Abstract
Melanosis developed in shrimp (Penaeus vannamei) is mainly initiated by polyphenoloxidase (PPO), thus understanding of the characteristics of PPO in shrimp is important for controlling the melanosis of shrimp. The shrimp cephalothorax turns black most rapidly amongst all the tissues during the chilled storage. Crude PPO extracted from this cephalothorax has an optimal pH of 6.0 and an optimal temperature of 50 °C. PPO is relatively stable under neutral and weak alkaline conditions (pH 5.5–9.0) and the temperature range of 25–35 °C. The kinetic parameters Km and Vmax were recorded as 3.02 mM and 54.3 U/mg of protein, respectively, using L-Dopa as a substrate. The molecular weight of PPO was estimated as 200–220 kDa by an activity staining test. A hydroxypyridinone derivative, 5-hydroxy-1-octyl-4-oxo-1,4-dihydropyridine-2-carbaldehyde O-ethyl oxime, was demonstrated to efficiently inhibit the PPO, indicating that this compound might find application as a shrimp preservative.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pacific white shrimp (Penaeus vannamei), primarily cultured in Thailand, is an important commercial aquatic product worldwide (Phonpala, 2010; Sae-Leaw et al., 2017). Fresh shrimp readily turn brown during postmortem storage. Prevention of such browning is a problem which needs to be solved urgently in the food industry. Enzymatic browning of shrimp is mainly caused by the melanosis initiated by the polyphenoloxidase (PPO)-catalyzed oxidation of tyrosine and its derivatives (Sae-Leaw et al., 2018). PPO, also known as tyrosinase, is a type-3 bifunctional enzyme containing two copper ions at its active site. It can catalyze two vital reactions in the biosynthesis of melanin, the o-hydroxylation of monophenols yielding o-diphenols (monophenoloxidase) and the subsequent oxidation of o-diphenols to o-quinones (diphenoloxidase) in the presence of oxygen (Nirmal and Benjakul, 2012a). The o-quinones undergo a series of non-enzymatic reactions to generate brown melanin pigment (Zamorano et al., 2009).
In order to maintain the quality (color, taste, nutrient, and so on) and to avoid melanosis of shrimp, it is necessary to study the enzymatic characteristics and kinetic properties of PPO (Simpson et al., 1988). PPO has been studied in different tissues from various crustacean species (Zamorano et al., 2009). However, PPOs from various species exhibit differences in molecular weight, enzymatic characteristics, and kinetic parameters (Montero et al., 2001; Nirmal and Benjakul, 2012b; Rolle et al., 1991). This study on shrimp PPO is designed to obtain a better understanding of the biochemical basis of PPO action and the melanosis phenomenon.
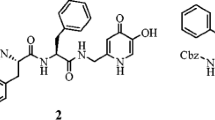
Hydroxypyridinones are efficient metal chelators, binding copper(II) with relatively high affinity and in principle can inhibit PPO by binding copper ions coordinated at the active site of the enzyme. Indeed, it has been demonstrated that some hydroxypyridinone derivatives effectively retard the browning of shrimp (Dai et al., 2016). Recently, a range of hydroxypyridinone derivatives containing an oxime ether moiety have been synthesized starting from kojic acid in our laboratory (Shao et al., 2018). Among them, 5-hydroxy-1-octyl-4-oxo-1,4-dihydropyridine-2-carbaldehyde O-ethyl oxime (1, Fig. 1) showed the most potent inhibitory effect on mushroom tyrosinase, with an IC50 value of 1.60 μM, which was sevenfold more potent than that of kojic acid, a well-known tyrosinase inhibitor. Compound 1 has been demonstrated to retard the browning of fresh-cut apple slices (Shao et al., 2018).
In the present study, the enzymatic characteristics of PPO, extracted from the cephalothorax of Penaeus vannamei, including the effects of pH and temperature on its activity, and kinetic parameters, were investigated. The potential of hydroxypyridinone derivative 1 as a shrimp preservative was explored by investigating its inhibitory effect and inhibitory kinetics against the PPO extract from shrimp (Penaeus vannamei). The copper(II) reduction capacity and copper chelating activity were also determined to identify the inhibition mechanism.
