body fat resulting from an imbalance of energy negatively affecting the health (Hill et al., 2012). It has emerged as a major public health problem worldwide thereby increasing the risk of type 2 diabetes, hypertension, cardiovascular disease, and several cancers (Chan and Woo, 2010). Fat accumulation usually occurs via adipogenesis, which is adipocyte differentiation in adipose tissues (Suh et al., 2015). Adipogenesis is controlled by the successive expression of various adipogenic factors which are responsible for lipid metabolism (Suh et al., 2015). Thus, the proper modulation of the adipogenic process is a suitable way to control obesity and obesity-mediated metabolic diseases.
ROS are inevitably produced from cellular metabolism, and play a crucial role in modulating the normal functions of cells (Halliwell et al., 1992). However, excessive ROS production damages cellular molecules and leads to oxidative stress, which is the cause of several pathological conditions (Halliwell et al., 1992). Oxidative stress is one of the main factors responsible for obesity, inflammation, and cancer (Fernández-Sánchez et al., 2011). However, cells have a defense mechanism against oxidative stress or cellular toxicity due to ROS (Kobayashi and Yamamoto, 2005). Nuclear factor (erythroid-derived 2)-like 2 (Nrf2)-kelch-like ECH associated protein 1 (Keap1) pathway is a protective system against oxidative stress. The regulation of this signaling pathway has been related to various pathogenic states (Kobayashi and Yamamoto, 2005). Since obesity or excessive fat accumulation is associated with excessive ROS production and increased oxidative stress, regulation of oxidative stress via the Nrf2/Keap1 signaling pathway may be important to control adipogenesis or obesity. In this study, the effects of parthenolide, a feverfew-derived phytochemical, on adipogenesis and ROS status along with the regulation of Nrf2/Keap1 signaling was investigated.
Materials and methods
Materials
PL was purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Bovine serum (BS), Dulbecco’s modified Eagle’s medium (DMEM), and insulin were purchased from Gibco (Gaithersburg, MD, USA). Fetal bovine serum (FBS), phosphate-buffered saline (PBS), and penicillin/streptomycin (PS) were obtained Hyclone (Logan, UT). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Amresco Inc. (Solon, OH, USA). 3-Isobutyl-1-methylxanthine (IBMX), dexamethasone (DEX), 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), and Oil Red O (ORO) were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO, USA). Nitroblue tetrazolium was obtained from Life Technology (Eugene, OR, USA).
Cell culture
3T3-L1 preadipocyte for growth and maintenance were cultured performed in DMEM containing 10% BS and 1% PS. For differentiation, after two days of confluency, the cells were incubated with DMEM with 1% PS, 10% FBS, and differentiation cocktail containing 1.67 μM insulin, 1 µM DEX, and 0.5 mM IBMX for 2 days (day 2). The culture was replaced with DMEM containing 1.67 μM insulin and 10% FBS for 2 more days (day 4) after which the culture was maintained in DMEM containing 10% FBS, and the media were changed every other day. PL, dimethylsulfoxide (DMSO, 0.1%), or hydrogen peroxide (50 µM) was treated every 2 days starting from differentiation. All cells were maintained at 37 °C in presence of 5% CO2 and 95% humidity.
Cell viability assay
To determine cell viability, 3T3-L1 (1.5 × 104 cells/well) were seeded in a 96-well plate for 24 h. The cells were treated with PL for 48 h, and the medium was discarded and MTT reagent (0.5 mg/mL) was added for 60 min at 37 °C. Dimethyl sulfoxide (DMSO) was added to solubilize MTT formazan. The color change of the wells was determined at 550 nm (Spectra Max M3; Molecular device, Sunnyvale, CA, USA).
ORO
The lipids accumulated within the adipocytes were stained on day 6 or 8 using ORO (0.35%) in 60% isopropanol. Briefly, the lipid-containing cells were fixed with 10% formalin for 1 h at 4 °C. After washing with distilled water, filtered ORO was added to the cells overnight at room temperature in the dark. The plate was then washed and dried for 8 h. The stained lipids were imaged and quantified via microscope and ImageJ program.
