Abstract
Long-term topical skin care by traditional anti-melanogenic agents can raise several safety concerns. An understanding of the molecular mechanisms of active compounds on melanogenesis is, therefore, necessary to address pigmentation issues. Here we revealed that stimulation with 1 mM betaine, an abundant component in rice bran, significantly reduced 21% of intracellular melanin content by suppressing tyrosinase activity and microphthalmia-associated transcription factor (MITF) expression in B16-F1 murine melanocytes. The expression of MITF was suppressed at both mRNA and protein levels by 43 and 44%, respectively. Subsequently, the betaine-stimulated melanocytes showed inhibition of PKA-CREB signaling axis but activation of extracellular-signal-regulated kinase and AKT-GSK3β signaling pathways. This inhibition and activation led to downregulation of MITF expression at both the transcriptional and post-translational levels to suppress melanin synthesis. These findings collectively suggested that betaine is a potential anti-melanogenic compound for functional foods and cosmetics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
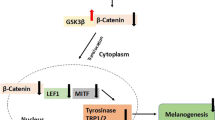
Melanin synthesis, termed as melanogenesis, is a prerequisite for defending the skin from the UV-induced DNA damage. However, overproduction of melanin could cause medical problems including Addison’s disease and melanoma [1]. Melanogenesis is a complex biological process triggered by both exogenous (UV ray, X-ray, and photoaging chemicals) and endogenous factors (hormones and cytokines) [2]. Melanogenic gene expression is regulated by multiple mechanisms, where microphthalmia-associated transcription factor (MITF) is a key transcriptional factor regulating tyrosinase and dopachrome tautomerase gene expressions, which are two key rate-limiting enzymes [1, 3]. MITF activity is regulated at both transcriptional and post-translational levels. The transcription of MITF is positively regulated by protein kinase A (PKA)-cAMP response element-binding (CREB) signaling axis [4, 5]. Alternatively, activation of the extracellular-signal-regulated kinase (ERK) has known to promote ubiquitin-dependent proteasome degradation of MITF, especially in the high activation of PKA-CREB signaling cascade [6,7,8,9]. In addition, activation of AKT is evidenced to suppress MITF activity by inhibitory phosphorylation on GSK3β [10, 11]. Thus, inhibition of PKA-CREB but activation of ERK and/or AKT-GSK3β pathways in melanocytes could reduce melanin synthesis and have hypopigmentation activity.
Natural and botanical compounds are widely used as traditional anti-pigmenting agents. However, long-term exposure of these traditional anti-pigmenting agents to skin often causes safety issues including atrophy, carcinogenesis, ochronosis, and other local or systemic side effects [12]. Thus, an understanding of the molecular mechanism of anti-melanogenic active compounds is necessary to develop novel depigmenting products. Previously, we screened an anti-melanogenic activity of several botanical extracts [13]. Our preliminary results revealed that betaine showed the most prominent activity among the 14 selected single compounds extracted from Korean rice bran (Oryza sativa sp.). Herein, we further investigated the efficacy and molecular mechanism of betaine on melanogenesis in B16-F1 murine melanocytes.
Materials and methods
Reagents and cell culture
Arbutin, mushroom tyrosinase, betaine, L-3,4-Dihydroxyphenylalanine, protease and phosphatase inhibitor cocktail, iQ SYBR Green Supermix, bovine serum albumin, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide were purchased from Sigma (USA). B16-F1 murine melanocytes were cultured and maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA) and were supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 0.1 mg/mL streptomycin (PEST, Welgene Inc., Korea) at 37 °C, 5% CO2 incubator.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
B16-F1 murine melanocytes were seeded in 96-well plate overnight (104 cells/well) and then treated with 1 mM arbutin and 0.5 mM or 1 mM betaine for 72 h. Cell viability was assayed using the MTT assay. The culture medium was removed from each well, and 200 µL of 10% MTT solution in culture medium was added and incubated for 3 h. The MTT solution was then removed and the cells were dried for 30 min. Then, 200 µL of dimethyl sulfoxide was added and incubated for 1 h with mild shaking. The absorbance at 570 nm was measured in a Multiskan GO microplate spectrophotometer (Thermo Fisher Scientific, USA).