Materials and methods
Reagents
L-3,4-Dihydroxyphenylalanine (L-DOPA) was purchased from Aladdin (Shanghai, China). The hydroxypyridinone derivative, 5-hydroxy-1-octyl-4-oxo-1,4-dihydropyridine-2-carbaldehyde O-ethyl oxime, was synthesized starting from kojic acid (Shao et al., 2018). Chemicals used for electrophoresis were purchased from Bio-Rad Laboratories (Hercules, CA, USA).
Shrimp collection and preparation
Shrimps (Penaeus vannamei) with an average weight of 10.0 ± 0.5 g and an average body length of 10.0 ± 0.5 cm were purchased from the Yonghui supermarket in Hangzhou, Zhejiang Province, China. The live shrimps were washed with ice water immediately when they arrived at laboratory for next experiments.
Storage of whole shrimp and the individual tissues
Whole shrimp, whole shrimp with the carapace removed, cephalothorax, carapace, cephalothorax with the carapace removed, whole abdomen, abdomen with the muscle removed, and corresponding muscle, telson, were stored at 4 °C. Development of melanosis in these samples was evaluated (day 0, day 2, day 4, and day 7). The entire study was repeated twice and there were at least three samples in each group.
Extraction of PPO from the cephalothorax of Penaeus vannamei
Crude PPO was extracted from the cephalothorax of Penaeus vannamei according to the method of Phonpala (2010). PPO activity was assayed according to the method of Augustin et al. (1985) with a slight modification. Briefly, to a solution of L-DOPA in deionized water (15 mM, 600 μL) was added 400 μL of sodium phosphate buffer (0.05 M, pH 6.0) and deionized water (100 μL). Then 100 μL of the crude PPO extract was added to the above mixture. The absorbance of the mixture was recorded at 35 °C at 475 nm for 8 min using a UV-2550 spectrophotometer (Shimadzu, Kyoto, Japan). One unit of PPO activity was defined as the increment in the rate of absorbance and an increase of 0.001 per min (A475/103/min). The protein content of crude PPO was measured according to the Chinese National Standard (GB 5009.5-2016) (2016) using Kjeldahl method.
Effects of pH and temperature on the activity and stability of PPO
The effect of pH on the activity of PPO was investigated in the buffers with different pH values (3.0–9.0) at 35 °C. The buffers, with pH values ranging from 3.0 to 7.0, were prepared with 0.2 M sodium phosphate and 0.1 M citric acid, while in the case of pH 8.0–9.0, the buffers were prepared with 0.2 M boric acid and 0.05 M borax. L-DOPA solution (120 μL, 15 mM, dissolved in the buffer solutions with different pH) was diluted with the corresponding buffer (100 μL), and incubated at 35 °C. After addition of the crude PPO extract (20 μL) to the above solution, the absorbance of the resulting mixture was monitored at 475 nm. The influence of pH on PPO stability was determined by incubating 20 μL of PPO extract with 100 μL of buffer (pH range and buffer composition was as above) for 30 min at room temperature. Then 15 mM L-DOPA in 0.2 M sodium phosphate and 0.1 M citric acid buffer (pH 6.0, 120 μL) at 35 °C was added, the residual PPO activity was measured as described above. The results expressed as the percentage of maximum PPO activity.
The activity of the crude PPO extract at different temperature (25 to 70 °C) was assayed in the buffer (pH 6.0; 0.2 M sodium phosphate—0.1 M citric acid). Briefly, a mixture of 120 μL of L-DOPA solution (15 mM, in the buffer mentioned above) and 100 μL of the same buffer was pre-incubated at different temperatures. Then 20 μL of the crude PPO extract was added to initiate the oxidation of L-DOPA. The enzyme activity was determined by recording the absorbance of the resulting solution at 475 nm. In order to determine the thermal stability of PPO, the PPO extract (20 μL) was diluted with the same buffer (100 μL) as above. The mixture was incubated at different temperatures (25 to 70 °C) for 30 min, then cooled with ice water (or heating with water bath) to 35 °C. Next, 120 μL of L-DOPA in the same buffer (15 mM, pH 6.0) pre-incubated at 35 °C was added to the above mixture. The absorbance of the resulting mixture was monitored at 475 nm. The residual activity of PPO was measured as described above. The results expressed as the percentage of maximum PPO activity.