ROS analysis
ROS analysis was performed by two methods, DCFH-DA, and nitroblue tetrazolium (NBT) assays. In NBT assay, after 6 days of differentiation, the cells were washed with PBS and incubated with 0.2% NBT solution for 90 min. The plates were washed and dried. ROS-mediated dark blue formazan was monitored and quantified via ImageJ program. In DCFH-DA assay, on the 6th day after differentiation, the plate was washed, and PBS containing 20 μM DCFH-DA was added and incubated for 30 min. After PBS was removed, the cell membranes were disrupted by adding DMSO. The fluorescence of DCF was analyzed at an excitation of 485 nm and an emission of 535 nm (SpectraMaxM3).
Real-time quantitative polymerase chain reaction (PCR)
The total RNA was harvested from the cells using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The total RNA was quantified using Nanodrop (Thermo Fisher Scientific, Waltham, USA). One microgram of RNA was used to synthesize cDNA using a cDNA kit (Thermo Fisher Scientific). The synthesized cDNA was mixed and amplified with SYBR Green PCR Master solution (Applied Biosystems) containing 100 ng/mL of the primers. PCR was performed in triplicate using AriaMx Real-Time PCR system (Agilent Technologies). The primers used in this study are following: C/EBPδ (forward, ATCGCTGCAGCTTCCTATGT; Reverse, AGTCATGCTTTCCCGTGTTC), KLF4 (forward, GTCCTTCTCCACGTTCGC; reverse, CCAGGAGGTCGTTGAACTC); KLF5 (forward, GCCAACTCTCCCACC TGTCA; reverse, GTGCACTTGTAGGGCTTCTCG), Krox20 (forward, TGACTATTGTGGCCGCAAGTT; reverse, TTCTGCCGAAGGTGGATCTT), Pref-1 (forward, GAGACCTTGACCGAGTCTGC; reverse, TTTGGATGGAGGAGGAG TTG); GAPDH (forward, CTGCGACTTCAACAGCAACT; reverse, GAGTTGGGATAGGGCCT CTC).
Subcellular fractionation
The cell lysates were prepared by using harvesting buffer [NaCl (50 mM), HEPES (10 mM, pH 7.9), EDTA (0.1 mM), sucrose (0.5 M), β-glycophosphate (17.5 mM), NaF (100 mM), tetrasodium pyrophosphate (10 mM), DTT (1 mM), benzamidine (1 mM), aprotinin (4 μg/mL), leupeptin (5 μg/mL), pepstatin A (2 μg/mL), and PMSF (1 mM)] and centrifuged at 500 × g for 5 min. The supernatant and pellet were used to prepare cytosolic and nuclear fractions, respectively. The pellets were resuspended in buffer A [KCl (10 mM), aprotinin (4 μg/mL), pepstatin A (2 μg/mL), PMSF (1 mM), HEPES (10 mM), EDTA (0.1 mM), and EGTA (0.1 mM)], pipetted again, and centrifuged at 500 × g for 5 min. The pellet was resuspended by vortexing for 10 min in buffer C [KCl (10 mM), aprotinin (4 μg/mL), pepstatin A (2 μg/mL), PMSF (1 mM), NP-40 (0.1%), HEPES (10 mM), EDTA (0.1 mM), and EGTA (0.1 mM)]. Finally, after centrifugation at 10,000 × g for 8 min, the supernatant obtained was the nuclear fraction. The nuclear fraction (200 μg) was additionally treated by ice-cold acetone and trichloroacetic acid (100%, w/v) with nuclear fraction, ice-cold acetone, and trichloroacetic acid in a ratio of 1:8:1 to enrich the nuclear proteins. For the cytoplasmic fraction, the supernatant obtained after centrifugation of the cell lysate was again centrifuged at 10,000×g for 10 min. The resulting supernatant was used as the cytoplasmic fraction.