Tyrosinase activity assay
Cellular tyrosinase activity was determined as described previously [13]. Briefly, cells were seeded and treated with 1 mM arbutin and 0.5 mM or 1 mM betaine for 72 h. Then the cells were washed twice with ice-cold PBS and lysed by using 300 µL of 20 mM Tris–HCl pH 7.5 supplemented with 0.1% Triton X-100 (Sigma, USA). The cell lysate was centrifuged at 12,000 rpm for 10 min at 4 °C. Then 50 µL of the supernatant was mixed with 140 µL reaction solution [0.1% L-3,4-Dihydroxyphenylalanine in 100 mM sodium phosphate (pH 6.0)] and 70 µL 400 U/mL mushroom tyrosinase in 96-well microplate. Tyrosinase activity was determined after incubation for 2 h at a wavelength of 475 nm. The activity was normalized to protein concentration.
Cell-free tyrosinase activity was determined as described previously [13]. Briefly, 50 µL of 0.03% (w/v) tyrosine solution in distilled water, 75 µL of 1 mM arbutin, 0.5 mM or 1 mM betaine in 0.1 M phosphate buffer, and 25 µL of 400 U/mL mushroom tyrosinase in 0.1 M phosphate buffer were added to each well in the 96-well microplate. The mixture was then incubated at 37 °C for 20 min, and the cell-free tyrosinase activity was determined at a wavelength of 475 nm. The activity was normalized to protein concentration.
Cellular melanin content
B16-F1 murine melanocytes were seeded overnight and then treated with 1 mM arbutin and 0.5 mM or 1 mM betaine for 72 h. Then, the cells were washed twice with ice-cold PBS and lysed using 100 µL of 20 mM Tris–HCl pH 7.5 supplemented with 0.1% Triton X-100 (Sigma, USA). The cell lysate was centrifuged at 12,000 rpm for 10 min at 4 °C. The pellets were collected and dissolved in 300 µL of 1 N NaOH at 60 °C for 1 h. Then, 200 µL of the solution was transferred to a 96-well microplate, and the cellular melanin contents were measured at a wavelength of 400 nm and normalized to protein concentration.
Intracellular melanin was stained following the Fontana-Masson method. Briefly, melanocytes were fixed with 4% paraformaldehyde for 30 min at ambient temperature. The cells were then stained with an ammoniacal silver solution at 60 °C and then treated with 0.1% gold chloride for 1 min followed by 5% sodium thiosulfate for 5 min. Images were obtained and analyzed by using a phase contrast microscope (Nikon, USA).
Total RNA isolation and quantitative real-time PCR (qPCR)
Total RNA was isolated from cells using the TRIzol reagent kit (Invitrogen, USA). The cDNA was synthesized from 2 μg of the total RNA with reverse transcriptase. The qPCR was performed using the iQ SYBR Green Supermix (Takara Bio Inc., Japan) as described elsewhere [14]. Data were recorded and analyzed by using the SYBR Green optic channel at a wavelength of 490 nm (iCycler iQ, Bio-Rad, USA).
Immunoblot
Cells were lysed in a RIPA buffer supplemented with cocktails of protease and phosphatase inhibitors at 4 °C. Equal protein concentration (40 μg) was used for the immunoblotting analysis. Samples were probed with appropriate primary antibodies in 100 mM Tris–HCl pH 7.4 supplemented with 0.1% Tween 20 (TBS-T) at 4 °C followed by washing for 30 min. Samples were incubated with the secondary antibody labeled with horseradish peroxidase for 1 h. Immunoreactive bands were determined by enhanced chemiluminescence immunoblotting detection reagents and quantified by using the ChemiDoc™ XRS system (Bio-Rad, USA).
Direct cAMP measurement and PKA kinase activity assay
Cells were lysed with 100 mM HCl for 10 min at ambient temperature to stop endogenous phosphodiesterase activity and to stabilize the released cAMP. Solutions were immediately analyzed in a microtiter plate with the cAMP enzyme-linked immunometric kit (Enzo Life Science Inc., USA) following the manufacturer’s description. The measured cAMP level was normalized to protein concentration. PKA kinase activity was determined by the enzyme-linked immonosorbant kit (Abcam, USA). Cell lysate was incubated with 3.3′,5,5′-tetramethylbenzidine substrate and the absorbance was measured at a wavelength of 450 nm. Activity from concentration range of purity PKA was used as a standard curve.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). Student’s t test or one-way ANOVA followed by Tukey test analyzed by GraphPad Prism were used for two or multiple group comparisons, respectively. A value of P < 0.05 was considered to be the statistically significant difference.