Inhibitory effect of hydroxypyridinone derivative on PPO activity
The total volume of the reaction system was 240 μL. A mixture of 20 μL of PPO extract and 20 μL of hydroxypyridinone derivative solution with different concentrations was incubated at 35 °C for 10 min. Then 80 μL of 0.05 M sodium phosphate (pH 6.0) and 120 μL of 15 mM L-DOPA in the same buffer pre-incubated at 35 °C were added to initiate the reaction (the final concentrations of 1 in the reaction system were 0.00, 7.35, 14.70, 29.40, 44.10, 58.80, and 99.96 µg/mL (0.00, 0.025, 0.05, 0.10, 0.15, 0.20, and 0.34 mM), respectively). The absorbance of the reaction solution at 475 nm was continuously recorded for 8 min.
PPO activity staining
Protein patterns of crude PPO extract were analyzed by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) according to the method of Laemmli (1970). A mixture of PPO extract (10 μL, approximately 25 μg protein) and the sample loading buffer (10 μL) was loaded onto the polyacrylamide gel which was prepared by 7.5% running gel and 5% stacking gel. Electrophoresis was conducted at a constant current of 15 mA per gel. After the completion of electrophoresis, the gel loaded with the mixture of PPO was immersed in 15 mM L-DOPA solution which was prepared by 0.2 M sodium phosphate and 0.1 M citric acid buffer (pH 6.0) at 25 °C for 25 min. Coomassie Brilliant Blue R-250 (0.125%) was used to stain another gel loaded with protein marker (Shanghai Beyotime Biotechnology Co., Ltd., China), then the gel was washed in a mixed solution containing 25% methanol and 10% acetic acid. The molecular weight of PPO was estimated by comparing its mobility with those of markers.
PPO extract was mixed with hydroxypyridinone derivative 1 (0, 44.10, and 99.96 µg/mL) at a ratio of 1:1 (v/v), and incubated at room temperature for 30 min. The resulting sample solution was loaded onto the polyacrylamide gel and subjected to electrophoresis, followed by staining and de-staining as described above.
Inhibition kinetics of the hydroxypyridinone derivative on PPO
The inhibition kinetics of 1 on crude PPO extract was investigated as previous report (Shao et al., 2018). The final concentrations of compound 1 were 0, 0.1, 0.2, and 0.3 mM (0, 29.4, 58.8 and 88.2 µg/mL), respectively; and the final concentrations of L-DOPA were 0.5, 1.0, 2.0, 4.0, and 6.0 mM, respectively, in this study. The kinetic parameters (Km and Vmax) were calculated based on Lineweaver–Burk plots (Lineweaver and Burk, 1934). The equilibrium constant of inhibitor for binding with free enzyme (KI) was obtained from the secondary plot of slope (Km/Vm) versus the concentration of the inhibitor, and the equilibrium constant with enzyme–substrate complex (KIS) was obtained from a plot of the vertical intercept (1/Vm) versus the concentration of the inhibitor (Chen et al., 2003).
Determination of copper(II) reducing capacity of hydroxypyridinone derivative
The determination of copper(II) reducing capacity of hydroxypyridinone derivative 1 was referred to the previous reports (Chen et al., 2017). A cupric sulphate solution (0.5 mL, 0.4 mM) was mixed with compound 1 solution (1 mL, 0–1.5 mM (0–441 µg/mL)). After incubation at 25 °C for 10 min, bathocuproine disulphonic acid solution (0.5 mL, 0.4 mM) was added to the above solution. The absorbance of the resulting mixture was determined at 483 nm after incubation at the same temperature for another 20 min. Kojic acid was used as a positive control under the same concentrations. Deionized water replaced compound 1 for blank.