Western blot
The cultured cells were washed with PBS and harvested using lysis buffer containing protease inhibitors and phosphatase inhibitors. The protein lysates were obtained as a supernatant after centrifugation at 8000 × g for 10 min. Bicinchoninic Acid (Thermo Scientific, USA) assay was used to estimate the total protein level, and protein extracts (50 μg) were separated by SDS-PAGE, and immunoblotted following a method described in a previous study (Kim et al., 2018).
Statistical analysis
All data are expressed as the mean ± standard error of the mean (SEM). All statistical analyses were performed by SPSS 12.0 K. One-way analysis of variance (ANOVA) was used for comparisons among the groups. Tukey’s multiple range test was used to estimate the significant difference among the mean values. Statistical significance was evaluated at p < 0.05.
Results and discussion
Effects of PL on 3T3-L1 cell viability
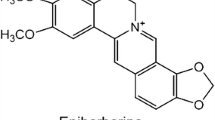
The effects of PL (Fig. 1A), a feverfew-derived phytochemical, on 3T3-L1 cell viability were investigated by MTT assay. When the cells were incubated with different concentrations (0.75, 1.56, 3.12, 6.25, 12.5, and 25 μM) of PL for 48 h, no cytotoxicity was observed up to a concentration of 12.5 μM. However, PL at a concentration of 25 μM reduced cell viability by 80% (Fig. 1B). Subsequent experiments with PL were conducted in the concentration range of 1–8 μM.
Effects of PL on 3T3-L1 adipocyte viability. Structure of Parthenolide (PL) (A). The cells were treated with PL or vehicle (DMSO) for 48 h (B). MTT assay was performed in triplicate according to the manufacturer’s protocol. Each value represents the mean ± SEM (n = 3). CON only DMSO treated without sample
Effects of PL on lipid storage during adipogenesis
PL significantly inhibited lipid accumulation as determined by ORO staining (Fig. 2A). PL dose-dependently reduced fat accumulation as compared to the control (CON); 9% at 2 μM, 46% at 4 μM and 54% at 8 μM concentration (Fig. 2B). This lipid-lowering effects of PL were associated with the inhibition of adipogenic factors. PL inhibited the protein abundance of adipogenic factors (PPARγ and C/EBPα) and its target protein FABP4 in a dose-dependent manner (Fig. 2B, C). A high concentration (8 μM) of PL reduced the levels of PPARγ, C/EBPα, and FABP4 by 98, 96, and 99%, respectively, as compared to the control (Fig. 2B, C), showing a similar result as that in the non-differentiation group. These results indicated that PL inhibited lipid accumulation by suppressing adipogenic factors during adipogenesis.
Effects of PL on lipid accumulation during adipogenesis. After differentiation with 1, 2, 4, and 8 μM PL or vehicle for 6 days, adipogenic lipid storage was evaluated by Oil red O staining and ImageJ software (A and B). The cells were treated with 1, 2, 4, and 8 μM PL during differentiation of 6 days. The total protein was obtained from the cells, and the abundances of PPARγ, C/EBPα, and FABP4 were analyzed by western blot (C). Their levels were quantified by ImageJ software (D). Each value represents the mean ± SEM (n = 3). Different characters indicate a significant difference among the samples (p < 0.05) based on one way ANOVA and the Tukey’s test. CON control, fully differentiation, ND non-differentiation
The differentiation process accompanies morphological changes from fibroblasts to circular adipocytes with the induction of adipogenic factors (Suh et al., 2015). Adipogenesis is experimentally induced by cocktails, such as IBMX, DEX, and insulin. These chemicals increase the cyclic adenosine monophosphate content and bind to glucocorticoids and insulin receptors (Kersten, 2001; Kim et al., 2018) to stimulate the expression of transcription factors, such as CCAAT/enhancer binding protein transcription factors (C/EBPβ and C/EBPδ), and Kruppel-like factors (KLF4 and KLF5) (Banerjee et al., 2003; Birsoy et al., 2008). The activation of these early adipogenic factors leads to the expression of the major adipogenic factors C/EBPα and peroxisome proliferator-activated receptor γ (PPARγ) (Tontonoz et al., 1994), which promotes the activation of lipid synthetic genes (Tontonoz et al., 1994). Many studies have reported the effects of food/natural plant-derived components on the adipogenic process and factors (Kim et al., 2018; Suh et al., 2015). Silibinin, a flavonoid found in milk thistle, has been known to suppress adipogenesis with downregulation of adipogenic factors (Suh et al., 2015). Dibenzoylmethane, a constituent of licorice, also showed anti-adipogenic effects (Kim et al., 2018). However, although most of the studies with PL have been focused on inflammation or cancer, there are no studies evaluating the effects of feverfew on adipogenesis or lipid accumulation.