Results and discussion
Betaine reduces intracellular melanin content in B16-F1 murine melanocytes
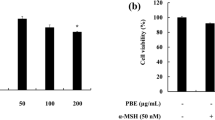
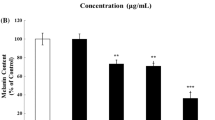
The preliminary anti-melanogenic activity assay revealed that betaine showed the most prominent activity among the 14 examined single compounds (Data not shown). The effects of betaine on melanogenesis were investigated in B16-F1 murine melanocytes. The MTT assay revealed that there was no cellular toxicity in melanocytes treated with 1 mM arbutin and 0.5 mM or 1 mM betaine (Fig. 1A). Treatment of 1 mM betaine significantly inactivated mushroom tyrosinase activity by 34% together with cellular tyrosinase activity by 22% compared with those of controls, whereas arbutin reduced the cellular enzyme activity by 10% (Fig. 1B). However, no significant reduction in tyrosinase activity was observed in melanocytes treated with lower concentration of betaine (0.5 mM). Tyrosinase is a critical melanogenic enzyme that catalyzes the first two steps of melanin production [3]. Therefore, next, we investigated the effects of betaine on melanin content. Stimulation of 1 mM betaine in melanocytes showed a significant reduction in the intracellular melanin content by 22% compared with control (Fig. 1C). Importantly, a similar reduction trend was observed in the cells stimulated with either 1 mM betaine or arbutin, a well-known anti-melanogenic agent (Fig. 1C). The reduction of intracellular melanin content by 1 mM betaine was also confirmed by the Fontana-Masson staining (Fig. 1D). These results indicated that stimulation of 1 mM but not 0.5 mM betaine reduces intracellular melanin content in B16-F1 murine melanocytes; thus, 1 mM betaine was used for further experiments. These results, therefore, suggested that betaine has a hypopigmentation activity via, at least in part, direct inhibition on the melanogenic enzyme activity.
Betaine reduces intracellular melanin content in B16-F1 murine melanocytes. (A) Cell viability of B16-F1 murine melanocytes stimulated with 1 mM arbutin and 0.5 mM or 1 mM betaine. (B) Tyrosinase activity. Cell-free mushroom tyrosinase and cellular tyrosinase activities were measured. (C) Cellular melanin content in B16-F1 murine melanocytes stimulated with 1 mM arbutin and 0.5 mM or 1 mM betaine. (D) Fontana-Masson staining of melanin contents in B16-F1 murine melanocytes stimulated with 1 mM arbutin or 1 mM betaine. Melanin (dark brown) was visualized after 72 h of the stimulation. P values compared with controls are denoted as *P < 0.05, **P < 0.01. (Color figure online)
Betaine suppresses MITF activity and melanogenic enzymes
MITF plays a key role in the regulation of two essential melanogenic enzymes, tyrosinase and dopachrome tautomerase [2, 3]. Thus, we assessed the gene and protein expression levels of MITF and its targets. The betaine stimulation reduced the MITF mRNA level by 43% compared with the control (Fig. 2A). Consistently, the mRNA expression levels of tyrosinase and dopachrome tautomerase were also decreased by 22 and 24%, respectively, in the betaine-stimulated melanocytes (Fig. 2A). We subsequently observed that the MITF protein level was significantly decreased by 44%, followed by the reduction of tyrosinase and dopachrome tautomerase protein levels by approximately 78 and 44%, respectively (Fig. 2B). These results suggested that betaine downregulates the expression of MITF followed by its transcriptional target genes in melanogenesis.