Determination of copper chelating capacity of hydroxypyridinone derivative
The copper chelating capacity of hydroxypyridinone derivative 1 was measured according to our previous report (Zhao et al., 2016). The hexamine buffer (10 mM, pH 5.0) containing 10.0 mM potassium chloride was used as a solvent for the preparation of copper sulfate (1.0 mM) and tetramethylmurexide (5.0 mM) solutions. A mixture of 1 solution (1.0 mL) with different concentrations, copper sulfate solution (1.0 mL) and tetramethylmurexide solution (0.2 mL) was incubated at 25 °C for 10 min. The absorbance of the resulting reaction solution was measured at 460 and 530 nm. The concentrations of free cupric ion in the solution were calculated based on the absorbance ratios of A460nm/A530nm and a standard curve of free cupric ion concentration (0–0.1 mM) vs absorbance ratio. Kojic acid was used as a positive control.
Statistical analyses
All assays were performed in triplicate. The data in the figures were analyzed with SPSS 16.0 software and expressed as mean ± SD. Significant differences between the treatments were examined by Tukey test. p < 0.05 was considered as statistically significant.
Results and discussion
Melanin formation in the shrimp during storage
As shown in Fig. 2, marked melanosis developed in the cephalothorax (including whole shrimp, with, and without the carapace removed) on day 2, while this phenomenon was not observed in other tissues, possibly suggesting that there is the highest activity of PPO in the cephalothorax. Black spots first appeared in the abdomen near the head during the chilled storage of whole shrimp, then the black spot distribution gradually spread to the whole abdomen. Removal of the carapace caused a faster and further melanosis development in the cephalothorax and whole shrimp, which could be attributed to the easier access of oxygen and contact of PPO with the substrates.
Melanosis appearance in different anatomical parts of Penaeus vannamei during 0, 2, 4, and 7 days of storage at 4 °C. (A) whole shrimp, (B) whole shrimp with the carapace removed, (C) cephalothorax, (D) carapace, (E) cephalothorax with the carapace removed, (F) whole abdomen, (G) abdomen with the muscle removed (right) and corresponding muscle (left), (H) telson
To determine the effect of digestive enzymes on development of melanosis, some other research (Ali et al., 1994; Montero et al., 2001; Zamorano et al., 2009) also carried out the storage study, and the highest PPO activity was observed in the carapace. However, in the present study no marked melanosis developed in the carapace during chilled storage. This indicates that the development of melanosis in the different tissues of the shrimp depends on several factors, in addition to the PPO levels, such as the activation of zymogen (proPPO) and the existence and concentration of substrates (Zamorano et al., 2009). The PPO in arthropods is originally synthesized in the form of proPPO, and then it is generated as an active form by limited proteolysis (Burmester, 2002). Thus, the accessibility of the proteolytic enzymes to proPPOs is likely to also be an important factor controlling melanosis in the different tissues. The cephalothorax was chosen for PPO extraction in this study.
Effect of pH on the activity and stability of PPO
The maximal activity of crude PPO extracted from the cephalothorax occurring at pH 6.0 (Fig. 3A). The result agrees with the report of Nirmal and Benjakul (2012b). The activity of crude PPO extract significantly decreased under both acidic and alkaline conditions (p < 0.05). At pH values ranging from 6.5 to 8.0, PPO activity was found to be 51.9–85.1% of the maximum, while at pH 9.0, PPO activity reduced to only 9.0% of the maximal activity, and PPO activity is absent at pH 3.0. The significant decrease in PPO activity under extreme acidic or alkaline conditions could be attributed to the unfolding of enzyme molecules (Benjakul et al., 2005). Rolle et al. (1991) reported that PPO extracted from cephalothorax of Taiwanese black tiger shrimp (Penaeus monodon) showed maximal activity for the oxidation of DOPA at pH 6.0.