Effects of PL on adipogenic stages and expression of early adipogenic factors
To investigate the effects of PL on adipogenic stages, PL was treated at different phases of adipocyte differentiation, and ORO staining was performed. PL (8 μM) was treated on days 0–2, 0–4, 0–6, 2–4, 2–6, and 4–6 of differentiation (Fig. 3A–C). PL effectively inhibited lipid accumulation in the early stages of adipogenesis (0–2 and 0–4 days) (Fig. 3B). PL treatment on 0–2, 0–4, and 0–6 days showed a 24%, 36%, and 50% reduction in ORO staining, respectively (Fig. 3C). However, PL treatment after 2 days was less effective (5– 17%) than treatment from day 0 (Fig. 3C). These results showed that PL mainly inhibited the early adipogenic stages during differentiation. C/EBPβ was mainly expressed in early adipogenic stage (0–2 days), and KLF2 protein was highly expressed at undifferentiation (day 0), but its level decreased with adipogenic differentiation (Fig. 3D). Thus, the effect of PL on early adipogenic factors (C/EBPβ and KLF2) was examined in 0–2 days of differentiation stage. PL modulated the levels of the early adipogenic factors. The protein level of C/EBPβ, a pro-early adipogenic factor, was reduced by 47% by PL treatment (8 μM), while the protein level of KLF2, an anti-early adipogenic factor, was increased by 4.5-fold in presence of protein abundance (Fig. 3D, E). In addition, the other early adipogenic factors, such as C/EBPδ, KLF4 (43%), KLF5 (42%), and Krox20 (48%) were significantly reduced by PL treatment (8 μM) in terms of the mRNA levels (Fig. 3F). However, the mRNA level of preadipocyte factor 1 (Pref-1), another anti-early adipogenic factor, increased in a concentration-dependent manner by PL treatment (Fig. 3G). These results showed that PL regulated early adipogenic factors to inhibit lipid storage during adipogenesis.
Effects of PL on adipogenic stages and expression of early adipogenic factors. The 3T3-L1 cells were differentiated with PL on days 0–2, 0–4, 0–6, 2–6, 2–4 or 4–6 (A). After 6 days, the effects of PL (8 μM) on lipid accumulation of the cells were examined by Oil red O staining and ImageJ software (B and C). The proteins of C/EBPβ and KLF2 were determined during adipogenic stages (D). PL (1, 2, 4, and 8 μM) was treated for 4 h in the 3T3-L1 differentiation, and the cells were harvested for protein extraction. The levels of C/EBPβ and KLF2 proteins were determined by western blot (E) and quantified using ImageJ software (F). The expression of C/EBPδ, KLF4, KLF5, Krox20, and Pref-1 was analyzed by real-time PCR and normalized to GAPDH (G and H). Each value represents the mean ± SEM (n = 3). Different characters indicate a significant difference among samples (p < 0.05) based on one way ANOVA and the Tukey’s test. CON control, fully differentiation, ND non-differentiation, Diff. differentiation
When the preadipocytes were differentiated to adipocytes, they undergo early, middle, and late stages (Suh et al., 2015). Each step involves the expression of specific transcription factors that stimulate adipogenesis. In particular, during the early period of adipogenesis, the cells undergo mitotic clonal expansion leading to a marked increase in cell number, which is a determinant of adipogenesis (Tang et al., 2003). C/EBPβ and δ, which are the main early adipogenic factors, promote the expression of PPARγ, an important transcription factor in the later stages (Moseti et al., 2016). KLFs are early adipogenic factors that differentially regulate the adipogenic process. KLF2 inhibits lipogenesis by suppressing PPARγ, whereas KLF4 and KLF5 promote adipocyte differentiation (Banerjee et al., 2003; Birsoy et al., 2008). Many phytochemicals have been known to inhibit lipid accumulation by suppressing the early stages of adipogenesis (Choi et al., 2012; Suh et al., 2015). However, several phytochemicals were reported to inhibit the maturing adipocytes in the middle of adipogenesis or promote lipolysis after adipocytes are matured (Szkudelska et al., 2002).