Betaine regulates cellular signaling pathways to suppress MITF activity
To explore the downregulation mechanism of MITF by betaine, we examined the effect of betaine on cellular signaling that regulates MITF expression in the melanocytes. It has been reported that MITF is regulated at both transcriptional and post-translational levels involving PKA-CREB [4, 5], AKT-GSK3β [10, 11], and ERK pathways [6,7,8,9]. In the betaine-stimulated cells, the results showed that cellular cAMP concentration was substantially reduced by 10% (Fig. 3A). Subsequently, the cellular PKA kinase activity was inhibited approximately 50% by betaine (Fig. 3B). Hence, we further examined phosphorylation of CREB, a PKA target and transcription factor, which upregulates MITF gene expression [5]. The immunoblot data showed that CREBSer133 phosphorylation was significantly reduced by 46% compared with control (Fig. 3C). Alternatively, we observed betaine markedly elevated the ERKThr202/Tyr204 phosphorylation level by 527% (Fig. 3C). ERK directly phosphorylates MITF at Serine 73 and indirectly phosphorylates MITF at Serine 409. Thus, ERK promotes ubiquitin-dependent proteasome degradation [6,7,8,9]. Therefore, activation of ERK can suppress the MITF protein expression level. In addition, immunoblot results also showed that betaine increased AKTSer473 phosphorylation by 17% and GSK3βSer9 phosphorylation by 61% (Fig. 3C). The GSK3β is evidenced to phosphorylate MITF at Serine 298 that induces the binding of MITF to the tyrosinase promoter and upregulates the gene expression of tyrosinase [10, 11]. Inhibitory phosphorylation on GSK3βSer9 by AKT, therefore, reduces MITF activity and suppresses MITF target gene expression.
Betaine regulates cellular signaling molecules suppressing MITF activity. (A) and (B) Cellular cAMP concentration and PKA kinase activity were quantified after 30 min and 1 h of the betaine stimulation, respectively. (C) Protein expression levels of CREB, ERK, AKT, GSK-3β and their phosphorylated forms
Next, we stimulated the melanocytes with phosphatidylinositol 3-kinase inhibitor, LY294002, which abrogates AKTSer473 phosphorylation. The results showed that betaine stimulation suppressed the expression of the MITF protein by 62%, but LY294002 induced MITF expression level by 28%. Importantly, co-treatment with both betaine and LY294002 abrogated the suppression of MITF protein expression by betaine (Fig. 4A). These data suggested that the suppression of MITF protein level by betaine requires activation of AKT-GSK3β signaling axis. Collectively, our results suggested that betaine reduces MITF gene expression by multiple mechanisms; inhibition of PKA-CREB and activation of ERK and AKT-GSK3β signaling pathways (Fig. 4B).
The existing skin-lightening agents have been reported to have several side effects to human skin; thus, the development of safe and natural compounds inhibiting melanogenesis has been actively progressed. In the present study, the natural and botanical compound betaine exhibited strong inhibitory effects for melanogenesis, whose activity is comparable to the well-known tyrosinase inhibitor, arbutin. Inhibitory effects of skin-lightening agents mainly function through two strategies: direct inhibition of tyrosinase enzyme activity or suppression of MITF and tyrosinase gene expression. Tyrosinase is the rate-limited enzyme involved in catalyzing the first two steps of melanin production [3]; thus, most of the skin-lightening agents are developed based on their inhibition effects on tyrosinase enzyme activity. In B16-F1 murine melanocytes, stimulation with 1 mM betaine significantly reduced tyrosinase enzyme activity, as well as the gene and protein expression levels of MITF and its downstream targets, tyrosinase and dopachrome tautomerase. Taken together, these results demonstrated that the inhibitory effects of betaine on melanogenesis are attributed to both the direct inhibition of tyrosinase enzyme activity and the suppression of MITF and tyrosinase gene expression.
The expression of MITF and its downstream targets tyrosinase and dopachrome tautomerase can be regulated by several signaling pathways, including cAMP-PKA-CREB, AKT-GSK3β, and ERK pathways [4,5,6,7,8,9,10,11]. The cAMP/PKA signaling induces CREB activity through phosphorylation at Ser133, which subsequently stimulates MITF promoter activity and its expression levels [4]. Thus, suppression of cAMP-PKA-CREB signaling pathway would decrease the expression of MITF and its downstream targets. AKT-GSK3β and ERK activation both induce MITF phosphorylation, which promotes the binding of MITF to tyrosinase promoter and MITF degradation, respectively, leading to the reduction of tyrosinase gene and protein expression [7,8,9,10]. Betaine stimulation decreases the phosphorylation level of CREB with activated AKT-GSK3β and ERK signaling pathways, leading to the reduction of MITF and its downstream targets tyrosinase and dopachrome tautomerase.