As shown in Fig. 3B, PPO exhibited a maximum stability at pH 6.0. PPO was stable in the pH range of 5.5–9.0 with the residual activity remaining above 80%. However, at pH 5.0 and 4.0, the remaining activities were only 57.4% and 33.8%, respectively; the PPO was very unstable at pH values below 4.0. The stability of PPO extracted from Penaeus vannamei cephalothorax under neutral and alkaline conditions (pH 5.5–9.0) indicated that the conformation of the active site of PPO was not affected by these conditions. Benjakul et al. (2005) reported that PPO from the Kuruma prawn (Penaeus japonicus) cephalothorax was stable over a wide pH range (3.0–10.0) with the remaining activity above 90%.
Effect of temperature on the activity and stability of PPO
PPO extracted from the cephalothorax exhibited maximum activity at 50 °C (Fig. 3C). The activity of PPO gradually increased with increasing temperature from 25 °C until 50 °C, after which the PPO activity decreased. Despite the occurrence of maximum activity at 50 °C, it was not advisable to determine enzyme activity at this level since the enzyme is unstable. Chen et al. (1991b) reported that the activity of PPOs, isolated from Florida spiny lobster (Panulirus argus) and Western Australian lobster (Panulirus cygnus), increased with increasing temperature up to 60 °C from 20 °C.
The PPO was found to exhibit higher stability in the range 25–35 °C at pH 6.0 (Fig. 3D), with the remaining activity of above 90%. The stability of PPO decreased at higher temperatures, losing 26.1% of the activity after incubation at 50 °C for 30 min. Furthermore, there was only 4.2% of remaining activity after 30 min incubation at 70 °C. Simpson et al. (1987) also reported that PPO from the carapace of shrimp (Penaeus setiferus) was stable at lower temperatures up to 50 °C but unstable at higher temperatures.
Inhibition of compound 1 on PPO activity
As shown in Fig. 4A, compound 1 exhibited inhibitory effect on PPO in a dose dependent manner. At concentrations of 44.10 and 99.96 µg/mL (0.15 and 0.34 mM), the inhibition rates of compound 1 on PPO were found to be 45% and 80%, respectively. The IC50 value of the hydroxypyridinone derivative for inhibiting PPO was calculated to be 47.04 µg/mL (0.16 mM). Structurally, compound 1 is closely related to kojic acid, which can also inhibit PPO by chelating the copper ions in the active site of PPO. Compared with our previous report (Shao et al., 2018), hydroxypyridinone derivative 1 was found to have a weaker inhibition on the crude PPO from the cephalothorax of shrimp than that on mushroom tyrosinase (IC50 7.99 μM). Although most of the PPOs possess a dinuclear copper centre at their active site, it has been demonstrated that the PPOs in animals, plants and fungi were different with respect to their sequences, size, glycosylation and activation (Gerdemann et al., 2002; Mayer, 2006). Therefore, the enzymatic properties of PPOs from different sources appreciably differ, which is believed to result from a variation in the substrate-binding pocket or the accessibility of the substrate to the active site (Matoba et al., 2006). This may offer an explanation for the differential inhibition of compound 1 on mushroom tyrosinase and PPO from shrimp.
Inhibition of compound 1 on PPO from the Penaeus vannamei cephalothorax. (A) Inhibitory effect of compound 1 on PPO activity; (B) activity staining of PPO in the absence or presence of compound 1 at different concentrations. Lane A: PPO crude extract; lane B: crude PPO extract with compound 1 (44.10 μg/mL); lane C: crude PPO extract with compound 1 (99.96 μg/mL)
In order to further evaluate the inhibitory effect of compound 1 on PPO, SDS-PAGE and activity staining of PPO in the presence and absence of 1 at the concentrations of 44.10 and 99.96 µg/mL (0.15 and 0.34 mM) were investigated (Fig. 4B). In the absence of 1, PPO effectively catalyzed the oxidation of substrate (DOPA) to produce quinone, and the intermediate products could easily polymerize to generate melanin. Hydroxypyridinone derivative 1 showed the inhibitory effect on PPO as indicated by the band with relatively weaker intensity (lane B and C), as compared to that of control (without compound 1, lane A). The result was in a good agreement with in vitro PPO inhibitory effect of compound 1 (Fig. 4A). One activity zone was observed as dark brown color at the apparent molecular weight of 200–220 kDa. This finding is similar to those of some reports (Gollas-Galván et al., 1999; Nirmal and Benjakul, 2011).