Effects of PL on adipokine production
The effects of PL on adipokine gene expression were examined by real-time PCR. The full differentiation of the adipocytes effectively induced gene expression of leptin, RBP4, resistin, and adiponectin. PL effectively regulated this adipokine expression. PL (8 μM) reduced the mRNA levels of leptin, RBP4, and resistin by 62%, 94%, and 42%, respectively, as compared to the control, while PL treatment (4 and 8 μM) increased adiponectin by 3–6 fold in terms of the mRNA level (Fig. 4A). These results showed that could can regulate adipocyte-derived adipokine production.
Effect of PL on adipokine production. PL (2, 4, and 8 μM) was treated for 6 days in the 3T3-L1 differentiation. The mRNA level of adiponectin was analyzed by real-time PCR after normalizing to GAPDH (A and B). Each value represents the mean ± SEM (n = 3). Different characters indicate a significant difference among samples (p < 0.05) based on one way ANOVA and the Tukey’s test. CON control, fully differentiation, ND non-differentiation
Besides functioning as energy storage sites, adipocytes are also known as endocrine organs releasing adipokines, the adipocyte-derived cytokines. These adipokines regulate energy metabolism and various hormones in the body and release cytokines to control inflammation (Festi et al., 2004). Adiponectin has anti-inflammatory and anti-obesity properties and is known to improve insulin sensitivity (Desprès and Lemieux, 2006; Kim et al., 2018). In contrast, resistin has been implicated in type 2 diabetes with pro-inflammatory properties, although the results are still contradictory (Kusminski et al., 2005). Leptin plays an important role in appetite and hunger, metabolism, energy intake, and food consumption behavior (Klok et al., 2007). Its chronic high level has been known to induce leptin resistance, leading to the insensitivity of leptin similar to insulin resistance (Pan et al., 2014). This reduced sensitivity of leptin results in not only obesity but also obesity-related diseases, such as systemic inflammation (Duntas and Biondi, 2013). In obesity, leptin sensitivity is reduced, resulting in an inability to sense satiety despite high levels of energy and leptin (Pan et al., 2014). RBP4 has recently been known to contribute to insulin resistance/diabetes in a rodent model (Yang et al., 2005). RBP4 has also been suspected to induce infiltration of macrophages to fat tissues causing local inflammation and leading to insulin resistance (Moraes-vieira et al., 2014). In this study, PL was shown to favorably regulate the expression of these adipokines, indicating that PL has a potential role to control obesity and obesity-related diseases.
Effects of PL on ROS production
To determine the effects of PL on intracellular ROS generation during differentiation, DCFH-DA and NBT assays were performed on the adipocytes. Differentiation-induced the elevation of ROS level in both the assays; ROS-derived fluorescence and NBT-stained formazan were increased by > eightfold and > twofold, respectively, as compared to the non-differentiation group (Fig. 5). Treatment with H2O2, a negative control, further increased the intracellular ROS level (Fig. 5). The adipocyte-derived ROS production was significantly reduced with PL treatment. PL (8 μM) decreased ROS-derived DCF level by 65% (Fig. 5A), and the formation of formazan was reduced by 50% with PL (8 μM) treatment (Fig. 5B). These results indicate that PL effectively suppressed ROS production during differentiation. The excessive increase in ROS is able to induce oxidative stress which is closely associated with adipogenesis or obesity (Denu and Hematti, 2016). In particular, nicotinamide adenine dinucleotide phosphate oxidase 4, which is known to be a ROS generating enzyme, is upregulated during adipocyte differentiation (Schroder et al., 2009).