In conclusion, our data suggested that betaine is a potential skin-lightening agent. Betaine reduces intracellular melanin content that was achieved by dual mechanisms: direct inhibition of tyrosinase enzyme activity and downregulation of MITF in terms of both transcriptional and post-translational levels (Fig. 4B). For that, three signaling pathways appear to be involved simultaneously: suppression of PKA-CREB, activation of AKT-GSK3β, and activation of ERK signaling pathways. Further studies should be performed to confirm such a detail function of betaine in each of the pathways of melanocytes.
References
Solano F, Briganti S, Picardo M, Ghanem G. Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res. 19(6): 550–571 (2006)
Cheli Y, Ohanna M, Ballotti R, Bertolotto C. Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment Cell Melanoma Res. 23(1): 27–40 (2010)
Gillbro JM, Olsson MJ. The melanogenesis and mechanisms of skin-lightening agents–existing and new approaches. Int. J. Cosmetic Sci. 33(3): 210–221 (2011)
Bertolotto C, Abbe P, Hemesath TJ, Bille K, Fisher DE, Ortonne JP, Ballotti R. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J. Cell Biol. 142(3): 827–835 (1998)
Busca R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 13(2): 60–69 (2000)
Wellbrock C, Marais R. Elevated expression of MITF counteracts B-RAF-stimulated melanocyte and melanoma cell proliferation. J. Cell Biol. 170(5): 703–708 (2005)
Wellbrock C, Weisser C, Geissinger E, Troppmair J, Schartl M. Activation of p59(Fyn) leads to melanocyte dedifferentiation by influencing MKP-1-regulated mitogen-activated protein kinase signaling. J. Biol. Chem. 277(8): 6443–6454 (2002)
Wu M, Hemesath TJ, Takemoto CM, Horstmann MA, Wells AG, Price ER, Fisher DZ, Fisher DE. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 14(3): 301–312 (2000)
Wellbrock C, Arozarena I. Microphthalmia-associated transcription factor in melanoma development and MAP-kinase pathway targeted therapy. Pigment Cell Melanoma Res. 28(4): 390–406 (2015)
Bellei B, Flori E, Izzo E, Maresca V, Picardo M. GSK3beta inhibition promotes melanogenesis in mouse B16 melanoma cells and normal human melanocytes. Cellular Signal. 20(10): 1750–1761 (2008)
Khaled M, Larribere L, Bille K, Aberdam E, Ortonne JP, Ballotti R, Bertolotto C. Glycogen synthase kinase 3beta is activated by cAMP and plays an active role in the regulation of melanogenesis. J. Biol. Chem. 277(37): 33690–33697 (2002)
Zhu W, Gao J. The use of botanical extracts as topical skin-lightening agents for the improvement of skin pigmentation disorders. J. Investig. Dermatol. Symp. Proc. 12(1): 20–24 (2008)
Jun HJ, Lee JH, Cho BR, Seo WD, Kang HW, Kim DW, Cho KJ, Lee SJ. Dual inhibition of gamma-oryzanol on cellular melanogenesis: inhibition of tyrosinase activity and reduction of melanogenic gene expression by a protein kinase A-dependent mechanism. J. Nat. Prod. 75(10): 1706–1711 (2012)
Jia Y, Kim JY, Jun HJ, Kim SJ, Lee JH, Hoang MH, Hwang KY, Um SJ, Chang HL, Lee SJ. The natural carotenoid astaxanthin, a PPAR-alpha agonist and PPAR-gamma antagonist, reduces hepatic lipid accumulation by rewiring the transcriptome in lipid-loaded hepatocytes. Mol. Nutr. Food Res. 56(6): 878–888 (2012)
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. NRF-2016R1A2A2A05005483), and a grant from Cooperative Research Program for Agriculture Science and Technology Development, Rural Development Administration, Republic of Korea (Project No. PJ011253042017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cho, BR., Jun, Hj., Thach, T.T. et al. Betaine reduces cellular melanin content via suppression of microphthalmia-associated transcription factor in B16-F1 murine melanocytes. Food Sci Biotechnol 26, 1391–1397 (2017). https://doi.org/10.1007/s10068-017-0171-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-017-0171-6