Inhibition kinetics of compound 1 on PPO
As shown in Fig. 5, the Lineweaver–Burk double-reciprocal plots of PPO yields a Km value for the catalysis oxidation of L-DOPA of 3.02 mM, and a Vmax of 54.3 U/mg of protein. Km values reflect the affinity of enzymes for their substrates. Higher Km value indicates a low catalytic efficiency of the enzyme towards the substrate. The result was similar to the reports for the PPOs from white shrimp (Panaeus setiferus) (Km 2.8 mM) (Simpson et al., 1988) and from Pacific white shrimp (Litopenaeus vannamei) (Km 2.43 mM) (Nirmal and Benjakul, 2011). Most of the PPOs isolated from crustaceans characterized so far possess relatively high apparent Km when L-DOPA was used as a substrate. The differences in Km and Vmax of PPO from different species were plausibly owing to the differences in molting stage, method of capture, handling, and storage conditions (Nirmal and Benjakul, 2012b).
Inhibitory kinetics of compound 1 on PPO from Penaeus vannamei cephalothorax. (A) Lineaweaver-Burk plots of PPO in the presence and absence of compound 1 at different concentrations. Curves 1–4 showed the concentrations of compound 1 were 0.0, 0.1, 0.2, and 0.3 mM, respectively. (B) The plot of slope versus the concentration of compound 1 for determining the inhibition constant KI. (C) The plot of intercept versus the concentration of inhibition for determining the inhibition constant KIS
With increasing concentration of the hydroxypyridinone derivative 1, the Km value increased and the Vmax value decreased (Fig. 5A). The plots of 1/v versus 1/[S] gave a group of straight lines with different slopes which intercept in the second quadrant, indicating that compound 1 was a mixed type inhibitor, namely it can bind to either free enzyme or enzyme–substrate complex. From Fig. 5B, C, the KI and KIS values were calculated as 74.6 μM and 146.4 μM, respectively, which indicated that the affinity of compound 1 for free enzyme was greater than that of enzyme–substrate complex. The result was in agreement with the inhibitory effect of compound 1 towards diphenolase of mushroom tyrosinase (Shao et al., 2018).
Copper reducing capability and copper chelating activity of compound 1
Evaluation of the copper reducing capability and copper chelating ability of compound 1 is helpful to understand its inhibitory mechanism on PPO. Bathocuproine disulphonic acid binds Cu+ to form a red complex, which has a maximum absorbance–wavelength of 483 nm. As shown in Fig. 6A, absorbance at 483 nm increases with increasing concentrations of compound 1 and kojic acid up to 0.25 mM (73.50 µg/mL) and 0.50 mM (71 µg/mL), respectively. Thereafter, no changes in absorbance were observed, indicating that no more Cu2+ ions were reduced to Cu+. Within the assayed concentration range (0.02–1.00 mM), compound 1 possessed a stronger copper reducing activity than that of kojic acid (p < 0.05). There are three isoforms of PPO, Oxy-PPO [Cu(II)Cu(II)O2], Met-PPO [Cu(II)Cu(II)] and Deoxy-PPO [Cu(I)Cu(I)] (Zhao et al., 2016). Met-PPO can be reduced to Deoxy-PPO, and the latter is further oxidized to form Oxy-PPO in the presence of O2. Oxy-PPO, a highly active isoform of PPO, can catalyze the oxidation of mono- or diphenol (Chen et al., 1991a). Copper in the active site of PPO plays a key role in the browning reaction. Thus, it was suggested that compound 1 could inhibit PPO activity by reducing Met-PPO to Deoxy-PPO.