Effects of PL on ROS generation. The 3T3-L1 cells were treated with different concentrations of PL or 100 μM hydrogen peroxide for 4 days and DCFH-DA (20 μM) was added for incubation for 30 min. The formation of fluorescent DCF was estimated at an excitation of 485 nm and an emission of 535 nm (A). The production of ROS was also determined by NBT assay and quantified using ImageJ software (B). Each value represents the mean ± SEM (n = 3). Different characters indicate a significant difference among samples (p < 0.05) based on one way ANOVA and the Tukey’s test. CON control, fully differentiation, ND non-differentiation
Adipogenesis was reported to be increased by high-dose hydrogen peroxide, a ROS, which can induce oxidative stress (Denu and Hematti, 2016). However, an antioxidant, such as N-acetylcysteine was found to suppress fat accumulation in the adipocytes (Pieralisi et al., 2016). These studies supported our data demonstrating the effects of PL on lipid accumulation during adipogenesis (Fig. 5). Since the systemic oxidative stress was shown to be associated with metabolic syndrome (Furukawa et al., 2004), PL is expected to exert an important role in preventing metabolic diseases by controlling oxidative stress.
Effects of parthenolide (PL) on the Nrf2/Keap1 pathway
To investigate the effects of PL on the Nrf2/Keap1 signaling pathway, Nrf2 and its target proteins were determined. Nrf2 and HO-1/NQO1 protein levels were effectively enhanced by PL as compared to the control group (CON) which has been fully differentiated (Fig. 6A). The increase in the Nrf2 and its target proteins in control group compared to ND (not differentiated)group (Fig. 6A) is recognized to be due to the adipogenesis-mediated ROS production (Fig. 5). PL (8 μM) increased the amount of Nrf2 by 50% as compared to the control. The HO-1 and NQO1 levels were also enhanced by 2.2- and 2.5-folds, respectively (Fig. 6B). However, the level of Keap1, a complex partner of Nrf2, was decreased by PL treatment (60%). In addition, PL augmented the movement of Nrf2 into the nucleus from the cytosol (Fig. 6C). PL (8 μM) increased the nuclear Nrf2 protein level by over fourfold as compared to the control (Fig. 6D), while the cytosolic Nrf2 protein level was decreased by 50% with PL treatment (8 μM). These results showed that PL activated the Nrf2/Keap1 signaling pathway by promoting the nuclear translocation of Nrf2.
Effects of PL on the Nrf2/Keap1 pathway. The 3T3-L1 cells were treated with 2, 4, and 8 μM PL during differentiation of 6 days. The expression levels of Nrf2, Keap1, HO-1, NQO1, and nuclear and cytosolic Nrf2 were analyzed by Western blot (A and C) and quantified by ImageJ software (B and D). Each value represents the mean ± SEM (n = 3). Different characters indicate a significant difference among samples (p < 0.05) based on one way ANOVA and the Tukey’s test. CON control, fully differentiation, ND non-differentiation
The Nrf2/Keap1 pathway is one of the mechanisms that regulate oxidative stress (Kobayashi and Yamamoto, 2005). In the absence of any stimulation, Nrf2, a key transcription factor in this signaling pathway, forms a complex with Keap1 in the cytoplasm (Kobayashi and Yamamoto, 2005). However, when the cells are exposed to signals, such as ROS, or electrophile, Keap1 is rapidly degraded by proteasome-mediated ubiquitination. The dissociated Nrf2 is introduced into the nucleus and binds to the antioxidant response element of the target gene to promote the expression of the target gene (Kobayashi and Yamamoto, 2005). Thus, PL-mediated ROS reduction during adipogenesis may be due to the activation of the Nrf2/Keap1 pathway. This is because direct ROS-scavenging activity of PL is able to be disregarded based on our results as shown in Supplementary Table 1.