The copper chelating ability of compound 1 and kojic acid increased with increasing concentration, but the copper chelating ability of compound 1 was stronger than that of kojic acid (p < 0.05) (Fig. 6B). At 2.5 mM, the copper chelating ability of compound 1 and kojic acid was 91.9% and 82.8%, respectively. The carbonyl group at position-4 and hydroxyl group at position-5 of pyridine ring in compound 1 form the chelating moieties for metal ions. The two copper ions in the active site of PPO directly involved in the catalytic activity. Therefore, copper chelation of compound 1 is one of the important mechanisms for the inhibition of PPO.
In conclusion, PPO from Penaeus vannamei cephalothorax, with a molecular weight of 200–220 kDa, exhibited maximal activity for the oxidation of L-DOPA under the conditions of pH 6.0 and 50 °C. The kinetic parameters Km and Vmax values were measured as 3.02 mM and 54.3 U/mg of protein, respectively. In vitro inhibitory effect and activity staining demonstrated hydroxypyridinone derivative 1 can effectively inhibit PPO. Kinetic investigation indicated that 1 is a mixed type inhibitor against PPO. Copper reduction and chelation could be the action modes of 1 in the inhibition on PPO. The present study on the enzymatic characteristics and inhibitory kinetics of PPO from the Penaeus vannamei cephalothorax provides useful information for shrimp preservation. Hydroxypyridinone derivative 1 could find application in controlling the quality of shrimps.
References
Ali MT, Gleeson RA, Wei CI, Marshall MR. Activation mechanisms of Pro-phenoloxidase on melanosis development in Florida Spiny lobster (Panulirus argus) cuticle. J. Food Sci. 59: 1024–1030 (1994)
Augustin MA, Ghazali HM, Hashim H. Polyphenoloxidase from Guava (Psidium guajava L.). J. Sci. Food Agric. 36: 1259–1265 (1985)
Benjakul S, Visessanguan W, Tanaka M. Properties of phenoloxidase isolated from the cephalothorax of Kuruma prawn (Penaeus japonicus). J. Food Biochem. 29: 470–485 (2005)
Burmester T. Origin and evolution of arthropod hemocyanins and related proteins. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 172: 95–107 (2002)
Chen Q, Chen QX, Qiu L, Song KK, Huang H. Inhibitory effect of 4-cyanobenzaldehyde and 4-cyanobenzoic acid on mushroom (Agaricus bisporus) tyrosinase. J. Protein Chem. 22: 607–612 (2003)
Chen JS, Rolle RS, Marshall MR, Wei CI. Comparison of phenoloxidase activity from Florida Spiny lobster and Western Australian lobster. J. Food Sci. 56: 154–157(1991b)
Chen JS, Wei CI, Marshall MR. Inhibition mechanism of kojic acid on polyphenol oxidase. J. Agric. Food Chem. 39: 1897–1901 (1991a)
Chen K, Zhao DY, Chen YL, Wei XY, Li YT, Kong LM, Hider RC, Zhou T. A novel inhibitor against mushroom tyrosinase with a double action mode and its application in controlling the browning of potato. Food Bioprocess. Technol. 10: 2146–2155 (2017)
Chinese National Standard (GB5009.5-2016). National Food Safety Standard: Determination of Protein in Foods. Chinese National Health and Family Planning Commission and China Food and Drug Administration, Beijing, China (2016)
Dai XY, Zhang MX, Wei XY, Hider RC, Zhou T. Novel multifunctional hydroxypyridinone derivatives as potential shrimp preservatives. Food Bioprocess. Technol. 9: 1079–1088 (2016)
Gerdemann C, Eicken C, Krebs B. The crystal structure of catechol oxidase: new insight into the function of type-3 copper proteins. Acc. Chem. Res. 35: 183–191 (2002)
Gollas-Galván T, Hernández-López J, Vargas-Albores F. Prophenoloxidase from brown shrimp (Penaeus californiensis) hemocytes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 122: 77–82 (1999)