Furthermore, HO-1, which is as a target molecule of Nrf2, has been reported to mediate ROS reduction in the cells (Ryter and Choi, 2005). The antioxidant enzymes, such as catalase, superoxide dismutase, and glutathione-metabolizing enzymes were also shown to be upregulated via Nrf2 (Zhong et al., 2013). Based on the data (Fig. 6), PL is thought to suppress ROS generation via Nrf2/Keap1 signaling during adipogenesis. This PL-mediated ROS control is recognized to be associated with inhibition of adipogenesis or lipid accumulation. However, many conflicting studies on the relationship between Nrf2 and adipogenesis have been reported (Gaikwad et al., 2001; Pi et al., 2010; Shin et al., 2007). The deficiency of Nrf2 has been involved in the reduction of adipogenesis and adipogenic factors, while Nrf2 deficiency has been found to markedly promote adipogenic differentiation which was reversed by the ectopic expression of Nrf2. The anti-adipogenic effects of sulforaphane, a vegetable phytochemical, are exerted by the activation of Nrf2 and its target gene NQO1 (Gaikwad et al., 2001). In the present study, PL-mediated activation of the Nrf2/Keap1 signaling pathway was thought to negatively regulate adipogenesis. However, to elucidate whether the anti-adipogenic effects of PL is dependently exerted via the Nrf2/Keap1 signaling pathway, further studies need to be performed in an Nrf2 deficient condition.
References
Banerjee SS, Feinberg MW, Watanabe M, Gray S, Haspel RL, Denkinger DJ, Kawahara R, Hauner H, Jain MK. The Krüppel-like factor KLF2 inhibits peroxisome proliferator-activated receptor-γ expression and adipogenesis. J. Biol. Chem. 278: 2581-2584 (2003)
Birsoy K, Chen Z, Friedman J. Transcriptional regulation of adipogenesis by KLF4. Cell Metab. 7: 339-347 (2008)
Chan RS, Woo J. Prevention of overweight and obesity: how effective is the current public health approach. Int. J. Environ. Res. Public Health 7: 765-783 (2010)
Choi KM, Lee YS, Sin DM, Lee S, Lee MK, Lee YM, Hong JT, Yun YP, Yoo HS. Sulforaphane inhibits mitotic clonal expansion during adipogenesis through cell cycle arrest. Obesity 20: 1365-1371 (2012)
Denu RA, Hematti P. Effects of oxidative stress on mesenchymal stem cell biology. Oxid. Med. Cell Longev. 2016: 2989076 (2016)
Desprès JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 444: 881-887 (2006)
Duntas LH, Biondi B. The interconnections between obesity, thyroid function, and autoimmunity: the multifold role of leptin. Thyroid 23: 646-653 (2013)
Feltenstein MW, Schühly W, Warnick JE, Fischer NH, Sufka KJ. Anti-inflammatory and anti-hyperalgesic effects of sesquiterpene lactones from Magnolia and Bear’s foot. Pharmacol. Biochem. Behav. 79: 299-302 (2004)
Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González A, Esquivel-Chirino C, Durante-Montiel I, Sánchez-Rivera G, Valadez-Vega C, Morales-González JA. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 12: 3117-3132 (2011)
Festi D, Colecchia A., Sacco T, Bondi M, Roda E, Marchesini G. Hepatic steatosis in obese patients: clinical aspects and prognostic significance. Obesity Rev. 5: 27-42 (2004)
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114: 1752-1761 (2004)
Gaikwad A, Long DJ 2nd, Stringer JL, Jaiswal AK. In vivo role of NAD(P)H:quinone oxidoreductase 1 (NQO1) in the regulation of intracellular redox state and accumulation of abdominal adipose tissue. J. Biol. Chem. 276: 22559-22564 (2001)
Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J. Lab. Clin. Med. 119: 598-620 (1992)
Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation 126: 126-132 (2012)
Kersten S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2: 282-286 (2001)
Kim JH, Kim CY, Kang B, Hong J, Choi HS. Dibenzoylmethane suppresses lipid accumulation and reactive oxygen species production through regulation of nuclear factor (erythroid-derived 2)-like 2 and insulin signaling in adipocytes. Biol. Pharm. Bull. 41: 680-689 (2018)
Klok M, Jakobsdottir S, Drent M. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obesity Rev. 8: 21-34 (2007)
Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signal. 7: 385-394 (2005)
Kusminski CM, McTernan PG, Kumar S. Role of resistin in obesity, insulin resistance and Type II diabetes. Clin. Sci. 109: 243-256 (2005)
Moraes-vieira PM, Yore MM, Dwyer PM, Syed I, Aryal P, Kahn BB. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 19: 512-526 (2014)
Moseti D, Regassa A, Kim WK. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int. J. Mol. Sci. 17: 124 (2016)
Nelson MH, Cobb SE, Shelton J. Variations in parthenolide content and daily dose of feverfew products. Am. J. Health Syst. Pharm. 59: 1527-1531 (2002)
Pan H, Guo J, Su Z. Advances in understanding the interrelations between leptin resistance and obesity. Physiol. Behav. 130: 157-169 (2014)
Pareek A, Suthar M, Rathore GS, Bansal V. Feverfew (Tanacetum parthenium L.): a systematic review. Pharmacogn. Rev. 5: 103-110 (2011)
Pi J, Leung L, Xue P, Wang W, Hou Y, Liu D, Yehuda-shinaidman E, Lee C, Lau J, Kurtz T W, CHAN JY. Deficiency in the nuclear factor E2-related factor-2 transcription factor results in impaired adipogenesis and protects against diet-induced obesity. J. Biol. Chem. 285: 9292-9300 (2010)
Pieralisi A, Martini C, Soto D, Vila MC, Calvo JC, Guerra LN. N-acetylcysteine inhibits lipid accumulation in mouse embryonic adipocytes. Redox Biol. 9: 39-44 (2016)
Ryter SW, Choi AM. Heme oxygenase-1: redox regulation of a stress protein in lung and cell culture models. Antioxid. Redox Signal. 7: 80-91 (2005)
Schroder K, Wandzioch K, Helmcke I, Brandes RP. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler. Thromb. Vasc. Biol. 29: 239-245 (2009)
Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, Yamamoto M, Kensler T W. NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol. Cell Biol. 27: 7188-7197 (2007)
Suh HJ, Cho SY, Kim EY, Choi HS. Blockade of lipid accumulation by silibinin in adipocytes and zebrafish. Chem. Biol. Interact. 227: 53-62 (2015)
Szkudelska K, Szkudelski T, Nogowski L. Daidzein, coumestrol and zearalenone affect lipogenesis and lipolysis in rat adipocytes. Phytomedicine 9: 338-345 (2002)
Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. 100: 44-49 (2003)
Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 8: 1224-1234 (1994)
Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436: 356-362 (2005)
Zhong Y, Liu T, Lai W, Tan Y, Tian D, Guo Z. Heme oxygenase-1-mediated reactive oxygen species reduction is involved in the inhibitory effect of curcumin on lipopolysaccharide-induced monocyte chemoattractant protein-1 production in RAW264.7 macrophages. Mol. Med. Rep. 7: 242-246 (2013)
Acknowledgements
This work was supported by the National Research Foundation of Korea, a Grant funded by the Korea government (the Ministry of Education) (NRF‐2015R1D1A1A01059729; 2016), and a research Grant from the Seoul Women’s University (2019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Human and animal rights
There is no animal or human experiment in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, C.Y., Kang, B., Hong, J. et al. Parthenolide inhibits lipid accumulation via activation of Nrf2/Keap1 signaling during adipocyte differentiation. Food Sci Biotechnol 29, 431–440 (2020). https://doi.org/10.1007/s10068-019-00672-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-019-00672-y