Laemmli UK. Cleavage of structure proteins during the assembly of the head of bacteriophage T4. Nature 277: 680–685 (1970)
Lineweaver H, Burk D. The determination of enzyme dissociation constant. J. Am. Chem. Soc. 56: 658–666 (1934)
Matoba Y, Kumagai T, Yamamoto A, Yoshitsu H, Sugiyama M. Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis. J. Biol. Chem. 281: 8981–8990 (2006)
Mayer AM. Polyphenol oxidases in plants and fungi: going places? A review. Phytochemistry 67: 2318–2331 (2006)
Montero P, Avalos A, Perez-Mateos M. Characterization of polyphenoloxidase of prawns (Penaeus japonicus). Alternatives to inhibition: additives and high-pressure treatment. Food Chem. 75: 317–324 (2001)
Nirmal NP, Benjakul S. Inhibitory effect of mimosine on polyphenoloxidase from cephalothoraxes of Pacific white shrimp (Litopenaeus vannamei). J. Agric. Food Chem. 59: 10256–10260 (2011)
Nirmal NP, Benjakul S. Inhibition kinetics of catechin and ferulic acid on polyphenoloxidase from cephalothorax of Pacific white shrimp (Litopenaeus vannamei). Food Chem. 131: 569–573 (2012a)
Nirmal NP, Benjakul S. Biochemical properties of polyphenoloxidase from the cephalothorax of Pacific white shrimp (Litopenaeus vannamei). Int. Aquat. Res. 4(1): article 6 (2012b)
Phonpala Y. Heated sulfur-cotainiing compounds: Properties and the uses for shelf-life extension of Pacific white shrimp (Litopenaeus vannamei) stored in ice. MS thesis, Prince of Songkla University, Hat Yai, Songkhla, Thailand (2010)
Rolle RS, Guizani N, Chen JS, Marshall MR, Yang JS, Wei CI. Purification and characterization of phenoloxidase isoforms from Taiwanese black tiger shrimp (Penaeus Monodon). J. Food Biochem. 15: 17–32 (1991)
Sae-Leaw T, Benjakul S, Simpson BK. Effect of catechin and its derivatives on inhibition of polyphenoloxidase and melanosis of Pacific white shrimp. J. Food Sci. Technol. 54: 1098–1107 (2017)
Sae-Leaw T, Benjakul S, Vongkamjan K. Retardation of melanosis and quality loss of pre-cooked Pacific white shrimp using epigallocatechin gallate with the aid of ultrasound. Food Control. 84: 75–82 (2018)
Shao LL, Wang XL, Chen K, Dong XW, Kong LM, Zhao DY, Hider RC, Zhou T. Novel hydroxypyridinone derivatives containing an oxime ether moiety: Synthesis, inhibition on mushroom tyrosinase and application in anti-browning of fresh-cut apples. Food Chem. 242: 174–181 (2018)
Simpson BK, Marshall MR, Otwell WS. Phenol oxidase from shrimp (Penaeus setiferus): purification and some properties. J. Agric. Food Chem. 35: 918–921 (1987)
Simpson BK, Marshall MR, Otwell WS. Phenoloxidases from pink and white shrimp: kinetic and other properties. J. Food Biochem. 12: 205–217 (1988)
Zamorano J, Martínez-álvarez O, Montero P, Gómezguillén MDC. Characterisation and tissue distribution of polyphenol oxidase of deepwater pink shrimp (Parapenaeus longirostris). Food Chem. 112: 104–111 (2009)
Zhao DY, Zhang MX, Dong XW, Hu YZ, Dai XY, Wei XY, Hider RC, Zhou T. Design and synthesis of novel hydroxypyridinone derivatives as potential tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 26: 3103–3108 (2016)
Acknowledgements
This research work was financially supported by the Natural Science Foundation of Zhejiang Province (No. LY17B020001), and Food Science and Engineering-the most important discipline of Zhejiang Province (2017SIAR216). .
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shao, LL., Zhou, JM., Zhu, Q. et al. Enzymatic characteristics of polyphenoloxidase from shrimp (Penaeus vannamei) and its inhibition by a novel hydroxypyridinone derivative. Food Sci Biotechnol 28, 1047–1055 (2019). https://doi.org/10.1007/s10068-018-00544-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-018-00544-x